Abstract
Desmoid tumors are uncommon benign tumors with locally aggressive behavior. Even with aggressive resection with or without radiotherapy, the relapse rate is relatively high. Surgical resection can be challenging especially in large size tumors, as the resultant post-operative wide wall defect can lead to cosmetically undesirable bulging and functional loss. The use of Ultrasound may be useful to aid in radical resection of the desmoid tumor with adequate margins. In our 30-year-old patient with recurrent abdominal wall desmoid tumor, intra-operative ultrasound was utilized in successful resection of the tumor followed by a minimal but adequate margin. The patient has recovered well and within the 6 months follow-up period and has not reported any functional limitations or recurrence.
INTRODUCTION
Desmoid tumors (also known as aggressive fibromatosis) are benign tumors with locally aggressive behavior that has no known metastatic potential. The incidence of desmoid tumors in the general population is two to four per million populations per year [1]. Desmoid tumors are rare. They make 0.03% of all neoplasms and less than 3% of all soft tissue tumors [2]. Even with aggressive resection with or without radiotherapy, the relapse rate is relatively high. Considerable number of women who present with desmoid-type fibromatosis (DF) have had a recent pregnancy. Pregnancy-related DF has better outcomes [3]. In ultrasound, desmoids frequently appear as oval, well or poorly defined solid soft tissue masses with variable echogenicity. [4]. Ultrasound may also be useful diagnostically and to aid radical resection of the desmoid tumor with adequate margins and minimal defect [5].
CASE PRESENTATION
A 30-year-old female with no known medical condition, underwent a caesarian section in 2014 and started to complain of abdominal mass 2 years later. She underwent resection in another institution and the pathology showed insufficient resection reaching the superior margin, <1 mm from the lateral margin, and 1 mm from inferior and deep margins. The patient was offered radiotherapy, however she refused.
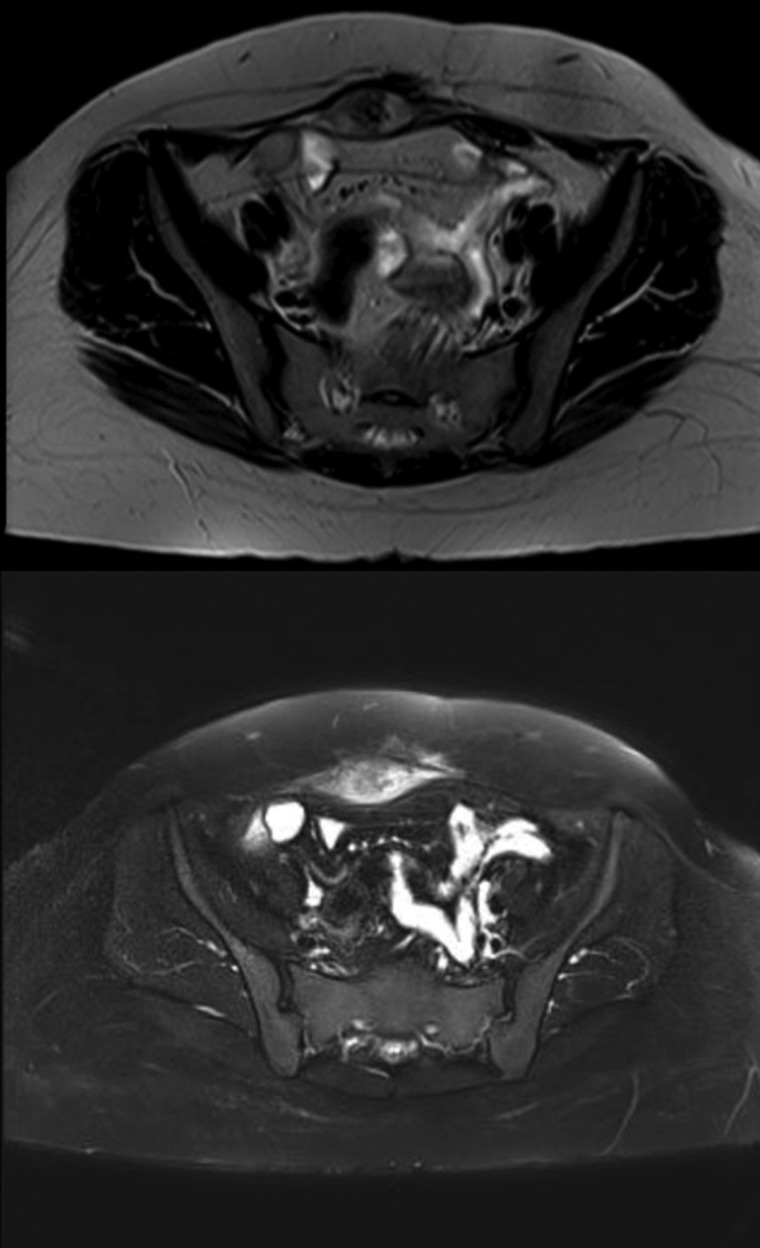
She was followed up with biannual MRI’s with no recurrence. Two years later, the patient noticed a growing mass. The follow-up MRI revealed development of a well-defined oval-shaped soft tissue lesion at the site of the previously resected anterior abdominal wall desmoid tumor measuring 4.3 × 2 cm, heterogeneously hypertense on T2, hypointense on T1 and significant enhancement on the delayed post contrast images, in keeping with a recurrent desmoid tumor (Fig. 1). Following the MRI, the patient was referred to our institution.
