Abstract
We studied the effects of varying light quality on the flowering, photosynthetic rate and fruit yield of everbearing strawberry plants (Fragaria×ananassa Duch. ‘HS138’), which are long-day plants, to increase the efficiency of fruit production in plant factories. The plants were grown under continuous lighting using three types of blue and red LEDs (blue light peak wavelength: 405, 450, and 470 nm; red light peak wavelength: 630, 660, and 685 nm) during the nursery period. All blue light from the various peak LED types promoted more flowering compared with red light (630 and 660 nm except for 685 nm). The longer wavelength among the red light range positively correlated with earlier flowering, whereas the number of days to anthesis did not significantly differ among blue LED treatment wavelengths, irrespective of peak wavelength. The result of a similar experiment using the perpetual flowering Fragaria vesca accession Hawaii-4 representing a model strawberry species showed almost the same pattern of flowering response to light quality. These results suggest that long-day strawberry plants show similar flowering response to light quality. The photosynthetic rate under red light (660 nm) was higher than that under blue light (450 nm). However, the plants grown under red light showed lower photosynthetic capacity than those grown under blue light. Although the light color used to grow the seedlings showed no difference in the daily fruit production, blue light irradiation during the nursery period hastened harvesting because of the advance in flowering.
Keywords: closed system, Fragaria vesca, harvest index, nursery plant, plant factory
Plant factories are closed plant production systems in which artificial lighting is used as a light source for growing plants without the use of sun light. The systems were conceptualized, developed, and implemented in Japan during the 1980s (Goto 2012). One advantage of the system is that environmental conditions, such as light, air temperature, humidity, and CO2 concentration, can be controlled for optimum growing conditions, which allow us to produce vegetables premeditatedly without being affected by weather conditions. In Japan, it is estimated that the number of plant factories using artificial light for commercial production of leafy vegetables, such as lettuce and spinach plants, had increased to >130 by the end of 2012 (Kozai 2013). Thus far, crops produced commercially by the system are mainly leafy vegetables and seedlings, which can be grown in a short time. Therefore, apart from leafy vegetables, it is necessary to establish the cultivation techniques of plants in the system.
Strawberry plants are one of the candidate crops for plant factories in the future because strawberry plants display appropriate characteristics. Strawberry plants are smaller in size than other horticultural crops, such as tomato and melon plants. The necessary light intensity for the cultivation of strawberry plants is lower than that for the cultivation of other crops (strawberry plants: 100–300 µmol m−2 s−1). Therefore, FL and LED, which are smaller in size than other light sources, such as high-intensity discharged (HID) lamps, are available for cultivating strawberry plants. Therefore, the necessary cultivation space per plant is small and strawberry plants can be cultivated using multi-layered shelving. As a result, the yield per cultivation area can be raised. Although there is little or no information about the cultivation of strawberry plants under artificial environment, an R&D project for producing of high-value materials using transgenic everbearing strawberry plants in a closed system was performed from 2006 to 2010 (Goto 2011; Hikosaka et al. 2009; Hikosaka et al. 2013; Miyazawa et al. 2009; Yoshida et al. 2012); this contributed to the accumulation of knowledge regarding environmental control and cultivation techniques for strawberry plants production in plant factories.
Commercial growers prefer a continuous or longer light period than that used usually for production of leafy vegetables in plant factories (Goto 2012). An extended light period can enhance the growth of plants, thereby increasing crop production and shortening the cultivation period. In addition, a long light period also decrease the initial cost of a lighting system by using combination of a lower light intensity (Goto 2012). However, it is difficult to grow the SD June-bearing strawberries under LD conditions for stable fruit production because an extended day inhibits flowering and promotes vegetative reproduction. Therefore, the everbearing strawberry plant for which growth and flowering is enhanced under an extended day is suitable for continuous high-yield fruit production in plant factories.
To optimize strawberry fruit production in plant factories, it is necessary to raise the efficiency of fruit production per unit of electricity consumption used for lighting, cooling, and other equipment. In particular, the electrical energy consumption for lighting comprises the majority of total energy expenditure used for growing plants in a plant factory. Strategies to raise the efficiency of fruit production from an engineering point of view include an improvement of energy-light conversion efficiency of lamps, the ratio of light energy received by leaves, and the coefficient of performance of air conditioning. In contrast, considering plant-based research, it is important to determine the optimal environmental conditions to promote photosynthesis and the translocation of photoassimilates to the salable part in terms of fruits.
LEDs have recently been introduced in many commercial plant factories in Japan and are expected to decrease the electricity costs of lighting and cooling because they are more efficient in converting electric power to light power and exerting lower cooling loads than conventional light sources (Goto 2013). In addition, LEDs are characterized by a narrow light-emitting spectrum, enabling the focus of light onto plants using only the required wavelength of light (Watanabe 2011). These capabilities imply that this technology potentially constitutes one of the most significant advances in horticultural lighting since the development of HID lamps (Morrow 2008).
