Summary
Stimulating oligodendrocyte (OL) production from endogenous progenitor cells is an important strategy for myelin repair and functional restoration after disease or injury-induced demyelination. Subventricular zone (SVZ) stem cells are multipotential, generating neurons and oligodendroglia. The factors that regulate the fate of these stem cells are poorly defined. In this study, we show that genetically increasing fibroblast growth factor receptor-3 (FGFR3) activity in adult SVZ stem cells transiently and dramatically redirects their differentiation from the neuronal to the oligodendroglial lineage after pathological demyelination. The increased SVZ-derived oligodendrogenesis leads to improved OL regeneration and myelin repair, not only in the corpus callosum (a normal destination for SVZ-derived oligodendroglial cells), but also in the lower cortical layers. This study identifies FGF signaling as a potent target for improving endogenous SVZ-derived OL regeneration and remyelination.
Keywords: adult neural stem cells, cell fate reprogramming in vivo, oligodendrogenesis, FGF receptor activity, remyelination, subventricular zone
Highlights
-
•
Adult neuronal progenitors with increased FGFR activity switch to gliogenesis in vivo
-
•
FGFR-induced increase in OPCs (30-fold) and oligodendrocytes (10-fold) is reversible
-
•
FGFR-induced increase in oligodendrocytes results in remyelination after chronic insult
In this article, Hébert and colleagues show that adult neural stem cells can be made to switch the cell type they produce in vivo, from neurons to oligodendrocytes, by increasing levels of FGFR3 activity in the stem cells. The result of this increased oligodendrogenesis is a greater ability to remyelinate axons after acute or chronic lesions.
Introduction
Demyelination, the loss of myelin sheaths that surround axons, occurs from infections, ischemia, exposure to toxins, or autoimmunity. In multiple sclerosis, demyelination occurs with loss of oligodendrocytes (OLs) reducing axon conductivity and neuron viability. Although precursor cells (OPCs) respond to damage and replace lost OLs, the extent of replacement is often insufficient to avoid degeneration. There is currently no treatment for increasing OLs to reverse disease-associated demyelination.
Two cell populations—parenchymal OPCs (pOPCs) and subventricular zone (SVZ) neural stem/progenitor cells (NSPCs)––contribute to OL replacement and remyelination in the cerebrum (Alizadeh et al., 2015, El Waly et al., 2014, Merson and Bourne, 2014). In response to demyelination, OPCs generated by NSPCs migrate long distances to replace OLs in the corpus callosum (Menn et al., 2006, Nait-Oumesmar et al., 1999, Picard-Riera et al., 2002, Xing et al., 2014). Given their migratory potential and ability to generate OLs, NSPCs represent an important endogenous cell source for OL repopulation and myelin repair.
Normally, in the rodent SVZ, NSPCs mainly generate interneuron precursors destined for the olfactory bulb (Doetsch and Hen, 2005, Ihrie and Alvarez-Buylla, 2011), but also generate a small number of OLIG2+ OPCs that migrate into white matter and differentiate into OLs (Menn et al., 2006). Upon demyelination, a subpopulation of NSPCs that normally generate neurons shift their fate to oligodendroglia to remedy OL death and myelin loss (Gonzalez-Perez and Alvarez-Buylla, 2011, Jablonska et al., 2010, Nait-Oumesmar et al., 1999, Picard-Riera et al., 2002). The factors that control this shift remain unclear.
Fibroblast growth factors (FGFs) and other factors regulate oligodendrogenesis during development and adulthood under normal conditions and after demyelination (Huang and Dreyfus, 2016, Naruse et al., 2017). In this study, we tested whether increasing fibroblast growth factor receptor-3 (FGFR3) activity in NSPCs of the adult SVZ affects the fate of the cells produced. The results suggest that increased FGF signaling redirects the differentiation of NSPCs from a neuronal to an oligodendroglial lineage, resulting in very enhanced oligodendrogenesis. Importantly, this increase led to improved axon remyelination after demyelination, at the transient expense of neurogenesis. This study provides evidence that levels of FGF signaling control the neuronal-oligodendroglial fate of adult NSPCs, identifying the FGF pathway as a potential therapeutic target for improving OL replacement and myelin repair.
Results
Increased FGFR Activity in NSPCs Preferentially Promotes Oligodendrogenesis in the Absence of Injury
To examine the effect of increased FGF signaling through FGFR3 in the adult SVZ, NSPCs of adult mice were targeted with Nestin-CreER to express a CAG-flox-stop-flox-Fgfr3TDII transgene. In these gain-of-function (GOF) mutants, a constitutively active, ligand-independent form of Fgfr3, Fgfr3TDII, becomes expressed on tamoxifen (TM) treatment (Kang et al., 2014). Consistent with an increase in canonical FGF signaling in NSPCs, levels of p-ERK increased in the SVZ of GOF mutants compared with controls (Figure S1A).
Note that the SVZ of loss-of-function Nestin-CreER;Fgfr1flox/flox;Fgfr2flox/flox;Fgfr3flox/flox mutants, in which all expressed FGFR genes are deleted with TM (and for which we previously described a strong phenotype in the hippocampal SGZ; Kang and Hébert, 2015), were also examined. However, in these loss-of-function mutants we could not detect significant changes in oligodendroglia or neuroblasts in the SVZ (Figure S1B), consistent with previous reports using single and double Fgfr knockouts (Furusho et al., 2012, Ishii et al., 2016, Oh et al., 2003, Zhou et al., 2012). Relevant to the current study, Nestin-CreER does not lead to recombination in pOPCs (Kang et al., 2014).