Figure 1:
MRI images demonstrating the recurrent abdominal wall desmoid tumor located in the right lower abdomen, which is heterogeneously hypertense on T2 and hypointense on T1.
The mass presented with a gradual increase in size and moderate pain. On physical examination there was a 5 × 3 cm hard, well-defined mass in the right lower quadrant. There were no skin changes. An MRI revealed a well-defined soft tissue mass confined to the right rectus abdominus muscle. A Tru-cut biopsy from the mass revealed a spindle cell neoplasm consistent with recurrent desmoid tumor. The patient’s family history was insignificant for malignancy. All her laboratory investigations and tumor markers were normal. After counseling the patient, resection was performed, using the advantage of intra-operative ultrasound (US) to localize the margin providing the patient maximal surgical benefit with minimal risk of a positive margin.
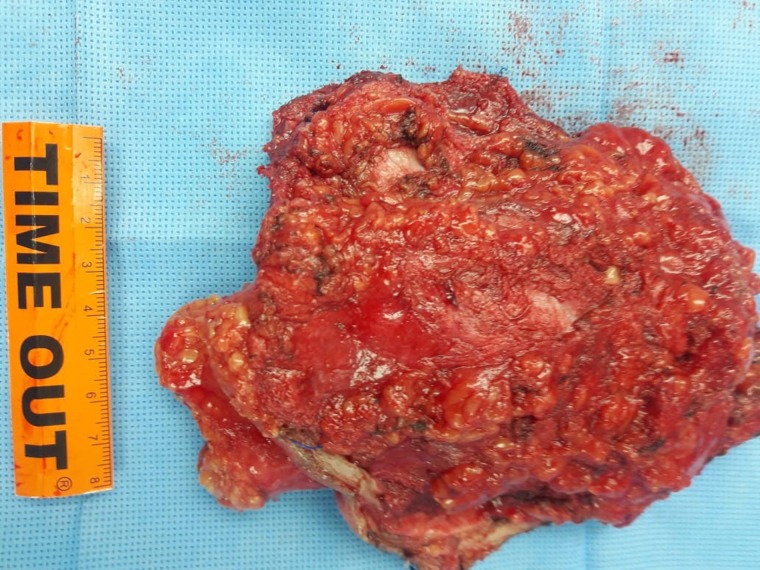
Rather than simply using palpation, intraoperative ultrasound guidance was used to determine the margins of the desmoid tumor. The ultrasound was used to define a 2 cm margin at each qudrant, which was then marked using electrocautery. The marks were then connected in a ring giving a 2 cm margin around the entire tumor via which excision was performed and an additional 2 cm were measured. Circumferential dissection was performed around the tumor until the previous surgical mesh (Fig. 2).
Figure 2:
An image of the surgical specimen after ultrasound aided resection of the desmoid tumor with 2 cm margin.
Final reconstruction of the abdominal wall using anterior or posterior wall component separation was postponed because of the uncertainty of the safety margin prior to final pathology and also because the patient plans further conception. A dual mesh was utilized in underlay fashion to bridge the large defect.
The postoperative course was uneventful, and the patient was discharged home on Day 5 in a stable condition. The patient was seen after 3 weeks and the final pathology report revealed completely free resection margins. In the 6 months follow-up, the patient reported no functional limitations or recurrence.
DISCUSSION
Pregnancy associated desmoid tumors have been a subject of interest. They have good outcomes and are not associated with increased risk in future pregnancies [3]. They have a variable clinical behavior. Some desmoids may grow progressively larger over time. It is common to experience indolent growth, periods of growth arrest and spontaneous regression [5–8]
Treatment options include active surveillance, surgical resection, radiotherapy or systemic treatment.
Active surveillance is increasingly being utilized as the treatment of choice for patients with minimal symptoms. Patients should be monitored every 3–6 months initially, and if disease is stable this can be extended [7]. For progressive or symptomatic cases, surgical resection with negative pathologic margins is the mainstay of therapy, though, the prognostic significance of achieving negative surgical margins remains a subject of debate, with some studies showing higher recurrence rates when negative margins are not achieved and others showing risk of recurrence is independent from margin status [8]. Post-operative radiotherapy is not typically recommended in the adjuvant setting after complete surgical resection. Utilization of radiotherapy in patients with close or positive margins is a subject of debate.
Systemic treatment options exist for locally advanced or unresectable desmoid tumors. Nonsteroidal anti-inflammatory drugs (sulindac or celecoxib), hormonal agents (tamoxifen and toremifene), and chemotherapy have all been described to have an efficacy for desmoid tumors [9]. Recently, imatinib and sorafenib have both demonstrated promise [10].
