Abstract
Liposarcoma is the most common retroperitoneal sarcoma and mesenchymal tumor in the abdomen. Usually, it presents with vague symptoms due to its large size and slow growth at the time of diagnosis. Liposarcoma is associated with a high local recurrence rate according to its histology, size and growth rate. Up till now, surgical resection is the only effective treatment for primary and recurrent abdominal liposarcoma.
Secondary mesenteric liposarcoma is an extremely rare entity and so far a small number of cases have been reported in the literature. In this article, we present a rare case of a 63-year-old female patient who was diagnosed with mesenteric liposarcoma after 3 years of complete excision of retroperitoneal liposarcoma, presenting primarily as abdominal mass causing mechanical intestinal obstruction.
INTRODUCTION
Soft tissue sarcoma (STS) accounts for <1% of all malignant tumors in adults with liposarcoma being the most common variant [1]. Retroperitoneum (RP) forms a major site for liposarcoma that accounts for 10–15% of the soft-tissue tumors and one-third of malignant tumors located in the RP [2]. Well-differentiated (WD) liposarcoma is the most common subtype, its growth is the slowest, has more gradual progression, lower recurrence rate, and better survival rates.
We report a case of a 63-year-old female patient who had a huge retroperitoneal sarcoma that was completely cured with surgical resection in 2015 and presented again in 2018 with mesenteric liposarcoma causing symptoms of intestinal obstruction.
CASE REPORT
We report a case of a 63-year-old female patient who was admitted to the surgical ward on the 10th of November in 2015 with a progressive volumetric increase of the abdomen over the last month with no GI symptoms, weight loss, pain, fever or vaginal bleeding, which failed to reduce with exercise and diet programs, so she sought medical advice.
She had a free past medical, surgical and family history of malignancy or liver disease. Physical examination revealed a distended abdomen with a palpable mass about 3 cm below the xiphoid process, about 20 cm in diameter, which was hard and had limited mobility. The values of laboratory tests, including tumor markers (carcinoembryonic antigen, alpha-fetoprotein, cancer antigen 125, and cancer antigen 15-3) on admission were within the normal ranges. Abdominal computed tomography (CT) showed two large lobulated masses in both sides of the pelvis with dominant heterogeneous mass lesion in the right side measuring 15x10 cm with central fatty component causing displacement of vessels and bowel and another small left Para-aortic lesion measured 5x5cm (Fig. 1); Therefore, elective laparotomy with midline incision was approached with gross total resection of 35×22×17 cm retroperitoneal mass with smooth regular surface and without resection of any adjacent organs (Fig. 2A and B).
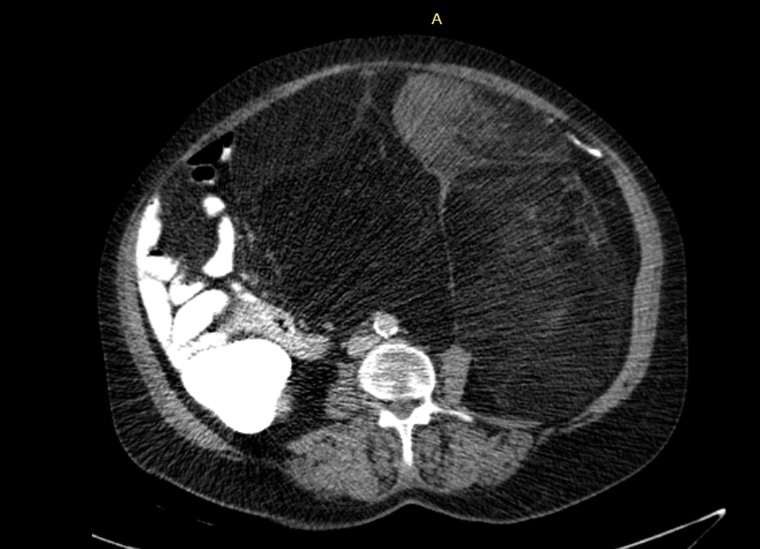
Figure 1:
Abdominal CT: The mesenteric fat in the Abdominal cavity is bulky and heterogeneous with soft tissue component anteriorly.
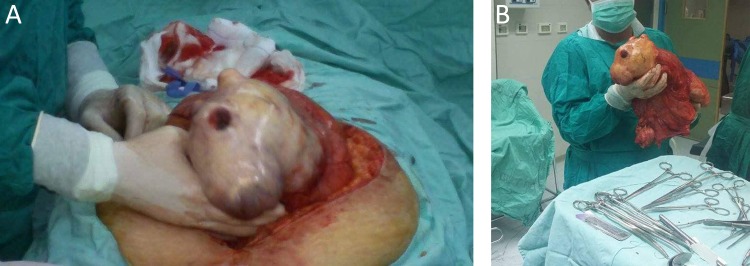
Figure 2:
A) laparotomy with midline incision was approached for resection. (B) Gross total resection of the 35×22×17 cm retroperitoneal mass with smooth regular surface and without resection of any adjacent organs.