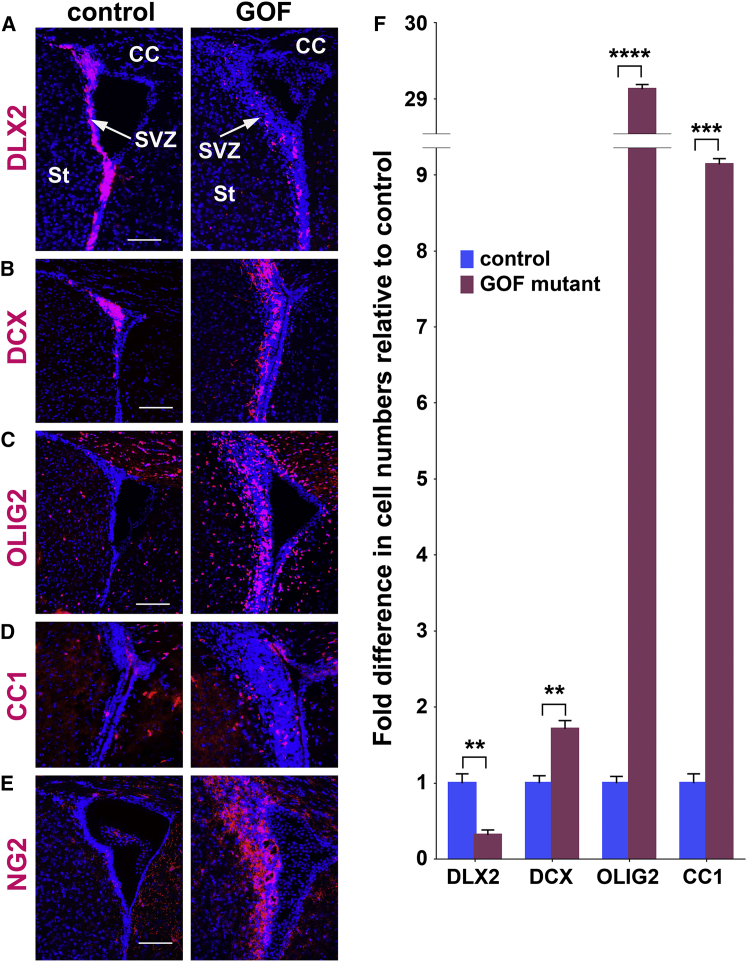
In GOF mutants, the number of DLX2-immunostained cells (neuronal precursors) in the SVZ was decreased compared with controls (controls: 17.39 ± 2.15 versus GOF mutants: 5.55 ± 1.16 cells/100 μm, p = 0.0042) (Figures 1A and 1F, shown as fold change relative to control for ease of comparison). In contrast, GOF mutants displayed modestly more doublecortin (DCX)+ neuroblasts (controls: 13.00 ± 1.29 versus GOF mutants: 22.29 ± 1.45 cells/100 μm, p = 0.0015) (Figures 1B and 1F), suggesting that, despite fewer DLX2+ neuronal progenitors, the DCX+ neuroblasts they produce are more proliferative with increased FGFR activity, as shown by DCX and Ki67 co-labeling (Figure S1C). In GOF mutants, the SVZ is much thicker by DAPI staining and the modest increase in neuroblasts seemed unlikely to account for this enlargement, prompting us to examine the oligodendroglia. We found that oligodendroglial cells (OLIG2+) were almost 30 times more numerous in the SVZ of GOF mutants (39.05 ± 0.89 cells/100 μm) than in controls (1.34 ± 0.122; p = 1.9 × 10−5) (Figures 1C and 1F). A minority of these cells were maturing CC1+ OLs (control: 0.78 ± 0.12; GOF mutants: 7.14 ± 0.8 cells/100 μm, p = 0.00078) (Figures 1D and 1F) and most were instead NG2+ OPCs (Figure 1E). This large expansion of OPCs suggests that increasing FGF signaling promotes oligodendroglia generation from NSPCs at the expense of DLX2+ neurogenic cells. Intriguingly, in the SVZ of controls, DLX2 and OLIG2 staining was mutually exclusive; whereas, in GOF mutants, double-labeled cells were present (control: 0/250 versus GOF mutants: 32/125 DLX2+OLIG2+/DLX2+ cells) (Figure S1D), suggesting a transition state between neurogenic and gliogenic progenitors. Our data do not exclude the possibility that selective survival, rather than a switch in fate, accounts for the shift in cell types that are ultimately produced, even though we do not detect significant cell death by TUNEL staining in either controls or mutants (Figure S1E).
Figure 1.
Increased FGFR Activity Enhances Oligodendroglia Generation While Reducing Neuronal Progenitors in the SVZ
(A–E) Coronal sections through the SVZ stained (red) for DLX2+ neural progenitors (A), DCX+ neuroblasts (B), OLIG2+ oligodendroglia (C), CC1+ OLs (D), and NG2+ oligo progenitors (E). DAPI counterstain (blue).
(F) Quantitation of differences in cell numbers relative to control shows large increases in oligodendroglia and decreases in neural progenitors. n ≥ 3, mean ± SEM; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
CC, corpus callosum; St, striatum. Scale bars, 100 μm. See also Figure S1.