Blue light emitted by LEDs with a peak wavelength of 450 nm promotes flowering of everbearing strawberry ‘HS138’ compared to red light emitted by LEDs with a peak wavelength of 660 nm (Yoshida et al. 2012); this is similar to the flowering response of Arabidopsis thaliana plant to light quality (Eskins 1992; Guo et al. 1998; Mockler et al. 1999). However, there are multiple blue and red LED types with varying peak wavelength range (400–500 and 600–700 nm, respectively). In single-peak LEDs, a small difference (20–40 nm) in peak wavelength sometimes causes a large difference in photosynthetic rate and growth (Goto 2013). Therefore, it is necessary to determine the optimal light quality for flowering, photosynthesis, growth and fruit yield of everbearing strawberry plants to choose appropriate LEDs from the various LED types.
In the present study, to determine appropriate LEDs as light sources during the nursery period, we revealed the effects of various light quality from single-peak blue and red LEDs on flowering and photosynthesis of everbearing strawberry plants ‘HS138’ used in previous research (Yoshida et al. 2012). We also investigated the flowering response and expression levels of the flowering-time related genes of F. vesca LD accession Hawaii-4 (H4) that were used in the molecular level studies of the flowering response as strawberry model species (Mouhu et al. 2009; Rantanen et al. 2014). Furthermore, as a practical research, we tested the effects of light quality during the nursery period on the growth and fruit yield of the everbearing strawberry plants.
Materials and methods
Plant material and growth conditions
We used micropropagated everbearing strawberry (Fragaria×ananassa Duch. ‘HS138’) (Hokusan Co., Ltd., Japan) plantlets, which were derived from the meristem of the mother plant. The plantlets were transplanted from a micropropagation system to a hydroponic culture system in a controlled environment room using a 16 h light period provided by white FLs (FHF32EX-N-H; Panasonic Co., Ltd., Japan) in a closed system. The plantlets were grown for 21 days under a PPF of 225 µmol m−2 s−1) on the culture panel using a quantum sensor (LI-190; Li-COR, Inc., USA). At 21 days after transplantation, the strawberry seedlings (with three to four unfolded leaves) were transplanted to a deep flow technique (DFT) hydroponic system equipped with air pumps. A standard commercial nutrient solution (Otsuka A treatment; Otsuka AgriTechno Co., Ltd., Japan) at the electrical conductivity level of 0.8 dS m−1 was used. In addition, we used the woodland strawberry (Fragaria vesca L.) perpetual flowering LD accession Hawaii 4 (H4). Seeds were sown in well-washed and wetted polyurethane foam and grown in a growth chamber. The environmental conditions were as follows: air temperature of 25°C, relative humidity of 70%, and light period of 12 h d−1, respectively. White FLs were used as the main light sources in a growth chamber for seed germination. The PPF at the top of plant canopy was set to 100 µmol m−2 s−1. The seedlings with two to three unfolded leaves were used as the plant materials. The seedlings were transplanted to a DFT hydroponic system equipped with air pumps.
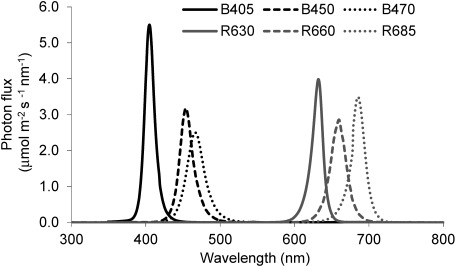
Four strawberry ‘HS138’ seedlings and nine ‘H4’ seedlings were grown under three blue LED panels (peak wavelength of 405, 450, and 470 nm; CCS, Inc., Japan), or three red LED panels (peak wavelength of 630, 660, and 685 nm; CCS, Inc., Japan) in the controlled environment room with continuous lighting (treatment codes: B405, B450, and B470 and R630, R660, and R685, respectively; Table 1). The spectral PF of each light source was calculated from the distribution of spectral energy which was measured using a spectroradiometer (LI-1800; Li-COR, Inc., USA) (Figure 1). Table 1 shows the spectral characteristics of each light source. The PPF was set to 80 µmol m−2 s−1 on the surface of the culture panel. The number of days from the onset of treatment to anthesis was measured by observing with the naked eye. We measured the number of leaves at 40 days (‘HS138’) and 30 days (H4) after anthesis of the first flower. We also measured the number of flower buds observed by 40 days (‘HS138’) and 30 days (H4) after anthesis of the first flower.