Histopathological testing revealed a low grade well differentiated multi-lobulated liposarcoma with minimal myxoid components and negative microscopic margins. immunohistochemical staining were performed and showed that the neoplastic cells are positive for S-100, while they are negative for CD 34 and C-kit.
After discussion of a multidisciplinary team and with consideration of the low grade, negative margins of the mass and repeated free Positron emission tomography (PET) scan, no radiotherapy or chemotherapy was administered and close follow-up has been performed. PET scan was done later on January 2016 and March 2017, the patient has remained healthy without any evidence of recurrence or metastasis either clinically or on PET scan.
After that, the patient stopped following up until September 2018, when she presented to the surgical ward with symptoms and signs of intestinal obstruction that progressed over the past week. On admission, ultrasound (US) showed 7.3×6 cm complex cystic mass in the right pelvic cavity with massive bowel gases all over, another assessment by abdominal CT scan was done and showed right lower abdominal 6.5×7 cm lobulated cystic lesion involving the terminal ileum with minimal wall enhancement, so the next day it was operated urgently to relieve the obstruction with gross total resection of the mass with 30 cm ilium segment and primary anastomosis was successful (Fig. 3).
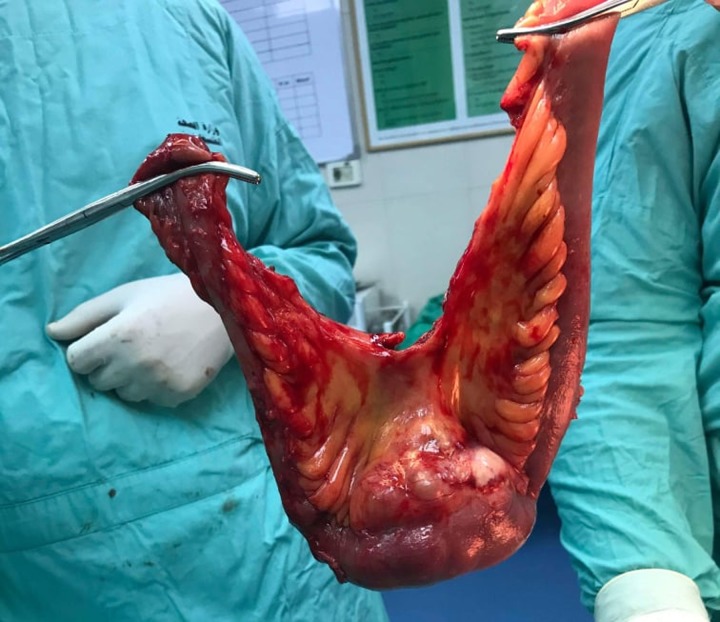
Figure 3:
Gross total resection of the mass with 30 cm ileum segment.
Histopathological testing results were as follows: CD 56, Smooth muscle actin and S100 protein were positive (Fig. 4A and B). The average Ki-67 proliferation index in tumor cells was 10%, stains for EMA, DOG1, CD34, and Desmin were negative, with three reactive lymph nodes; confirmed as well differentiated mesenteric liposarcoma with free margins. The postoperative course was uneventful and the patient was discharged on the eighth postoperative day.
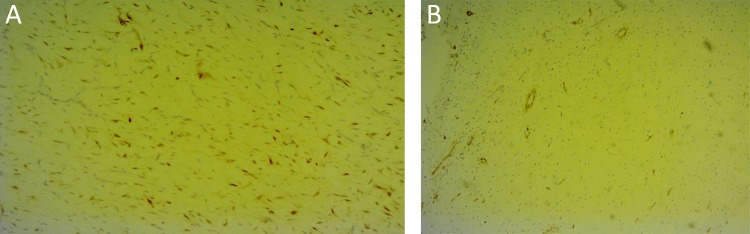
Figure 4:
A) Immunohistochemistry: Adipocytic cells exhibit S-100 protein immunoreactivity highlighting the presence of lipoblasts. (B) smooth muscle actin.