Increased FGFR Activity Redirects NSPCs to Produce Oligodendroglia on Demyelination
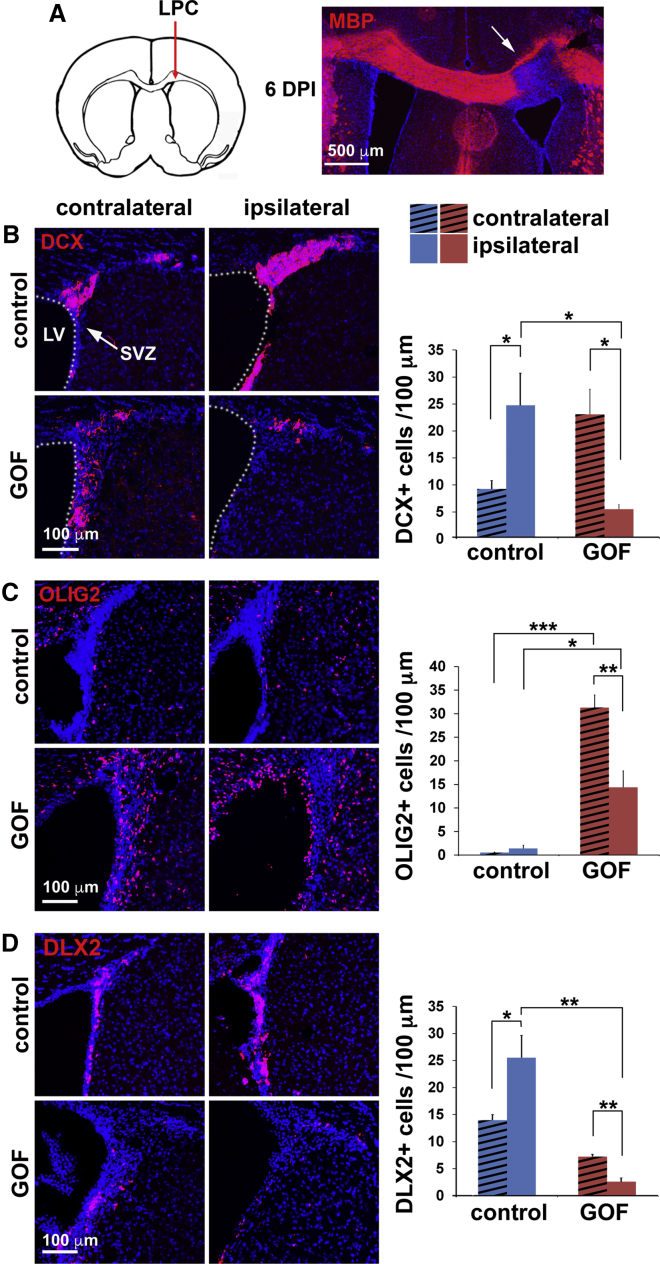
Lineage plasticity of NSPCs in the SVZ is induced by demyelination (Gonzalez-Perez and Alvarez-Buylla, 2011, Jablonska et al., 2010, Nait-Oumesmar et al., 1999, Picard-Riera et al., 2002). The enhanced oligodendroglia generation we observed in FGFR GOF mutants prompted us to ask whether increased FGFR activity following demyelination can further redirect NSPCs from producing neurons to oligodendroglia. First, acute focal demyelination was induced with an injection of lysophosphatidylcholine (LPC) in one side of the corpus callosum. Six days post-injection, a clearly demarcated area of the injected side had invariably lost myelin basic protein (MBP) staining (Figure 2A), indicating demyelination.
Figure 2.
Increased FGFR Activity Promotes Oligodendrogenesis at the Expense of Neurogenesis after Focal Demyelination
(A) Left: depiction of corpus callosum LPC injection. Right: at 6 days post-injection (DPI), myelin staining (MBP) (red) shows focal demyelination (arrow).
(B–D) Coronal sections through the SVZ at 14 days post-LPC stained (red) for DCX (B), OLIG2 (C), and DLX2 (D). Quantitation on the right shows decreased neuroblasts and neural progenitors with concurrent increased oligodendroglia on the injected ipsilateral side in mutants compared with controls.
LV, ventricle. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Normally, most progenitors in the SVZ generate DCX+ neuroblasts. Consistent with this, the ratio of OLIG2+ to DCX+ cells in the uninjured contralateral side of control mice was ∼1:17 (Figures 2B and 2C) (OLIG2: 0.55 ± 0.073; DCX: 9.25 ± 1.54 cells/100 μm). At 14 days post-demyelination (ipsilateral SVZ), despite an increase in neurogenic cells, the number of OLIG2+ cells remained small in control mice (Figure 2C), with a 1:18 ratio of OLIG2+ cells to DCX+ cells (OLIG2: 1.41 ± 0.66; DCX: 24.7 ± 5.97 cells/100 μm). In contrast, in GOF mutants, in the absence of injury, oligodendroglial cells were drastically more numerous and the main cell type in the SVZ (contralateral SVZ, Figures 2B and 2C), with a 1.4:1 ratio of OLIG2+ cells to DCX+ cells (OLIG2: 31.3 ± 2.64; DCX: 23.05 ± 4.68 cells/100 μm). After demyelination, unlike in control mice, in GOF mutants, DCX+ cells were reduced ∼4-fold, and the cells around the ventricle were mostly oligodendroglial (ipsilateral SVZ, Figures 2B and 2C). The decrease of DCX+ cells on demyelination led to an even greater ratio of OLIG2+ cells to DCX+ cells, 2.6:1 (OLIG2: 14.38 ± 3.5; DCX: 5.46 ± 0.85 cells/100 μm), indicating a demyelination-induced change in the lineage composition of SVZ cells. Note that the fewer OLIG2+ cells in the SVZ of the injected side compared with the non-injected side in Figure 2C likely reflects the recruitment of these cells away from the SVZ to the demyelinated corpus callosum. Similar to the DCX+ neuroblasts, the DLX2+ neuronal precursors in the GOF mutants were also further decreased after LPC injection. Together these results suggest that FGF signaling in NSPCs induces a switch from generating predominantly neuronal cells to predominantly oligodendroglia, especially following demyelination.
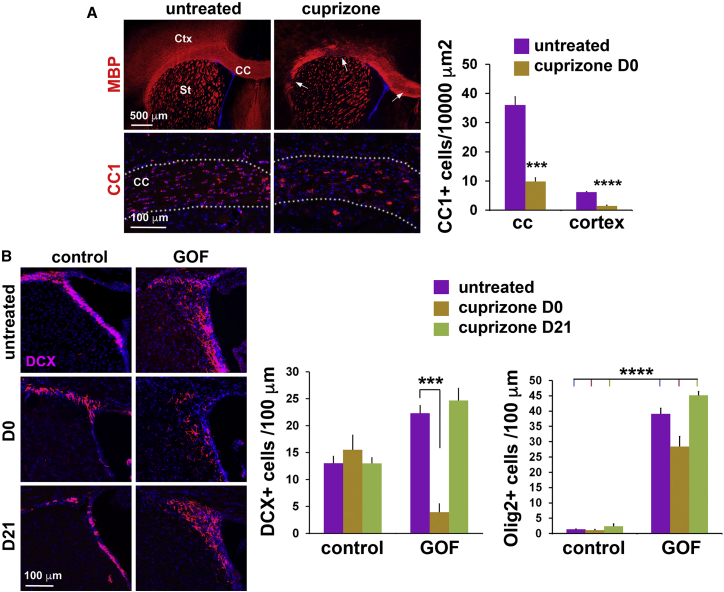
To test whether FGF signaling can also redirect NSPCs from neurogenesis to oligodendrogenesis after chronic global, rather than acute local, demyelination and in the absence of any injection damage, mice were fed a 0.2% cuprizone diet for 12 weeks. The day after cuprizone cessation (day 0), presumably with the lowest level of myelin before recovery, patchy areas with loss of MBP staining were found in the corpus callosum, and staining in the cortex was reduced in both controls and GOF mutants (Figure 3A). Most CC1+ OLs were lost in the corpus callosum and cortex. At day 0, DCX+ neuroblast numbers in treated controls were comparable with those in untreated mice, whereas, in GOF mutants, the number of neuroblasts decreased ∼6-fold. At this time, oligodendroglia in GOF mutants remained abundant in the SVZ (Figure 3B). Similar to LPC-induced demyelination, cuprizone resulted in a slight reduction in the absolute number of OLIG2+ cells after demyelination, which again may be due to the emigration of these cells to demyelinating areas. Together, the data obtained using cuprizone support an FGFR3-induced fate switch from neurogenic to oligodendrogenic following demyelination. Interestingly, 3 weeks after ceasing cuprizone (D21), DCX+ cells in GOF mutants recovered to numbers in untreated mice (Figure 3B), indicating that the FGF-induced switch from neurogenesis to oligodendrogenesis is reversible on remyelination. Since astrocytes upregulate GFAP on demyelination, we examined their activation state in LPC- and cuprizone-treated mice and found no difference between GOF mutants and controls (Figure S2).
