Abstract
Infection after total knee replacement (IATJ) is a rare complication. It is associated with increased morbidity and mortality increasing the final costs. Gram positive coccus and Staphylococcus coagulase-negative and Staphylococcus aureus are the most common isolated germs (>50% of the cases). Conditions related to the patient, to the surgical procedure and even to the post op have been identified as risk factors to IATJ. Many complementary methods together with clinical symptoms are useful to a proper diagnosis. Treatment for IATJ must be individualized but generally is a combination of systemic antibiotic therapy and surgical treatment. Prosthesis exchange in one or two stages is the first choice procedure. Debridement with prosthesis retention is an option in acute cases with stable implants and antibiotic sensible germs.
Keywords: Anti-bacterial agents; Arthroplasty, replacement, knee; Debridement; Infection
Resumo
Infecção após artroplastia total do joelho (IATJ) é complicação incomum. Está associada a aumento da morbimortalidade e dos custos de internação. Cocos gram-positivos, sobretudo Staphylococcus coagulase-negative e Staphylococcus aureus, são os germes mais comumente isolados (> 50% de todos os casos). Condições ligadas ao paciente, ao procedimento cirúrgico e mesmo ao pós-operatório têm sido identificadas como fatores de risco para IATJ. Vários são os métodos complementares que se somam à investigação clínica para o diagnóstico infeccioso e melhor caracterização do quadro. O tratamento para a IATJ deve ser individualizado, mas geralmente envolve a combinação da antibioticoterapia sistêmica com o tratamento cirúrgico. A troca do implante em um ou dois estágios é o procedimento de escolha. Desbridamento com retenção da prótese é opção em casos agudos, com implantes estáveis e com germes sensíveis aos agentes antimicrobianos.
Palavras-chave: Antibacterianos, Artroplastia do joelho, Desbridamento, Infecção
Introduction
Infection after total knee arthroplasty (TKA) is a topic of great interest for orthopedists and infectologists. Alternatives for diminishing the TKA infection rate have long been sought, given that these rates continue to be between 0.4% and 2% after primary arthroplasty and between 3.2% and 5.6% after revision arthroplasty.1, 2, 3, 4, 5 Long-term follow-up has shown a periprosthetic infection rate of 1.55% over the first two years after TKA and 0.46% per year after this period, until the tenth year.6, 7 TKA is a procedure performed worldwide, with 600,000 surgical procedures per year in the USA and a mean survival rate of 95% over 15 years.8, 9, 10 Kurtz et al.10 predicted that there would be an increase in the demand for TKA of 673% by 2030. Although the TKA infection rate may seem low, the number of such injuries tends to increase with increasing numbers of procedures.
Clinical complications and increased costs associated with TKA injuries have been of growing concern. The mortality rate among patients over the age of 65 years who were awaiting a surgical procedure for treating TKA infection has ranged from 0.4% to 1.2%, and between 2% and 7% among patients aged over 80 years.11 The mean cost of treating TKA infections has been estimated as 50,000 dollars per patient and 250 million dollars per year, in the United States.12, 13
The microorganisms most commonly encountered in TKA infection cultures are coagulase-negative Staphylococcus (30–43%) and Staphylococcus aureus (12–23%), followed by contamination due to mixed flora (10%), Streptococcus (9–10%), Gram-negative bacilli (3–6%) and anaerobic bacilli (2–4%). No germ is isolated in around 11% of the cases.14, 15
This review had the aim of discussing the diagnosis and treatment of patients with a condition of TKA infection.