Table 1. Spectral characteristics of each treatment.
| B405 | B450 | B470 | R630 | R660 | R685 | |
|---|---|---|---|---|---|---|
| Photon flux (µmol m−2 s−1) | ||||||
| 300–400 nm (UV) | 24.3 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 |
| 400–500 nm (B) | 79.4 | 79.2 | 76.8 | 0.0 | 0.0 | 0.0 |
| 500–600 nm (G) | 0.3 | 0.7 | 3.1 | 1.2 | 0.2 | 0.0 |
| 600–700 nm (R) | 0.4 | 0.1 | 0.1 | 78.8 | 79.8 | 80.0 |
| 700–800 nm (FR) | 0.0 | 0.0 | 0.0 | 0.1 | 0.6 | 7.6 |
| PPF | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
Figure 1. Spectral photon distribution (SPD) of LED at PPF of 80 µmol m−2 s−1. The SPD was calculated from the spectral energy distribution of the light source, which was measured using a spectroradiometer (LI-1800; Li-COR, Inc., USA).
Real-time quantitative RT-PCR analysis
Leaf samples were collected for RT-PCR during the light period at the time points of 2, 7, and 12 days from each LED wavelength treatment. For the leaf samples, middle leaflets of the youngest completely opened leaves and youngest initials leaves were pooled from several individuals. Total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen GmbH, Germany). The first strand of cDNA was synthesized using the PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio Inc., Japan). Quantitative RT-PCR was performed using the Stratagene Mx3005P instrument (Agilent Technologies, USA) and KOD SYBER qPCR Mix (Toyobo Co., Ltd., Japan). cDNA (equivalent to 10 ng of total RNA) was amplified using a genetic specific primer set in a 20 µl reaction volume, according to the manufacturer’s instructions. The real time PCR program was 2 min at 98°C, followed by 40 cycles of 10 s at 98°C, 11 s at 60°C and 30 s at 68°C, followed by 1 min at 98°C, 30 s at 68°C and 30 s at 98°C. Three technical replicates were analyzed in each experiment. The used gene specific primers used were: FvMSI1-F, 5′-TCC CCA CAC CTT TGA TTG CCA-3′; FvMSI1-R, 5′-ACA CCA TCA GTC TCC TGC CAA G-3′; FvFT1-F, 5′-CAA TCT CTT GGC CGA AAA CT-3′; FvFT1-R, 5′-TGA GCT CAA ACC TTC CCA AG-3′; FvCO-F, 5′-GAC ATC CAC TCC GCC AAC-3′; and FvCO-R, 5′-GTG GAC CCC ACC ACT ATC TG-3′. Primer sets for FvMSI1, FvFT1, and FvCO were described by Rantanen et al. (2014).
Gas-exchange measurements
We used four strawberry seedlings ‘HS138’ grown under white FLs. The gas-exchange of a leaf of each strawberry plants was measured by using a portable photosynthesis measuring system (LI-6400; Li-COR, Inc., USA). Light was provided from three blue LED panels (peak wavelength of 405, 450, and 470 nm; CCS, Inc., Japan), or three red LED panels (peak wavelength of 630, 660, and 685 nm; CCS, Inc., Japan). The measurements were conducted at different PF levels, at 25°C, and the CO2 concentration was maintained at 1,000 µmol−1 mol−1.
Light quality during nursery period on growth and fruit yield
Nine strawberry seedlings ‘HS138’ grown under each treatment were transplanted to a controlled environment room with continuous lighting. Blue LED panels [LHL-1200 (450); peak wavelength at 450 nm; Iwasaki Electric Co., Ltd., Japan], and red LED panels [LHL-1200 (660); peak wavelength at 660 nm; Iwasaki Electric Co., Ltd., Japan] were used as light sources. The experiment consisted of three treatments [Blue (B), red (R) and B→R treatments] using the two different light sources. In B→R treatments, the first light sources were blue LEDs until 20 days after the onset of treatment, after which the light source was changed from blue to red LEDs. The PPF was set to 150 µmol m−2 s−1 on the surface of the plants.
We measured the net photosynthetic rate under cultivation light (PPF: 150 µmol m−2 s−1) and SPAD value at 11, 21, 31, and 41 days after initiating treatment and net photosynthetic rate under saturated light (PPF: 1500 µmol m−2 s−1) provided by blue and red LEDs (6400-02B; Li-COR, Inc., USA) at 12, 22, 32, and 42 days after initiating treatment. SPAD value was measured using a SPAD-502 m (Konica Minolta, Inc., Japan).
The dry weights, number of leaves, leaf area and leaf petiole length of the three plants from each treatment were measured 10 days after anthesis. The dry weights of removed runners and leaves during treatment period were also measured. The remaining 3 plants from each treatment were grown under 19.4 mol m−2 d−1 of daily light integral (DLI) conditions (Light period: 24 h d−1; PPF: 225 µmol m−2 s−1) under white FLs for the fruit yield survey, which lasted until 60 days after anthesis.