Figure 3.
Increased FGFR Activity Induces a Transient Decrease in Neurogenesis in the SVZ after Global Demyelination
(A) MBP (myelin) and CC1 (OLs) staining in the corpus callosum (CC) (dotted outline) and cortex (Ctx) in untreated and cuprizone-treated mice at day 0 (D0) when treatment was stopped. Arrows point to areas with significant loss of myelin. Quantitation shows a loss of CC1+ OLs in the corpus callosum and cortex in treated compared with untreated control mice.
(B) DCX+ neuroblasts in the SVZ in both controls and GOF mutants with or without cuprizone treatment. In treated mice, samples were collected at D0 or D21 after the stop of cuprizone. Quantitation shows decreased numbers of neuroblasts in GOF mutants at D0, but the decrease recovers at D21. Much larger numbers of oligodendroglia are present in the SVZ of untreated and treated GOF mutants at D0 and D21 than in controls.
∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S2.
Increased FGFR Activity Promotes OL Regeneration and Axon Remyelination
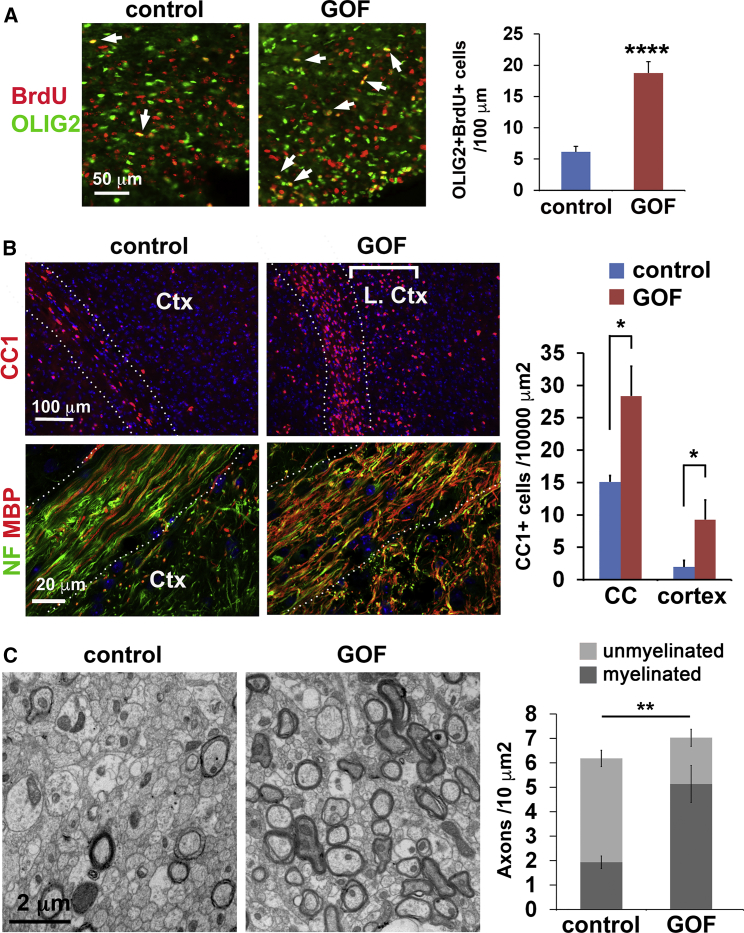
The shift in NSPC lineages after demyelination raises the possibility that increasing FGFR activity could improve OL regeneration and myelin repair. At 6 days post-LPC, OLIG2+ cells accumulated in the demyelinated corpus callosum of both controls and GOF mutants. Long-term bromodeoxyuridine (BrdU) pulse labeling revealed three times more new OLIG2+ BrdU+ cells in the lesioned corpus callosum of GOF mutants than controls, consistent with improved regeneration induced by FGFR activity (Figure 4A). The increase in new oligodendroglia (OLIG2+BrdU+) in GOF mutants was maintained from 6 days to 3 weeks post-LPC (Figure S3A). Although the double-labeled cells could in theory originate from both pOPCs and NSPCs, the additional newborn oligodendroglia were likely generated from NSPCs owing to the specific targeting of NSPCs by Nestin-CreER. An increase in new BrdU+ CC1+ OLs was also found at the lesion site in GOF mutants 6 days post-LPC, indicating that FGFR-induced SVZ-derived new OPCs can differentiate into OLs. By 3 weeks, however, similar levels of BrdU+ CC1+ cells were observed at lesion sites in control and mutants showing that OLs eventually also regenerate in controls (Figure S3A). Together, our results suggest that, on LPC-induced demyelination, increased FGFR activity in NSPCs improves the speed of OL regeneration.
Figure 4.
Increased FGFR Activity Promotes OL Regeneration and Remyelination
(A) More new oligodendroglia (BrdU+ OLIG2+, arrows) are observed at sites of LPC demyelination in the corpus callosum 6 days post-injection in GOF mutants compared with controls.