Risk and prevention factors
TKA infection has been correlated with a number of risk factors: diabetes, malnutrition, smoking, use of steroids, poor control over anticoagulation, obesity, cancer, alcoholism, urinary tract infections, multiple blood transfusions and revision surgery. The current guidance is that such factors should be identified and multidisciplinary intervention should be implemented before performing any procedure, with the aim of getting the patient into a better condition.16
Use of antimicrobial prophylaxis, care in preparing the patient's skin before the operation and use of laminar flow in surgical theaters have reduced the intraoperative contamination rates. Forty years ago, for every 10 patients who underwent TKA, one would develop infection.17, 18
Malinzak et al.19 reported that the infection rate was 0.51% among 8494 hip and knee arthroplasty procedures. They found that the risk factors for infection were obesity, early age and diabetes mellitus. Patients with body mass index greater than 40 and those with diabetes presented a 3.3 and 3.1 times greater chance of TKA infection, respectively. Glycemic control has been a topic greatly discussed. The benefits of rigorous control, both before and after the operation, were reported by Marchant et al.20 and Van den Berghe et al.21
Obesity is a risk factor and is also correlated with wound complications, as demonstrated by Winiarsky et al.,22 in a study in which 22% of the obese group of patients presented infection of the surgical wound and higher prevalence of deep infection. Obesity is not necessarily synonymous with nutrition, and evaluating transferrin, albumin and leukocytes has been important in these cases.
Persistence of drainage during the postoperative period and wound complications are also factors associated with infection. Galat et al.23 reported that the infection rate was higher in the group of patients in whom there was hematoma formation. This was also reported by Parvizi et al.,24 who indicated that the infection rate was higher in cases with persistent drainage through the surgical wound and in patients who presented RNI > 1.5.
Clinical presentation and diagnosis
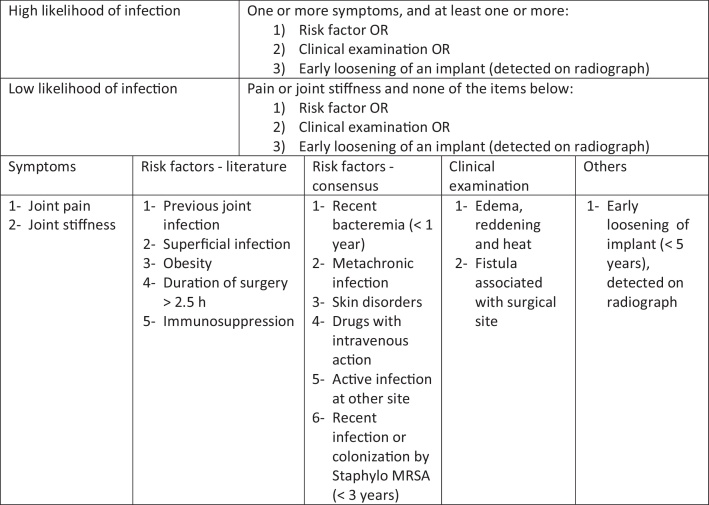
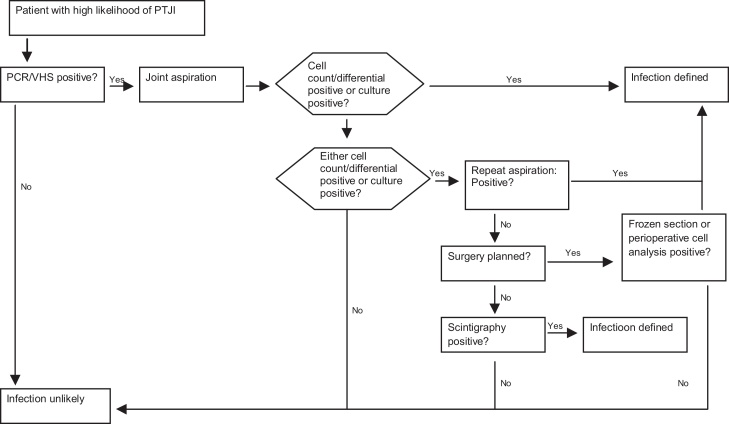
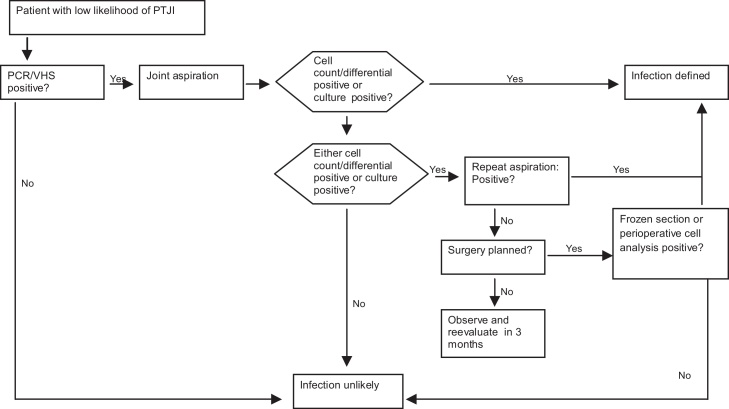
The evaluation and management of patients with TKA infection should follow a logical, clear and reproducible sequence. The American Academy of Orthopedic Surgeons (AAOS) has developed clinical practice guidelines for this process (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Stratification of the risk factors.