Results and discussion
Flowering response to various light quality
All blue light emitted from the various peak LED types promoted more flowering of everbearing strawberry ‘HS138’ compared with red light (630 and 660 nm except for 685 nm) (Figure 2A). The longer wavelength among the red light range positively correlated with earlier flowering, whereas the number of days to anthesis did not significantly differ among all types of blue LEDs and red LEDs with a peak wavelength of 685 nm. Therefore, it is believed that using blue LEDs or red LEDs with a peak wavelength of 685 nm was suitable for promoting flowering of everbearing strawberry plants. The results of a similar experiment using the H4 showed almost the same pattern of flowering responses to light quality (Figure 2B). These results suggested that LD type strawberry plants showed similar flowering responses to light quality. The number of leaves of ‘HS138’ and H4 were investigated at 40 days and 30 days after anthesis, respectively (Figure 2C). The number of leaves of ‘HS138’ was much greater in red light than that in blue light; the same tendency was observed in H4 except for R630 treatment. We also measured the number of flower buds observed at 40 (‘HS138’) and 30 (H4) days after anthesis (Figures 2E and F). The number of fruit buds of ‘HS138’ was also higher in red light; however, the same tendency was not observed in H4. Hidaka et al. (2015) reported that supplemental lighting increased the number of flowers in various strawberry cultivars. They explained that this increase in the number of flowers was caused by an increased supply of carbohydrates to bud primordia by supplemental lighting. Therefore, the reason for the increase in the number of buds of ‘HS138’ grown under red light may be the accelerated photosynthesis per plant due to increase in the number of leaves. On the other hand, because we observed extreme stem elongation in H4 grown under red light (data not shown), it was thought that aggravation of light intercepting characteristic in H4 grown under red light reduced photosynthesis, without increasing the number of buds.
Figure 2. Effects of varying light quality from a single-peak LED on days to anthesis (A and B) and number of leaves (C and D) as well as the number of buds (E and F) of everbearing strawberry ‘HS138’ and Fragaria vesca accession Hawaii-4 (H4). The number of leaves and buds of ‘HS138’ and H4 were investigated 40 days and 30 days after anthesis, respectively. The light sources of the B405, B450, and B470 and R630, R660, and R685 treatments were blue LEDs (peak wavelength: 405, 450, and 470 nm), and red LEDs (peak wavelength: 635, 660, and 685 nm), respectively. Different letters indicate significant differences between treatments at p<0.05 as determined with the Tukey–Kramer’s test. Vertical bars indicate S.E. (n=4–9).
Expression Levels of FvFT1 and FvCO under each LED wavelength treatment
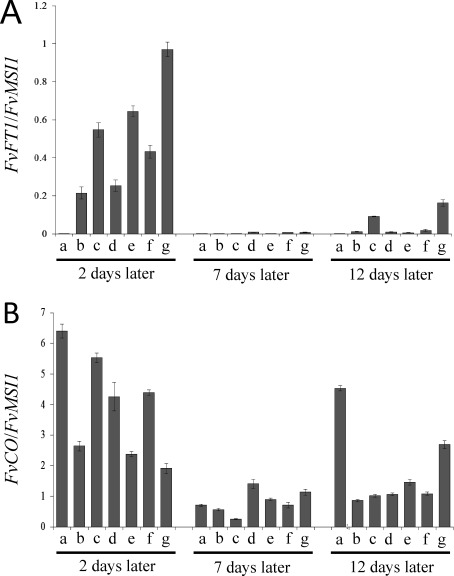
We analyzed the expression of FvFT1 and FvCO of LD F. vesca leaves under each light wavelength treatment (Figure 3). FvFT1 of SD treatment as a control was scarcely expressed during all time points, and these samples did not generate flowers. In addition, we found an abundant level of FvFT1 expression under each LED wavelength treatment at 2 days compared with that 7 and 12 days. However, no relationship between the level of FvFT1 and flowering time was detected, although the FvFT1 mRNA level at 2 days was different among each LED treatments. Rantanen et al. (2014) reported that the expression of FvFT1 of the leaves of F. vesca accession H4 was high in far-red and incandescent light treatment that promoted flowering, 1 week (7 days) after the starting of the end-of-day (EOD) light quality treatments. In our study, the expression of FvFT1 was unclear to assess different timings of flowering under LED treatments. Our continuous lighting irradiated with higher light intensity than that with EOD lighting. As a hypothesis, we could suggest that continuous lighting with high light intensity could stimulate floral bud formation regardless of the light quality. However, it seems that far-red light is a strong signal to induce higher FvFT1 expression, because the expression of FvFT1 becomes higher than other red light treatment under the 685-nm LED. As shown in Figure 1 and Table 1, light from the 685-nm LED includes much far-red light spectrum than other LED. Rantanen et al. (2014) reported that far-red EOD was a strong signal to induce FvFT1 expression. In our study, higher level of far-red in 685-nm LED treatment could lead to higher FvFT1 expression. To confirm the effects of continuous lighting, we should evaluate the circadian change in FvFT1 expression and the effects of light quality on it.