(B) More OLs labeled by CC1 and myelinated axons labeled by NF1 and MBP viewed by confocal imaging in the corpus callosum and lower cortical layers (L. Ctx) at 10 days post-cuprizone are observed in the GOF mutants.
(C) Transmission electron micrographs showing sections through the corpus callosum with a greater number of myelinated axons in the GOF mutants than controls.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figures S3 and S4.
We also examined the effects of increased FGFR activity on remyelination after cuprizone treatment. On cuprizone cessation, normally the demyelinated areas gradually recover as OLs are regenerated. As expected, in controls at 10 days post-cuprizone, partial recovery of CC1+ OLs was observed in the medial corpus callosum with little recovery in lateral regions or in lower cortical layers (Figures 4B and S3B), which may be due to fewer SVZ-derived OPCs migrating to these areas. In contrast, in GOF mutants many more CC1+ cells were found in the medial and lateral corpus callosum and lower cortical layers compared with controls between 10 days and 3 weeks post-cuprizone (Figures 4B and S4A), indicating greater cell regeneration. In the upper layers, both controls and mutants showed no obvious recovery in OLs (Figure 4B). By 6 weeks after cuprizone treatment, controls had recovered CC1+ cells to levels comparable with mutants in all regions (Figure S4A). Together, the data suggest that increased FGFR3 activity in NSPCs causes faster OL regeneration in both corpus callosum and lower cortical layers after cuprizone-induced demyelination.
The increase in OLs observed in the lateral corpus callosum could in theory be due to paracrine influences on resident OPCs rather than migration of new OPCs from the SVZ. However, no increase in the proliferation state of oligodendroglial (OLIG2+Ki67+) cells was detected 0, 10, and 21 days post-cuprizone in the lateral corpus callosum of GOF mutants compared with controls, and no increase in oligodendroglia before treatment in either the lateral corpus callosum or lower cortex could be detected (Figures S4B–S4E).
Importantly, consistent with improved OL regeneration, the corpus callosum and lower neocortex showed much better remyelination in GOF mutants than controls. In controls at day 10, most axons labeled by Neurofilament in the lateral corpus callosum and lower cortical layer remained without a surrounding myelin sheath, while in mutants, most axons were myelinated (Figure 4B). Transmission electron microscopy (TEM) confirmed a greater number of myelinated axons in the corpus callosum in GOF mutants than controls at 14 days post-cuprizone (Figure 4C), although the g ratios when comparing only myelinated axons in mutants and controls was similar (controls: 0.79 ± 0.01 versus GOF mutants: 0.76 ± 0.018, p = 0.129). These results indicate that, following demyelination, increasing FGFR activity in NSPCs improves OL replacement and increases the number of remyelinated axons in the corpus callosum and lower neocortical layer.
Discussion
Using a conditional genetic approach to increase FGFR3 activity in NSPCs of the adult mouse SVZ, we revealed an ability for FGF signaling through this receptor to promote a switch in the cell type produced from neurogenic to oligodendrogenic. Since individual FGFRs can activate different intracellular transduction pathways, activation of FGFR1 or FGFR2 may not reproduce the effects described here for FGFR3. Arguing against different roles for FGFRs, however, is the finding that a double knockout of Fgfr1 and Fgfr2 results in reduced remyelination after cuprizone treatment (Furusho et al., 2015). Irrespective of which FGFRs can increase oligodendrogenesis, our findings are consistent with observations that, at low FGF2 concentrations, cortical stem cells generate neurons in cell culture, while at higher concentrations they generate oligodendroglia (Qian et al., 1997), and that experimentally increasing FGF2 in vivo expands OPCs in adulthood (Azim et al., 2012, Ruffini et al., 2001). Interestingly, the shift to oligodendrogenesis by NSPCs induced by increased FGF signaling following demyelination occurs transiently during remyelination before reversing back to its neurogenic state after remyelination. This finding suggests that although activated FGFR3 enhances oligodendrogenesis at the expense of neurogenesis during remyelination, the capability of NSPCs to generate neurons is not irrevocably compromised.
Despite very similar properties of NSPCs from either the SVZ or the hippocampal SGZ in culture, our results clearly demonstrate different effects of perturbing FGF signaling in the two niches in vivo. First, in contrast to the requirement for FGF signaling in SGZ neurogenesis (Kang and Hébert, 2015, Stevens et al., 2012, Werner et al., 2011, Zhao et al., 2007), FGF signaling is not essential for neurogenesis in the SVZ. Second, while increasing FGFR activity using the same transgenic mice described here leads to increased neurogenesis in the hippocampal SGZ (Jin et al., 2003, Kang and Hébert, 2015, Rai et al., 2007), we show here that increasing FGFR activity in SVZ NSPCs redirects these cells from neurogenic to oligodendrogenic. These differences may be due to distinct lineage potentials of NSPCs in the two locations or to environmental factors.
In vivo fate mapping showed that following cuprizone-induced demyelination, NSPC-derived oligodendroglia were recruited to the corpus callosum adjacent to the SVZ, suggesting a limited migratory potential (Xing et al., 2014). Our current study confirms that, in controls, slower OL replacement occurs in the more lateral regions of the corpus callosum, with limited if any replacement in lower cortical layers. However, in GOF mutants, OL replacement and remyelination in the lateral corpus callosum and lower cortical layers was greatly increased, suggesting that increased FGF signaling may promote migration of SVZ-derived OPCs to more distal areas. The absence of significant OL replacement and remyelination in the upper neocortical layers in both controls and GOF mutants suggests a different myelin repair mechanism, likely dependent on pOPCs. Although the FGFR-induced increase in OLs observed here leads to more remyelinated axons without an increase in myelin thickness, suggesting a greater capacity to initiate myelination, NSPC-derived OLs can also lead to increased myelin thickness (Xing et al., 2014).
