Abstract
Tilapia skin has non-infectious microbiota, high amounts of type I collagen, and similar morphological structure to human skin, so it has been suggested as a potential xenograft for the management of burn wounds. A 23-year-old male patient, with no comorbidities, arrived at our burn treatment center after a thermal injury caused by contact with flames from a gunpowder explosion. Superficial partial thickness burns were present in his right upper limb and deep partial thickness burns were present in his left upper limb. Tilapia skin was applied to the lesions, leading to complete reepithelialization within 12 and 17 days of treatment, respectively. No dressing changes were needed and no side effects were observed. Tilapia skin carries the promise of an innovative, easy-to-apply and highly available product that can become the first nationally studied animal skin registered by the National Sanitary Surveillance Agency for use in the treatment of burns.
INTRODUCTION
According to the World Health Organization, burns are responsible for 180 000 deaths annually, the majority in low- and middle-income countries, group in which Brazil is included. Moreover, non-fatal burns account for prolonged hospitalization, disfigurement, and disability, coupled with stigma and rejection [1].
Nile Tilapia Fish Skin (NTFS) has been suggested as an option of biological material for the management of burns. The Colony Forming Units found in samples of NTFS before the process of chemical sterilization indicated the presence of normal, non-infectious microbiota [2]. NTFS also presented a large composition of type I collagen, morphology similar to human skin and high resistance and tensile extension at the break [3]. Despite these characteristics and their enormous significance and potential, no description is present so far in any medical literature of NTFS use in treatment of human burns.
CASE REPORT
A 23-year-old male patient, with no comorbidities, arrived at a burn treatment center in Fortaleza, Brazil after a thermal injury caused by contact with flames from a gunpowder explosion. Superficial partial thickness burns (SPTB) were present in right upper limb (Fig. 1) and deep partial thickness burns (DPTB) were present in left upper limb, (Fig. 2) face and anterior and posterior thorax. Involvement of 16% of total body surface area was calculated with the Lund and Browder chart. After admission as inpatient, he was resuscitated with intravenous fluids using the Parkland formula and remained hemodynamically stable. Local Institutional Review Board approval and written permission from the patient were obtained, in accordance with the Declaration of Helsinki. No conflicts of interest are present.
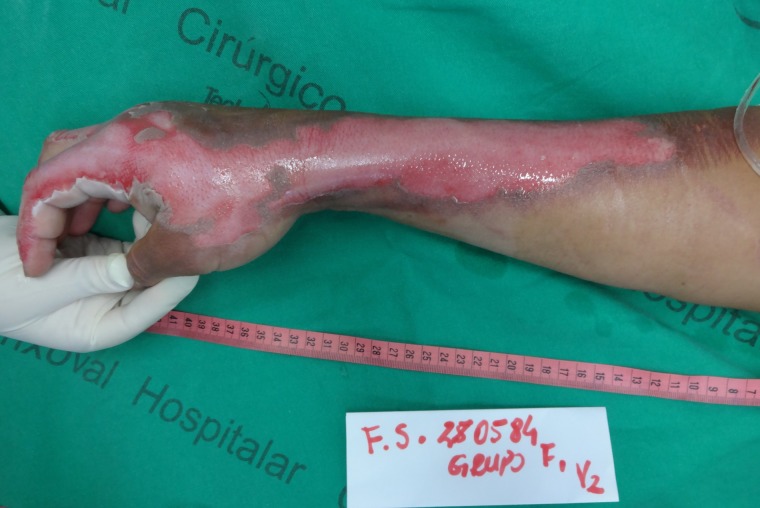
Figure 1:
Superficial partial thickness burn in the right upper limb, after cleaning of the lesion.
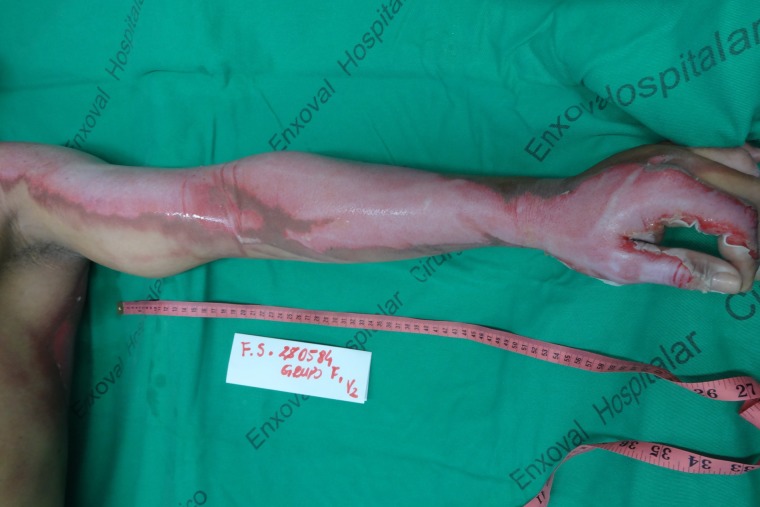
Figure 2:
Deep partial thickness burn in the left upper limb, after cleaning of the lesion.