Figure 3. Relative expression of FvFT1 (A) and FvCO (B) in the leaves of wild strawberry (Fragaria vesca) by qRT-PCR analysis. Each sample was collected at different time points (2, 7 and 12 days) after starting each LED wavelength treatment. Each LED wavelength treatment was as follows: a, short day (Light period: 12 h d−1) provided by white FLs; b, 405 nm; c, 450 nm; d, 470 nm; e, 630 nm; f, 660 nm; and g, 685 nm. Real-time quantitative RT-PCR amplification of FvMSI1 was used to normalize the gene expression under identical conditions. Three technical replicates were analyzed, and all results are shown as mean±SE.
Photosynthetic rate under various light quality
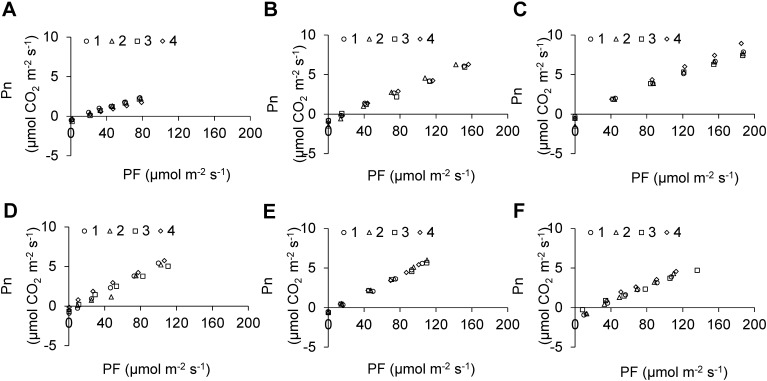
In the present study, we investigated the photosynthetic rate under a light-limitation level of <200 µmol m−2 s−1, so that a linear relationship between PF and Pn was observed in all treatments (Figure 4). We determined the slope of each line, i.e., we calculated Pn/PF under each light quality (Figure 5). The results showed that red light of 630 and 660 nm promoted photosynthesis compared with red light of 685 nm and all blue light wavelength used. McCree (1972) and Inada (1976) showed that the quantum yield of photosynthesis was higher under red light than under blue or green light, and Yanagi et al. (1996) revealed that the strawberry plants grown under red LEDs underwent more photosynthesis than those grown under the blue LEDs. In addition, in the blue light range, blue light of 405 nm inhibited photosynthesis compared with blue light of 450 and 470 nm (Figure 5). Similarly, in the red light range, red light of 685 nm inhibited photosynthesis compared to red light of 630 and 660 nm. In addition, these tendencies of photosynthetic response to narrow range wavelength were consistent with the results reported by McCree (1972) and Inada (1976). Therefore, it was believed that using a red LED with peaks in 630 or 660 nm was more efficient for the photosynthesis of everbearing strawberry plants in the case of short time exposure.
Figure 4. Effects of varying light quality (A: 405 nm, B: 450 nm, C: 470 nm, D: 630 nm, E: 660 nm, and F: 685 nm) on net photosynthetic rate (Pn) of everbearing strawberry plants ‘HS138’ grown under white FLs (100 µmol m−2 s−1). PF indicates photon flux. Net photosynthetic rates were measured using a portable photosynthesis system (LI-6400; Li-COR, Inc., USA). The legends indicate plant number used by measuring Pn.
Figure 5. Effects of varying light quality on light utilization efficiency (net photosynthetic rate/photon flux; Pn/PF) of everbearing strawberry plants ‘HS138’ grown under white fluorescent lamps (100 µmol m−2 s−1). Vertical bars indicate S.E. Different letters indicate significant differences between treatments at p<0.05 as determined with the Tukey–Kramer’s test (n=4).
Effects of Light quality during nursery period on growth and fruit yield
We chose blue (450 nm) and red (660 nm) LEDs as light sources to promote flowering and photosynthesis, respectively, for cultivation during nursery period and to evaluate the effects of light quality during the nursery period on fruit production. In addition, we established the B→R treatment, expecting promotion of flowering and photosynthesis during the first and later half of the nursery period, respectively. The plants under the B and B→R treatments flowered significantly earlier than those under the R treatment (Table 2). The results were accordant with the results reported by Yoshida et al. (2012). Table 2 also shows morphogenesis variables of plants in the B, R and B→R treatments at 10 days after anthesis and Figure 6 shows the phenotype. The number of leaves in the R treatment was greater than that in the B and B→R treatments due to late anthesis. Similarly, the leaf area in the R treatment was bigger than that in the B and B→R treatments. However, the averaged leaf area per leaf in the B treatment was significantly higher than that in the R and B→R treatments, and the specific leaf weight, which signifies dry weight per leaf area, was higher in R and B→R treatments than that in the B treatment. Furthermore, blue light promoted the elongation of the leaf petiole.