Although FGF signaling promotes OPC expansion, it can inhibit their differentiation into OLs (Armstrong et al., 2002, Butt and Dinsdale, 2005, Murtie et al., 2005, Zhou et al., 2012, Zhou et al., 2006). In contrast, in FGFR3 GOF mutants, we observed an increase not only in OPCs, but also myelinating OLs. However, because CMV-β-actin-based promoters can be silenced on cell differentiation or over time (Gray et al., 2011, Vroemen et al., 2005), we cannot exclude the possibility that our FGFR3 transgene undergoes silencing, which relieves the FGFR block on OPC differentiation. It is also possible that the discrepancies observed in OL production using different genetic approaches reflect distinct mechanisms regulating SVZ-derived OPCs, which we specifically targeted, and pOPCs, since previous studies did not distinguish between these two sources of OLs.
Several benefits of increasing FGF signaling in the brain have been reported. First, as shown here, increasing FGFR activity dramatically increases OPC numbers after demyelination, with only transient effects on neurogenesis. Second, FGFR activity in already mature OLs increases myelin production (Ishii et al., 2014, Furusho et al., 2017, Furusho et al., 2012). Third, FGFR activity can suppress the reactive state of astrocytes (Kang et al., 2014, Oh et al., 2003), which contribute to inflammation in demyelinating conditions, such as multiple sclerosis, and have been proposed to exacerbate remyelination (Li et al., 2016, Ludwin et al., 2016). Finally, FGF activity can also rescue age-related declines in hippocampal neurogenesis (Kang and Hébert, 2015). Therefore, repeated transient upregulation of FGF signaling is expected to have overall beneficial effects on remyelination: high signaling inducing increased OPC numbers and myelin production followed by low signaling allowing the exuberant OPCs to differentiate. Altogether, our findings further support FGF signaling as a therapeutic target for stimulating OL regeneration and myelin repair caused by disease or injury.
Experimental Procedures
Mice
All experiments herein have been approved by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee. Mouse lines are detailed in the Supplemental Experimental Procedures. Mice were 2–3 months old at the start of experiments. All mice received tamoxifen (TM) (5 mg/35 g body weight) every other day for a total of five doses. New cells in the LPC-induced demyelination model were labeled with BrdU twice a day for 5 days starting the day after LPC injection.
Demyelination
LPC: LPC was injected at “day 0” in the corpus callosum 3–4 weeks after the last dose of TM. Cuprizone: mice were fed cuprizone chow for 12 weeks. TM was administered every other day for five doses with the last dose given 2 weeks before the end of cuprizone.
Immunostaining
Cryosections were treated for antigen retrieval, blocked, incubated with primary antibodies (see Supplemental Experimental Procedures), and incubated with secondary fluorescent antibodies. For BrdU staining, slides were acid treated before antigen retrieval.
TEM
Mice were perfused with fixative. Brains were postfixed, washed, and vibratome sliced. The corpus callosum was dissected, treated with osmium tetroxide, stained with uranyl acetate, dehydrated, and embedded in resin. Ultrathin sections were stained with uranyl acetate, lead citrate, and imaged.
Quantitation and Statistics
Cells were counted in the SVZ or the corpus callosum in coronal sections positionally matched in the rostocaudal axis. For cell counts, the SVZ was defined as the visibly denser layer of DAPI+ nuclei lining the ventricle. Statistical analyses were with the Student's t test. At least three sections per hemisphere for each of three mice per genotype were used. Numbers were averaged and compared between mutant and control littermates. Data were presented as mean ± SEM.
Acknowledgments
We thank Xheni Nishku for sectioning and microscopy, David Hall for TEM support, Marta Gronska-Peski and Nachiket Kamatkar for critical reading of the manuscript. This study was supported by NIH MH070596 and MH083804. The authors declare no competing financial interests.
Published: June 11, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.05.006.
Supplemental Information
References
- Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front. Mol. Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. (2015) Myelin damage and repair in pathologic CNS: challenges and prospects. Front. Mol. Neurosci. 8:35. [DOI] [PMC free article] [PubMed]
- Armstrong R.C., Le T.Q., Frost E.E., Borke R.C., Vana A.C. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J. Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Armstrong R.C., Le T.Q., Frost E.E., Borke R.C., Vana A.C.. (2002) Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J. Neurosci.. 22:8574-8585. [DOI] [PMC free article] [PubMed]
- Azim K., Raineteau O., Butt A.M. Intraventricular injection of FGF-2 promotes generation of OL-lineage cells in the postnatal and adult forebrain. Glia. 2012;60:1977–1990. doi: 10.1002/glia.22413. [DOI] [PubMed] [Google Scholar]; Azim K., Raineteau O., Butt A.M.. (2012). Intraventricular injection of FGF-2 promotes generation of OL-lineage cells in the postnatal and adult forebrain. Glia 60:1977-1990. [DOI] [PubMed]
- Butt A.M., Dinsdale J. Fibroblast growth factor 2 induces loss of adult OLs and myelin in vivo. Exp. Neurol. 2005;192:125–133. doi: 10.1016/j.expneurol.2004.11.007. [DOI] [PubMed] [Google Scholar]; Butt A.M., Dinsdale J.. (2005) Fibroblast growth factor 2 induces loss of adult OLs and myelin in vivo. Exp. Neurol.. 192:125-133. [DOI] [PubMed]
- Doetsch F., Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]; Doetsch F., Hen R. (2005) Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 15:121-128. [DOI] [PubMed]
- El Waly B., Macchi M., Cayre M., Durbec P. Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 2014;8:145. doi: 10.3389/fnins.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]; El Waly B., Macchi M., Cayre M., Durbec P. (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 8:145. [DOI] [PMC free article] [PubMed]
- Furusho M., Dupree J.L., Nave K.A., Bansal R. Fibroblast growth factor receptor signaling in OLs regulates myelin sheath thickness. J. Neurosci. 2012;32:6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Furusho M., Dupree J.L., Nave K.A., Bansal R.. (2012). Fibroblast growth factor receptor signaling in OLs regulates myelin sheath thickness. J. Neurosci.. 32: 6631-6641. [DOI] [PMC free article] [PubMed]