Factors associated with increased recurrence rate include: site (extremities having the worst prognosis, trunk/abdominal wall tumors have a lower rate of recurrence than either intra-abdominal or extra-abdominal disease [8], size >7 cm, female gender, and younger age [10].
Our patient first developed abdominal desmoid tumor 2 years after caesarian section. She had a primary resection in a different institute with positive margin </=1 mm. She had been offered adjuvant radiotherapy ± hormonal treatment but she refused, so wait and watch approach was adopted with biannual MRI done as follow-up till another recurrence was evident almost 2 years after primary surgery. The recurrence was less than 7 cm and painful, so surgery was discussed.
In abdominal desmoid tumors, surgical resection can be challenging and new approaches should be considered to obtain oncological radicality, minimal tissue loss to avoid wide wall defect, postoperative wall bulging and functional loss. One of these approaches is using the Ultrasound in radical resection of desmoid tumor. Diagnostically, MRI is the gold standard for abdominal wall desmoid tumors identification, however Ultrasound could be helpful in defining the tumor with variable echogenicity [3].
‘Ultrasound guided sparing resection’ is useful to identify minimal tissue safe margins (1–2 cm), an oncologically correct resection, and the best reconstruction opportunity.
Surgical technique is usually based on preoperative or intraoperative US exploration using a probe to define the lesion margins and depth before cutting. Tumor wall thickness can be measured by deep ‘wall intraoperative US exploration’ (WIOUS), which is a beneficial method used in the literature to describe circumferential margin underling the transition area between the tumor and surrounding free muscle tissue with a distance from the lesion radius. Determining the wall thickness accurately via intraoperative ultrasound facilitates safe surgical margins without sacrificing excessive normal tissue [1].
The desmoid tumor shape is recognizable by US ‘fig2’, allowing well defined free margin to be obtained [1, 4]. Contradictory data regarding the surgical margin as independent factor for local recurrence led to no consensus in regard to the optimal minimum margin to be followed to achieve negative pathological margin. In the only study describing this approach, a 1 cm negative margin was adopted as the minimal tissue safe margin [4], however for our patient, taking into consideration that we are resecting local recurrence, we preferred to extend our minimal margin to 2 cm in all directions, aided by the ultrasound, which barely helped in having negative margins in all four sides.
Frozen section failed to confirm negative margins microscopically because of excessive fibrosis, which also affected the decision to proceed with definitive abdominal wall reconstruction in the same setting. The literature review about desmoid type fibromatosis within the abdominal wall showed no consensus regarding the surgical technique to close abdominal defects.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bolzon S, Vagliasindi A, Zanzi F, Negri M, Guerrini GP, Rossi C, et al. Abdominal wall desmoid tumors: a proposal for US-guided resection. Int J Surg Case Rep 2015;9:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonvalot S, Ternès N, Fiore M, Bitsakou G, Colombo C, Honoré C, et al. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann Surg Oncol 2013;20:4096–4102. 10.1245/s10434-013-3197-x. [DOI] [PubMed] [Google Scholar]
- 3. Burtenshaw SM, Cannell AJ, McAlister ED, Siddique S, Kandel R, Blackstein ME, et al. Toward observation as first-line management in abdominal desmoid tumors. Ann Surg Oncol 2016;23:2212–9. 10.1245/s10434-016-5159-6. [DOI] [PubMed] [Google Scholar]
- 4. Crago AM, Denton B, Salas S, Dufresne A, Mezhir AJ, Hameed M, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg Oncol 2013;258:347–53. 10.1097/SLA.0b013e31828c8a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Cian F, Delay E, Rudigoz RC, Ranchère D, Rivoire M. Desmoid tumor arising in a cesarean section scar during pregnancy: monitoring and management. Gynecol Oncol 1999;75:145–8. [DOI] [PubMed] [Google Scholar]
- 6. Fiore M, Coppola S, Cannell AJ, Colombo C, Bertagnolli MM, George S, et al. Desmoid-type fibromatosis and pregnancy. Ann Surg 2014;259:973–8. [DOI] [PubMed] [Google Scholar]
- 7. Garbay D, Le Cesne A, Penel N, Chevreau C, Marec-Berard P, Blay JY, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG). Ann Oncol 2012;23:182–6. 10.1093/annonc/mdr051.mdr051. [DOI] [PubMed] [Google Scholar]
- 8. Lou L, Teng J, Qi H, Ban Y. Sonographic appearances of desmoid tumors. J Ultrasound Med 2014;33:1519–25. [DOI] [PubMed] [Google Scholar]
- 9. Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol 2011;22:452–7. 10.1093/annonc/mdq341. [DOI] [PubMed] [Google Scholar]
- 10. Salas S, Dufresne A, Bui B, Blay JY, Terrier P, Ranchere-Vince D, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: A wait-and-see policy according to tumor presentation. J Clin Oncol 2011;29:3553–8. 10.1200/JCO.2010.33.5489. [DOI] [PubMed] [Google Scholar]