Table 2. Effects of varying light quality on growth and development of everbearing strawberry plants ‘HS138’ at 10 days after anthesis.
| Treatment | Days to anthesis | Number of leaves | Number of buds | Leaf area per plant (cm2/plant) | Average leaf area per leaf (cm2/leaf) | SLW* (mg DW cm−2) | Leaf petiole length (cm) |
|---|---|---|---|---|---|---|---|
| B | 40 b | 29 b | 72 a | 1782 a | 62.2 a | 4.5 b | 11.1 a |
| R | 49 a | 45 a | 36 b | 2140 a | 47.2 b | 5.8 a | 9.4 b |
| B→R | 41 b | 34 ab | 76 a | 1717 a | 50.7 b | 5.6 a | 9.0 b |
* Specific leaf weight. Different letters indicate significant differences between treatments at p<0.05 as determined with the Tukey–Kramer’s test (n=3).
Figure 6. Phenotype of everbearing strawberry ‘HS138’ grown under varying light quality at 10 days after anthesis. Blue (B) and Red (R): under continuous lighting illuminated by blue LEDs and red LEDs, respectively, B→R: under continuous lighting illuminated by blue LEDs substituted with red LEDs at 20 days after initiating treatment. The numbers of days to anthesis of B, R, and B→R were 40, 49, and 41, respectively.
Table 3 shows dry weight at 10 and 100 days after anthesis. Dry weight at both 10 and 100 days after anthesis in the R treatment was highest among the treatments, enhancing the ratio of dry matter increment per day between 10 and 100 days after anthesis compared with other treatments. Red light irradiation during the nursery period increased the ratio by 10% as compared with blue light.
Table 3. Dry weight and dry matter increment during the nursery and harvesting period.
| Treatment | Dry weight at 10 days after anthesis (gDW) | Dry matter during the nursery periodz (gDW) | Fruit yield (gFW) | Dry matter of fruity (gDW) | Dry weight at 100 days after anthesis (gDW) | Dry matter during all periodx (gDW) | Dry matter increment during harvesting periodw (g/90 days) | Dry matter increment per dayv (g/day) |
|---|---|---|---|---|---|---|---|---|
| B | 17.6 b | 19.1 b | 813.7 a | 69.2 a | 34.5 b | 112.6 a | 93.5 a | 1.04 a |
| R | 23.1 a | 25.7 a | 793.2 a | 67.4 a | 52.9 a | 130.0 a | 104.3 a | 1.16 a |
| B→R | 19.7 ab | 21.8 ab | 731.9 a | 62.2 a | 44.7 ab | 114.9 a | 93.2 a | 1.04 a |
z Dry matter during the nursery period was calculated by adding the dry weight of removed leaves and runners to total dry weight at 10 days after anthesis. y Dry matter of fruit was calculated by multiplying fruit yield by fruit dry matter ratio (0.085). x Dry matter during all period was calculated by adding the dry weight of removed leaves and dry matter of fruit to total dry weight at 100 days after anthesis. w Dry matter increment during harvest period was calculated by subtracting total dry matter during the nursery period from total dry matter during all period. v Dry matter increment per day was calculated by dividing dry matter increment during harvest period by days of the harvesting period (90 days). Different letters indicate significant differences between treatments at p<0.05 as determined with the Tukey–Kramer’s test (n=3).
At 11 days after the initiating treatment, Pn under the cultivation light of the R treatment was more significantly higher than that under the cultivation light of the B treatment (Figure 7A). However, after the 21 days, Pn under the cultivation light of the R treatment was significantly smaller than that under the cultivation light of the B treatment or did not differ between treatments. Pn under the saturated light of the R treatment decreased similar to Pn under the cultivation light after the 22 days in comparison with that at 12 days (Figure 7B). In contrast, Pn under the saturated light of the B treatment was maintained highly during the treatment period. Pn under the cultivation light of the B→R treatment of 21 days was significantly higher than that under the cultivation light of the other treatments, and approximately 80% higher than that under the cultivation of the B treatment. The SPAD value (chlorophyll content of leaves) of the plants grown only under red light was lower than that of the plants grown under blue light (Figure 7C). Matsuda et al. (2004) reported that in rice plants grown under red light supplemented with blue light, Pn measured under white light at 1,600 and 250 µmol m−2 s−1 were higher than those in the plants grown under red light alone and this was associated with a higher total N content. In this study, because SPAD value is high under blue light, it is thought that Pn under saturated light was high under blue light similar to the reason of results of Matsuda et al. (2004).
Figure 7. Net Photosynthetic rate (Pn) under cultivation light quality conditions at 150 µmol m−2 s−1 (A), and saturated light at 1500 µmol m−2 s−1 (B) and SPAD value (C) of everbearing strawberry plants ‘HS138’ grown under varying light qualities. Pn was measured using portable a photosynthesis system (LI-6400; Li-COR, Inc., USA). Saturated light was provided from blue and red LEDs (6400-02B; Li-COR, Inc., USA). Different letters indicate significant differences between treatments at p<0.05 as determined with the Tukey–Kramer’s test. Vertical bars indicate S.E. (n=9).