- Furusho M., Roulois A.J., Franklin R.J.M., Bansal R. Fibroblast growth factor signaling in OL-lineage cells facilitates recovery of chronically demyelinated lesions but is redundant in acute lesions. Glia. 2015;63:1714–1728. doi: 10.1002/glia.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]; Furusho M., Roulois A.J., Franklin R.J.M., Bansal R.. (2015). Fibroblast growth factor signaling in OL-lineage cells facilitates recovery of chronically demyelinated lesions but is redundant in acute lesions. Glia 63:1714-1728. [DOI] [PMC free article] [PubMed]
- Furusho M., Ishii A., Bansal R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2–MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 2017;37:2931–2946. doi: 10.1523/JNEUROSCI.3316-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Furusho M., Ishii A., Bansal R.. (2017). Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci.. 37:2931-2946. [DOI] [PMC free article] [PubMed]
- Gonzalez-Perez O., Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 2011;67:147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gonzalez-Perez O., Alvarez-Buylla A. (2011) Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 67:147-156. [DOI] [PMC free article] [PubMed]
- Gray S.J., Foti S.B., Schwartz J.W., Bachaboina L., Taylor-Blake B., Coleman J., Ehlers M.D., Zylka M.J., McCown T.J., Samulski R.J. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gray S.J., Foti S.B., Schwartz J.W., Bachaboina L., Taylor-Blake B., Coleman J., Ehlers M.D., Zylka M.J., McCown T.J., Samulski R.J.. (2011) Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther.. 22:1143-1153. [DOI] [PMC free article] [PubMed]
- Huang Y., Dreyfus C.F. The role of growth factors as a therapeutic approach to demyelinating disease. Exp. Neurol. 2016;283(Pt B):531–540. doi: 10.1016/j.expneurol.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huang Y., Dreyfus C.F.. (2016) The role of growth factors as a therapeutic approach to demyelinating disease. Exp. Neurol. 283(Pt B):531-540. S0014-4886(16) 30039-5. [DOI] [PMC free article] [PubMed]
- Ihrie R.A., Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ihrie R.A., Alvarez-Buylla A. (2011) Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70:674-686. [DOI] [PMC free article] [PubMed]
- Ishii A., Furusho M., Dupree J.L., Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J. Neurosci. 2014;34:16031–16045. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishii A., Furusho M., Dupree J.L., Bansal R.. (2014). Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J. Neurosci.. 34:16031-16045. [DOI] [PMC free article] [PubMed]
- Ishii A., Furusho M., Dupree J.L., Bansal R. Strength of ERK1/2 MAPK activation determines its effect on myelin and axonal integrity in the adult CNS. J. Neurosci. 2016;36:6471–6487. doi: 10.1523/JNEUROSCI.0299-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishii A., Furusho M., Dupree J.L., Bansal R.. (2016). Strength of ERK1/2 MAPK activation determines its effect on myelin and axonal integrity in the adult CNS. J. Neurosci.. 36:6471-6487. [DOI] [PMC free article] [PubMed]
- Jablonska B., Aguirre A., Raymond M., Szabo G., Kitabatake Y., Sailor K.A., Ming G.L., Song H., Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jablonska B., Aguirre A., Raymond M., Szabo G., Kitabatake Y., Sailor K.A., Ming G.L., Song H., Gallo V. (2010) Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 13:541-550. [DOI] [PMC free article] [PubMed]
- Jin K., Sun Y., Xie L., Batteur S., Mao X.O., Smelick C., Logvinova A., Greenberg D.A. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]; Jin K., Sun Y., Xie L., Batteur S., Mao X.O., Smelick C., Logvinova A., Greenberg D.A. (2003) Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2:175-183. [DOI] [PubMed]
- Kang W., Hébert J.M. FGF signaling is necessary for neurogenesis in young mice and sufficient to reverse its decline in old mice. J. Neurosci. 2015;35:10217–10223. doi: 10.1523/JNEUROSCI.1469-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kang W., Hebert J.M. (2015) FGF signaling is necessary for neurogenesis in young mice and sufficient to reverse its decline in old mice. J. Neurosci. : the official journal of the Society for Neuroscience 35:10217-10223. [DOI] [PMC free article] [PubMed]
- Kang W., Balordi F., Su N., Chen L., Fishell G., Hebert J.M. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc. Natl. Acad. Sci. U S A. 2014;111:E2987–E2995. doi: 10.1073/pnas.1320401111. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kang W., Balordi F., Su N., Chen L., Fishell G., Hebert J.M. (2014) Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc. Natl. Acad. Sci. U S A 111:E2987-E2995. [DOI] [PMC free article] [PubMed]
- Li J., Zhang L., Chu Y., Namaka M., Deng B., Kong J., Bi X. Astrocytes in OL lineage development and white matter pathology. Front. Cell. Neurosci. 2016;10:119. doi: 10.3389/fncel.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li J., Zhang L., Chu Y., Namaka M., Deng B., Kong J., Bi X.. (2016). Astrocytes in OL lineage development and white matter pathology. Front. Cell. Neurosci.. 10:119. [DOI] [PMC free article] [PubMed]
- Ludwin A.K., Rao V.T.S., Moore C.S., Antel J.P. Astrocytes in multiple sclerosis. Mult. Scler. 2016;22:1114–1124. doi: 10.1177/1352458516643396. [DOI] [PubMed] [Google Scholar]; Ludwin A.K., Rao V.T.S., Moore C.S., Antel J.P.. (2016). Astrocytes in multiple sclerosis. Mult. Scler.. 22:1114-1124. [DOI] [PubMed]
- Menn B., Garcia-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. Origin of OLs in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Menn B., Garcia-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. (2006) Origin of OLs in the subventricular zone of the adult brain. J. Neurosci. : the official journal of the Society for Neuroscience 26:7907-7918. [DOI] [PMC free article] [PubMed]
- Merson T.D., Bourne J.A. Endogenous neurogenesis following ischaemic brain injury: insights for therapeutic strategies. Int. J. Biochem. Cell Biol. 2014;56:4–19. doi: 10.1016/j.biocel.2014.08.003. [DOI] [PubMed] [Google Scholar]; Merson T.D., Bourne J.A. (2014) Endogenous neurogenesis following ischaemic brain injury: insights for therapeutic strategies. Int. J. Biochem. Cell Biol. 56:4-19. [DOI] [PubMed]