NTFS was subjected to a rigorous process of chemical sterilization, glycerolization and irradiation, followed by microbiological tests for bacteria and fungi, before storage in refrigerated sterile packaging. Prior to its use in the patient, the skin was washed in sterile 0.9% saline for 5 minutes, with this process being repeated three times in a row. Lima et al. [6], apart from describing the steps of NTFS preparation, showed the biomaterial did not present variations in its microscopic and tensiometric structure after chemical sterilization and irradiation and recovered its natural consistency after the rehydration process [3].
The patient was submitted to anesthesia and analgesia with 150 mg of ketamine, 10 mg of midazolam and 200 mg of tramadol. After cleaning the lesion with tap water and 2% chlorhexidine gluconate and removing necrotic and fibrinous tissue, (Fig. 3) the biomaterial was applied to the upper limbs of the patient. (Fig. 4) Coverage of at least 1 cm of healthy skin in the borders of the wound and superposition of at least 1 cm between NTFS pieces are needed, both to ensure that eventual movement in the first days of treatment will not lead to uncovering of any area of the burn. Due to the still experimental nature of the treatment with NTFS, the researchers decided to apply silver sulfadiazine cream 1%, still used as standard treatment by almost all Brazilian burn centers, to the rest of the burned areas. Finally, firm coverage of the wounds with gauze and bandage was performed.
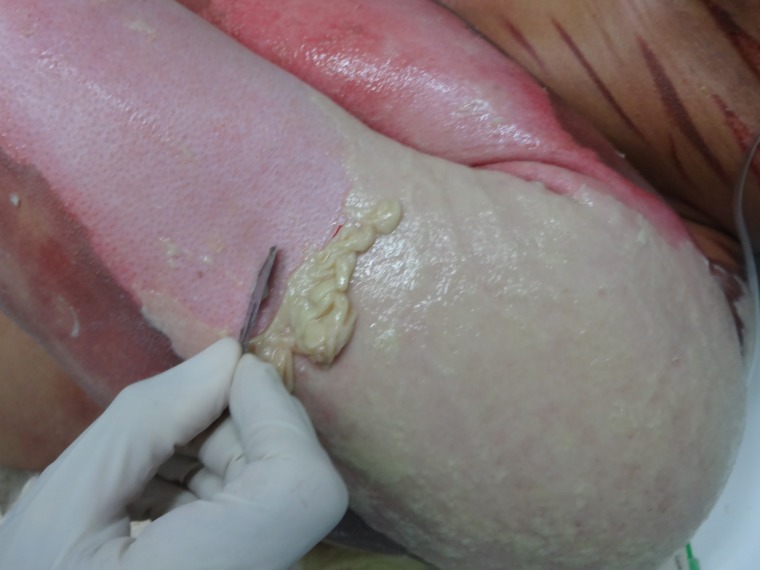
Figure 3:
Process of removing necrotic and fibrinous tissue from the lesion, an essential step to allow maximal contact between NTFS and the wound bed.