A strong correlation was observed between Pn under saturated light and Pn under cultivation red light (Figure 8B), whereas there was no correlation between Pn under saturated light and Pn under cultivation blue light (Figure 8A). In addition, there was a weak correlation between the SPAD value and Pn under both blue and red cultivation light (Figures 8C and D). These results indicated that the red light had a high light use efficiency; however it became clear that photosynthetic rate was greatly influenced by the ability for photosynthesis of the leaf and that the photosynthetic ability decreased when plants grown only under red light. Furthermore, we suggested that the photosynthetic rate was raised by irradiating red with blue together or irradiating it in turn.
Figure 8. Relationship between net photosynthetic rate (Pn) at saturated light (A, B) or SPAD value (C, D) and Pn under blue (450 nm) or red (660 nm) LED at PPF of 150 µmol m−2 s−1 of everbearing strawberry plants ‘HS138.’ r is the correlation (n=36).
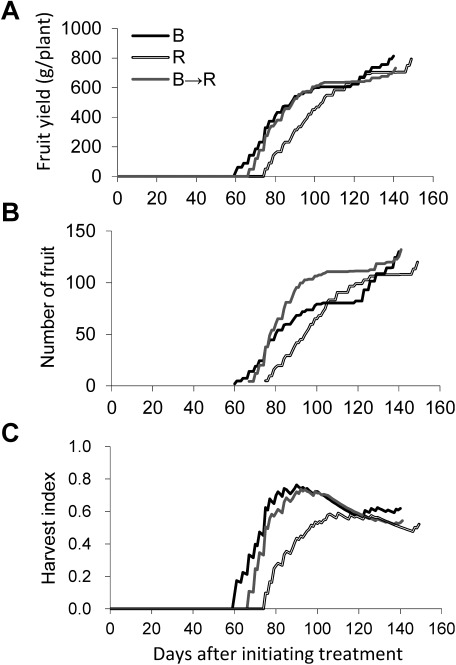
At 10 days after anthesis, we transplanted the nursery plants in the B, R and B→R treatments to the same light condition (Light period: 24 h d−1; PPF: 225 µmol m−2 s−1) provide by white FLs. In the B and B→R treatments, flowering was earlier than that in the R treatment (Table 2), and the time of harvest occurred earlier by approximately 10–15 days (Figure 9A). When the fruit yield reached approximately 600 g in the B, R, and B→R treatments, the speed of production of fruit decreased and a temporally limit to the yield was reached (Figure 9A). At approximately 40 days after starting harvesting, yields exceeded 600 g in these treatments, and subsequently, the speed of fruit production decreased. Although the dry weight at 10 days after anthesis was smaller than that in the R treatment (Table 3), the B and B→R treatments showed a similar speed of production of fruit compared with that in the R treatment. The time of harvesting fruits was early in the B and B→R treatments, thereby efficiently enhancing production of fruit.
Figure 9. Fruit yield (A), number of fruit (B) and harvest index (C) of everbearing strawberry plants ‘HS138.’ The harvest index was estimated by dividing fruit dry weight by estimated dry matter at each day. Estimated dry matter at each day was calculated by assuming that dry matter increased linearly at the rate of dry matter increment per day (Table 3). Blue (B) and Red (R): under continuous lighting illuminated by blue LEDs and red LEDs, respectively, B→R: under continuous lighting illuminated by blue LEDs substituted with red LEDs at 20 days after initiating treatment. The numbers of days to anthesis of B, R, and B→R were 40, 49, and 41, respectively. After 10 days after anthesis, the plants were grown under continuous lighting illuminated by white fluorescent lamps (PPF: 225 µmol m−2 s−1).
The number of fruits in the B→R treatment rapidly increased at approximately 20 days after harvesting compared with the B treatment (Figure 9B). This was probably due to the change from blue light into red light at 20 days after initiating treatment. It is believed that the number of flower buds was influenced by the growth rate at the flower inductive stage as discussed above. In fact, the photosynthetic rate of the B→R treatment increased exponentially when changing to red light from blue light (Figure 7A).
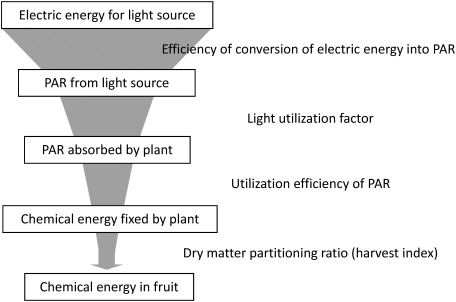
Harvest index represent the partitioning of photoassimilate between fruits and the vegetative part such as leaves, roots and stems. The value greatly changed in all treatments by the cultivation days, and the value of approximately 0.8 was achieved in the B and B→R treatments after initiating examination for 90 days (50 days after anthesis). This result means that approximately 80% of chemical energy which converted light energy absorbed by leaves into were accumulated to fruits. All treatments lasted 50–55 days before reaching the maximum of the harvest index from anthesis, and there were not many differences. On the other hand, the days passed until the flowering of the R treatment was longer than that in the B and B→R treatments. Therefore, the harvest index of the R treatment was lowered in comparison with B and B→R treatments. The translocation ratio of photoassimilate to fruit greatly influences the efficiency of electrical energy in plant factories (Figure 10). Thus, blue light irradiation during the nursery period is an effective technique for improving the production of fruit efficiency in plant factories.