- Murtie J.C., Zhou Y.X., Le T.Q., Vana A.C., Armstrong R.C. PDGF and FGF2 pathways regulate distinct OL lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol. Dis. 2005;19:171–182. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]; Murtie J.C., Zhou Y.X., Le T.Q., Vana A.C., Armstrong R.C.. (2005) PDGF and FGF2 pathways regulate distinct OL lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol. Dis.. 19:171-182. [DOI] [PubMed]
- Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C., Baron-Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into OLs after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]; Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C., Baron-Van Evercooren A. (1999) Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into OLs after demyelination. Eur. J. Neurosci. 11:4357-4366. [DOI] [PubMed]
- Naruse M., Ishizaki Y., Ikenaka K., Tanaka A., Hitoshi S. Origin of OLs in mammalian forebrains: a revised perspective. J. Physiol. Sci. 2017;67(1):63–70. doi: 10.1007/s12576-016-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naruse M., Ishizaki Y., Ikenaka K., Tanaka A., Hitoshi S.. (2017). Origin of OLs in mammalian forebrains: a revised perspective. J. Physiol. Sci. 2017 Jan;67(1):63-70. [DOI] [PMC free article] [PubMed]
- Oh L.Y.S., Denninger A., Colvin J.S., Vyas A., Tole S., Ornitz D.M., Bansal R. Fibroblast growth factor receptor 3 signaling regulates the onset of OL terminal differentiation. J. Neurosci. 2003;23:883–894. doi: 10.1523/JNEUROSCI.23-03-00883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oh L.Y.S., Denninger A., Colvin J.S., Vyas A., Tole S., Ornitz D.M., Bansal R.. (2003). Fibroblast growth factor receptor 3 signaling regulates the onset of OL terminal differentiation J. Neurosci. 23: 883-894. [DOI] [PMC free article] [PubMed]
- Picard-Riera N., Decker L., Delarasse C., Goude K., Nait-Oumesmar B., Liblau R., Pham-Dinh D., Baron-Van Evercooren A. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]; Picard-Riera N., Decker L., Delarasse C., Goude K., Nait-Oumesmar B., Liblau R., Pham-Dinh D., Baron-Van Evercooren A. (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. U S A 99:13211-13216. [DOI] [PMC free article] [PubMed]
- Qian X., Davis A.A., Goderie S.K., Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]; Qian X., Davis A.A., Goderie S.K., Temple S. (1997) FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron 18:81-93. [DOI] [PubMed]
- Rai K.S., Hattiangady B., Shetty A.K. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]; Rai K.S., Hattiangady B., Shetty A.K. (2007) Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 26:1765-1779. [DOI] [PubMed]
- Ruffini F., Furlan R., Poliani P.L., Brambilla E., Marconi P.C., Bergami A., Desina G., Glorioso J.C., Comi G., Martino G. Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 2001;8:1207–1213. doi: 10.1038/sj.gt.3301523. [DOI] [PubMed] [Google Scholar]; Ruffini F., Furlan R., Poliani P.L., Brambilla E., Marconi P.C., Bergami A., Desina G., Glorioso J.C., Comi G., Martino G.. (2001) Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther.. 8:1207-1213. [DOI] [PubMed]
- Stevens H.E., Jiang G.Y., Schwartz M.L., Vaccarino F.M. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol. Psychiatry. 2012;71:1090–1098. doi: 10.1016/j.biopsych.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stevens H.E., Jiang G.Y., Schwartz M.L., Vaccarino F.M. (2012) Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol. Psychiatry 71:1090-1098. [DOI] [PMC free article] [PubMed]
- Vroemen M., Weidner N., Blesch A. Loss of gene expression in lentivirus- and retrovirus-transduced neural progenitor cells is correlated to migration and differentiation in the adult spinal cord. Exp. Neurol. 2005;195:127–139. doi: 10.1016/j.expneurol.2005.04.012. [DOI] [PubMed] [Google Scholar]; Vroemen M., Weidner N., Blesch A.. (2005) Loss of gene expression in lentivirus- and retrovirus-transduced neural progenitor cells is correlated to migration and differentiation in the adult spinal cord. Exp. Neurol.. 195:127-139. [DOI] [PubMed]
- Werner S., Unsicker K., von Bohlen und Halbach O. Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J. Neurosci. Res. 2011;89:1605–1617. doi: 10.1002/jnr.22680. [DOI] [PubMed] [Google Scholar]; Werner S., Unsicker K., von Bohlen und Halbach O. (2011) Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J. Neurosci. Res. 89:1605-1617. [DOI] [PubMed]
- Xing Y.L., Roth P.T., Stratton J.A., Chuang B.H., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., Merson T.D. Adult neural precursor cells from the subventricular zone contribute significantly to OL regeneration and remyelination. J. Neurosci. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xing Y.L., Roth P.T., Stratton J.A., Chuang B.H., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., Merson T.D. (2014) Adult neural precursor cells from the subventricular zone contribute significantly to OL regeneration and remyelination. J. Neurosci. : the official journal of the Society for Neuroscience 34:14128-14146. [DOI] [PMC free article] [PubMed]
- Zhao M., Li D., Shimazu K., Zhou Y.X., Lu B., Deng C.X. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol. Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]; Zhao M., Li D., Shimazu K., Zhou Y.X., Lu B., Deng C.X. (2007) Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol. Psychiatry 62:381-390. [DOI] [PubMed]
- Zhou Y.X., Flint N.C., Murtie J.C., Le T.Q., Armstrong R.C. Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of OL progenitor differentiation. Glia. 2006;54:578–590. doi: 10.1002/glia.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou Y.X., Flint N.C., Murtie J.C., Le T.Q., Armstrong R.C.. (2006). Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of OL progenitor differentiation. Glia 54:578-590. [DOI] [PMC free article] [PubMed]
- Zhou Y.X., Pannu R., Le T.Q., Armstrong R.C. Fibroblast growth factor 1 (FGFR1) modulation regulates repair capacity of OL progenitor cells following chronic demyelination. Neurobiol. Dis. 2012;45:196–205. doi: 10.1016/j.nbd.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou Y.X., Pannu R., Le T.Q., Armstrong R.C.. (2012) Fibroblast growth factor 1 (FGFR1) modulation regulates repair capacity of OL progenitor cells following chronic demyelination. Neurobiol. Dis.. 45:196-205. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.