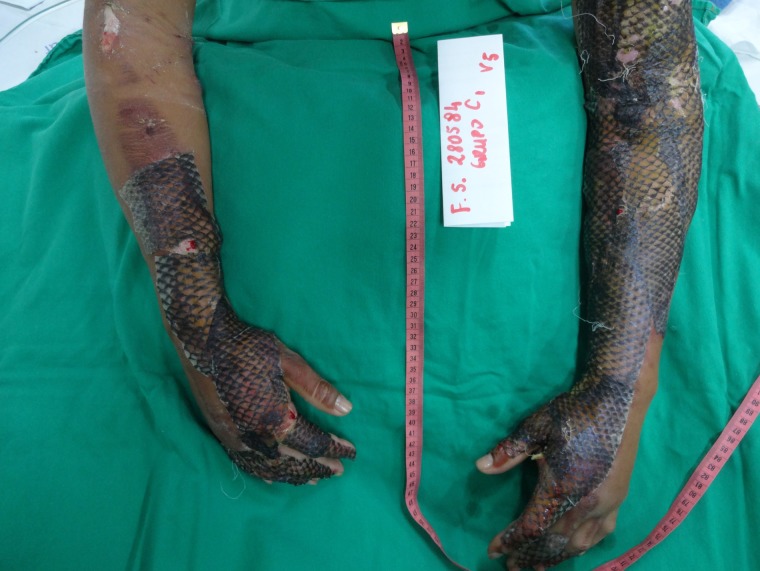
Figure 4:
Appearance of the left upper limb after NTFS application.
Daily collection of blood samples was performed and no significant alterations were found. Also, vital signs and clinical status of the patient remained stable. Gauze and bandage of upper limbs were cut off every 72 hours for the first week of treatment to evaluate for NTFS adherence. The biomaterial showed good adherence to the wound bed with no need of dressing changes. (Fig. 5)
Figure 5:
Appearance of the dressing on the sixth day of treatment. Good adherence of NTFS to the wound bed was detected.
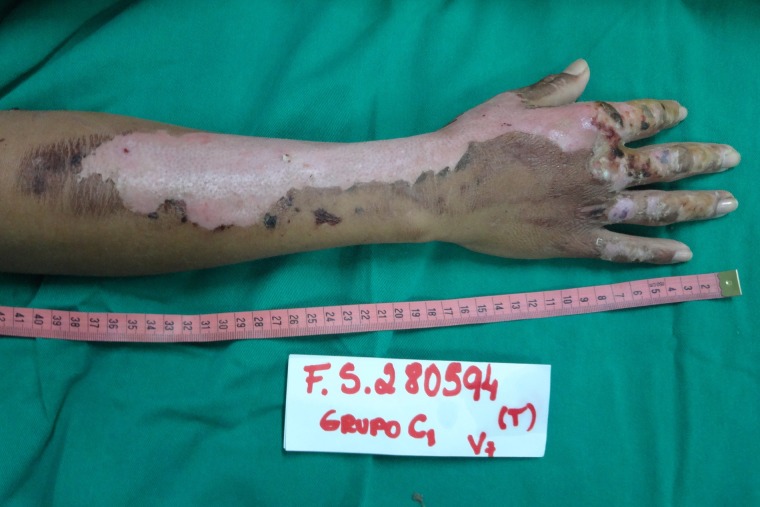
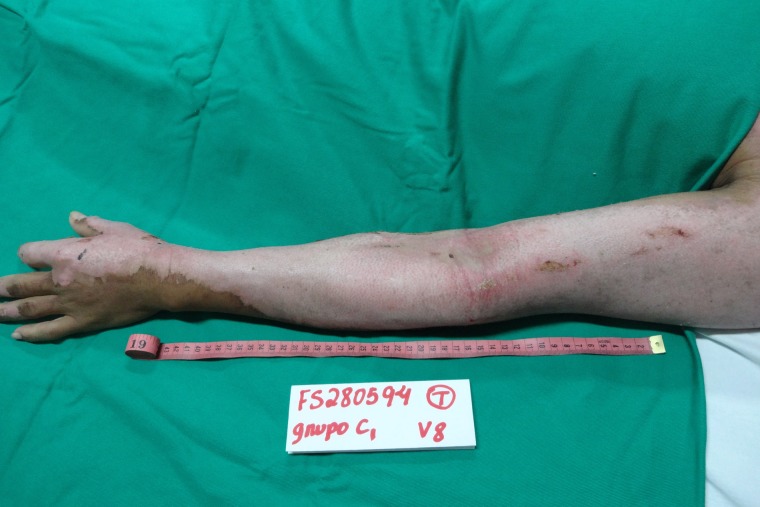
On the 12th day of treatment, NTFS had a dried and hardened appearance and started to slough off from the patient’s right upper limb. Thus, the researchers decided to remove NTFS in the area. The patient’s limb was placed under a shower and the wound was soaked with water. The hydration process led to weakening, breaking and slippage of the NTFS, with exposure of the underlying healed skin. (Fig. 6) On the 17th day of treatment, a similar process was performed, allowing NTFS removal from the left upper limb. (Fig. 7) No side effects were noted.