Figure 10. Schematic diagram indicating that the electric energy for light source is fixed by plants as chemical energy in fruits.
Acknowledgments
The part of this research project was entrusted by Ministry of Economy, Technology and Industry of Japan (METI) as “Developmental of fundamental technologies for production of high-value materials using transgenic plants (2006 to 2010)”. We thank Dr. Takeshi Kurokura (Utsunomiya University, Japan) to kindly provide the seeds of the woodland strawberry (F.vesca).
Abbreviations
- FL
Fluorescent lamp
- FvCO
F. vesca homolog of CONSTANS
- FvFT1
F. vesca homolog of FLOWERING LOCUS T
- LD
long-day
- LED
Light-emitting diode
- PAR
photosynthetic active radiation
- PF
photon flux
- Pn
net photosynthetic rate
- PPF
photosynthetic photon flux
- SD
short-day
References
- Eskins K (1992) Light-quality effects on Arabidopsis development. Red, blue and far-red regulation of flowering and morpohology. Physiol Plant 86: 439–444 [Google Scholar]
- Goto E (2011) Production of pharmaceutical materials using genetically modified plants grown under artificial lighting. Acta Hortic 907: 45–52 [Google Scholar]
- Goto E (2012) Plant production in a closed plant factory with artificial lighting. Acta Hortic 956: 37–49 [Google Scholar]
- Goto E (2013) Measurements of the photosynthetic rates in vegetables under various qualities of light from light-emitting diodes. Acta Hortic 1037: 261–268 [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Hidaka K, Dan K, Imamura H, Takayama T, Sameshima K, Okimura M (2015) Variety comparison of effect of supplemental lighting with LED on growth and yield in forcing culture of strawberry. Environ Control in Biol 53: 135–143 [Google Scholar]
- Hikosaka S, Sasaki K, Goto E, Aoki T (2009) Effects of in vitro culture methods during the rooting stage and light quality during the seedling stage on the growth of hydroponic everbearing strawberry. Acta Hortic 842: 1011–1014 [Google Scholar]
- Hikosaka S, Yoshida H, Goto E, Tabayashi N, Matsumura T (2013) Effects of light quality on the concentration of human adiponectin in transgenic everbearing strawberry. Environ Control in Biol 51: 31–33 [Google Scholar]
- Inada K (1976) Action spectra for photosynthesis in higher plants. Plant Cell Physiol 17: 355–365 [Google Scholar]
- Kozai T (2013) Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc Jpn Acad Ser B 89: 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R, Ohashi-Kaneko K, Fujiwara K, Goto E, Kurata K (2004) Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol 45: 1870–1874 [DOI] [PubMed] [Google Scholar]
- McCree KJ (1972) The action spectrum, absorbance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9: 191–216 [Google Scholar]
- Miyazawa Y, Hikosaka S, Goto E, Aoki T (2009) Effects of light conditions and air temperature on the growth of everbearing strawberry during the vegetative stage. Acta Hortic 842: 817–820 [Google Scholar]
- Morrow CR (2008) LED lighting in horticulture. HortScience 43: 1947–1950 [Google Scholar]
- Mouhu K, Hytönen T, Folta K, Rantanen M, Pailin L, Auvinen P, Elomaa P (2009) Identification of flowering genes in strawberry, perennial SD plant. BMC Plant Biol 99: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126: 2073–2082 [DOI] [PubMed] [Google Scholar]
- Rantanen M, Kurokura T, Mouhu K, Pinho P, Tetri E, Halonen L, Palonen P, Elomaa P, Hytönen T (2014) Light quality regulates flowering in FvFT1/FvTFL1 dependent manner in the woodland strawberry Fragaria vesca. Front Plant Sci 5: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H (2011) Light-controlled plant cultivation system in Japan-development of a vegetable factory using LEDs as a light source for plants. Acta Hortic 907: 37–44 [Google Scholar]
- Yanagi T, Okamoto K, Takita S (1996) Effect of blue and red light intensity on photosynthetic rate of strawberry leaves. Acta Hortic 440: 371–376 [Google Scholar]
- Yoshida H, Hikosaka S, Goto E, Takasuna H, Kudou T (2012) Effects of light quality and light period on flowering of everbearing strawberry in a closed plant production system. Acta Hortic 956: 107–112 [Google Scholar]