DISCUSSION
Liposarcomas are malignant tumors arising from mesenchymal cells and account for more than 20% of all sarcomas in adults. [1] According to WHO classification, the various subtypes of liposarcoma are well-differentiated, pleomorphic, round-cell, myxoid and differentiated subtypes. The well-differentiated is the most common type and includes different histological variants [3]. Approximately 75% of well-differentiated liposarcomas develop in the deep soft tissues of the limbs, followed by 20% in the retroperitoneum. Primary mesenteric liposarcoma is an extremely rare entity and so far a small number of cases have been reported in the literature [4].
Retroperitoneal liposarcoma often manifests as a palpable abdominal mass with abdominal discomfort, less commonly with advanced disease it presents with pain, early satiety or symptoms of bowel obstruction [5].
Despite the large tumor size and the difficult nature of surgery due to the proximity to vital structures, complete tumor resection remains the most effective treatment for the majority of patients with primary retroperitoneal liposarcoma [2] and that is approached in our case as primary management. One-third of well-differentiated liposarcomas recur locally, while the metastatic spread is virtually never seen unless de-differentiation occurs. The sites of reported metastasis and/or recurrence of liposarcoma were local, cardiac, hepatic, mesenteric, bone, and pulmonary [6].
The mesentery is a frequent avenue of spread for malignant neoplasms through the peritoneal cavity and between the peritoneal spaces and the retroperitoneum [7], which is shown in our case., when the retroperitoneal mass recurs as a mesenteric mass that most probably spread by seeding through the peritoneum or intraoperative.
On the other hand, the imaging appearance of liposarcoma varies, depending on the tumor group. Well-differentiated liposarcomas appear as well-defined predominantly fat-containing lesions contain thin septa with low attenuation at computed tomography (−10 to −100 HU). But Magnetic resonance imaging considered more ideal imaging modality because it has better soft-tissue image contrast and higher sensitivity for depicting microscopic and macroscopic fat [8].
The diagnosis of the above-mentioned case was confounded due to the clinical finding of an intra-abdominal lump with limited mobility that required more assessment with CT scan which demonstrated the presence of right lower abdominal cystic lesion involving the terminal ileum with minimal wall enhancement. The only curative treatment for a mesenteric liposarcoma consists of a wide excision and clear surgical margins followed by adjuvant radiotherapy in high-risk patients [6].
PET scan with fluorine 18 Fluorodeoxyglucose (FDG) has the potential to allow differentiation of liposarcoma from other fat-containing lesions as it shows low to intermediate FDG uptake, with the value depending on histologic subtype and well-differentiated liposarcomas display the least uptake (median standardized uptake value, 2.0; range, 1.2–3.2) [8]. The follow up in our case was done with PET scan, showing no evidence of abnormal uptake or active malignant disease until 16 months post resection.
It is worthy to mention that mortality is close to 0% for lesions arising in non-visceral (somatic) soft tissues, compared to nearly 80% for tumors occurring in the retroperitoneum or other visceral sites, where the risk of multiple local recurrences approaches 100% [9].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1. Matthyssens LE, Creytens D, Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg 2015;2:4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu YX, Liu JY, Liu JJ, Yan P, Tang B, Cui YH, et al. . A retrospective, single-center cohort study on 65 patients with primary retroperitoneal liposarcoma. Oncol Lett 2018;15:1799–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al Saanna G, Bovée J, Hornick J, Lazar A A Review of the WHO Classification of Tumours of Soft Tissue and Bone. An ESUN Book Review by The World Health Organization (WHO) classification system for cancer represents the common nomenclature for cancer worldwide. 2013.
- 4. Mitra A, Jain Kumar S, Gupta D, Chandra Murthy Kaza R. Giant angiomyolipoma of the kidney presenting as anaemia-a rare presentation. Open J Urol 2012;2:75–7. [Google Scholar]
- 5. Liles JS, Tzeng CW, Short JJ, Kulesza P, Heslin MJ. Retroperitoneal and intra-abdominal sarcoma. Curr Probl Surg 2009;46:445–503. [DOI] [PubMed] [Google Scholar]
- 6. Amar M, Gravante G, Louise S, Rai D, Bowrey J, Haynes G. A case of recurrent mesocolon myxoid liposarcoma and review of the literature. Case Rep Oncol Med 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics 2003;23:457535–473456. [DOI] [PubMed] [Google Scholar]
- 8. Shaaban AM, Rezvani M, Tubay M, Elsayes KM, Woodward PJ, Menias CO. Fat-containing retroperitoneal lesions: imaging characteristics, localization, and differential diagnosis. Radiographics 2016;36:710–34. [DOI] [PubMed] [Google Scholar]
- 9. Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology 2014;64:38–52. [DOI] [PubMed] [Google Scholar]