Figure 6:
Appearance of the right upper limb lesion after removal of NTFS, with a total of 12 days required for complete reepithelialization of the SPTB.
Figure 7:
Appearance of the left upper limb lesion after removal of NTFS, with a total of 17 days required for complete reepithelialization of the DPTB.
DISCUSSION
Since allografts and xenografts appear to be equally effective, xenografts might be a superior choice for their increased safety and reduced price [4]. Although frog skin was previously used as a burn treatment in Brazil, it was never registered by the National Sanitary Surveillance Agency (ANVISA) [5]. Porcine skin was neither registered nationally for the use in burn wounds and has very low availability in the specialized centers. NTFS can become the first nationally studied animal skin registered by ANVISA for use in burn treatment.
When used as a xenograft for treatment of experimental burns in rats, NTFS led to improvements in the healing process and no relevant changes in hematological and biochemical parameters [6]. Tilapia collagen nano-fibers were also found to enhance skin wound healing speed in rats by promoting cell adhesion, proliferation and differentiation [7]. Another study found promising results with the application of marine collagen peptides from tilapia skin on deep partial-thickness scalds in rabbits [8]. A recent study showed tilapia collagen significantly induces epidermal growth factor and fibroblast growth factor expression, which can promote proliferation and differentiation of fibroblasts and keratinocytes [9].
Healing is expected within 2 weeks for SPTB and usually takes longer than 3 weeks for DPTB [10]. The periods of 12 and 17 days required, respectively, for complete reepithelialization of this patient’s wounds in the right upper limb and the left upper limb, suggest effectiveness of NTFS as a xenograft for the treatment of human burns. Also, no dressing changes were needed in the areas covered with NTFS, due to good adherence of the biomaterial to the wound bed, suggesting its potential in reducing burn patient pain, hospital costs and overall workload of the health care team.
We recognize the limitations of analyzing these results in a single patient, but we are currently working on phase II and phase III randomized controlled trials, which will allow NTFS to firm itself as a relevant option in the therapeutic arsenal of burn wounds, producing financial and social impact for the health system.
CONFLICTS OF INTEREST
None.
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. World Health Organization Burns. Available at: http://www.who.int/news-room/fact-sheets/detail/burns Accessed 2 October, 2018.
- 2. Lima EM Junior, Bandeira TJPG, Miranda MJB, Ferreira GE, Parente EA, Piccolo NS, et al. . Characterization of the microbiota of the skin and oral cavity of Oreochromis niloticus. J Heal Biol Sci 2016;4:193–7. [Google Scholar]
- 3. Alves APNN, Lima EM Júnior, Piccolo NS, de Miranda MJB, Lima Verde MEQ, Ferreira AEC Júnior, et al. . Study of tensiometric properties, microbiological and collagen content in nile tilapia skin submitted to different sterilization methods. Cell Tissue Bank 2018;19:373–82. [DOI] [PubMed] [Google Scholar]
- 4. Hermans MHE. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: Is there a clinical difference? Burns 2014;40:408–15. [DOI] [PubMed] [Google Scholar]
- 5. Piccolo N, Piccolo MS, Piccolo MTS. Uso de pele de rã como curativo biológico como substituto temporário da pele em queimaduras. Rev Bras Queim 2002;2:18–24. [Google Scholar]
- 6. Lima Junior EM, Picollo NS, Miranda MJB, Ribeiro WLC, Alves APNN, Ferreira GE, et al. . Uso da pele de tilápia (Oreochromis niloticus), como curativo biológico oclusivo, no tratamento de queimaduras. Rev Bras Queimaduras 2017;16:10–7. [Google Scholar]
- 7. Wang N, Ding T, Zhou T, Liu X, Sun J, Xue Y, et al. . Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf B: Biointerfaces 2016;143:415–22. [DOI] [PubMed] [Google Scholar]
- 8. Zhou C, Hong P, Li S, Hu Z, Yang P. Marine collagen peptides from the skin of nile tilapia (Oreochromis niloticus): characterization and wound healing evaluation. Mar Drugs 2017;15:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Gao K, Liu S, Wang S, Elango J, Bao B, et al. . Fish collagen surgical compress repairing characteristics on wound healing process in vivo. Mar Drugs 2019;17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan S. Burn blister fluids in the neovascularization stage of burn wound healing: a comparison between superficial and deep partial-thickness burn wounds. Burn Trauma 2013;1:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]