Reproduced with modifications from “The diagnosis of periprosthetic joint infections of the hip and knee. Guideline and evidence report”. Adopted by the American Academy of Orthopedic Surgeons Board of Directors, June 18, 2010. American Academy of Orthopedic Surgeons, 2010;18(12):760–770 (with permission).
Fig. 2.
Algorithm for managing patients with a high likelihood of infection following TKA.
Reproduced with modifications from “The diagnosis of periprosthetic joint infections of the hip and knee. Guideline and evidence report”. American Academy of Orthopedic Surgeons, 2010;18(12):760–770 (with permission).
Fig. 3.
Algorithm for managing patients with a low likelihood of infection following TKA.
Reproduced with modifications from “The diagnosis of periprosthetic joint infections of the hip and knee. Guideline and evidence report”. American Academy of Orthopedic Surgeons, 2010;18(12):760–770 (with permission).
TKA infections can be temporally divided into three types: acute (less than three months), subacute (three to 24 months) and chronic (>24 months).25, 26 The time period analyzed relates to the start of the infectious condition and is important in determining the treatment. The first two forms of presentation are linked to the surgical procedure and the last to bacteremia, generally relating to the skin, teeth or genitourinary tract.27 Acute infections are characterized by pain, edema, heat, erythema and fever, commonly caused by virulent germs such as S. aureus and Gram-negative bacilli. Patients with subacute conditions (coagulase-negative Staphylococcus and P. acnes) usually have signs and symptoms that are non-evident and may present persistent pain, implant loosening or both, which makes aseptic loosening a differential diagnosis.14 The chronic condition has variable presentation, with signs and symptoms that are similar to those reported in the acute and subacute conditions. From the assessment and the clinical history, it can be defined whether the patient has high or low likelihood of infection, which is important for the subsequent propaedeutics.
After clinical and temporal characterization, laboratory tests form part of the investigation of infections. C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR) are evaluated in patients with suspected TKA infection. Carvalho Junior et al.28 demonstrated that CRP and ESR return to levels lower than the preoperative levels in 30 and 80 days, respectively, after non-complicated TKA. Piper et al.29 reported that the cutoff values for CRP and ESR were 14.5 mg/L and 19 mm/h, respectively, for diagnosing TKA infection. Another important laboratory tool for the diagnosis has been interleukin 6 (IL-6). A recent meta-analysis showed that the diagnostic accuracy was best using IL-6 values, followed by CRP, ESR and leukocyte counts.30 Other markers (alpha-1 glycoprotein acid and procalcitonin) have emerged, although still without applicability within clinical practice.
Imaging examinations can also be used to complement the evaluation, but are not essential for diagnosing the infection, nor do they rule it out. Simple anteroposterior and lateral radiographs are useful when evaluated comparatively with previous images.31 Periosteal reactions, component migration and osteolysis are signs of possible involvement of infection. Bone scintigraphy, computed tomography, magnetic resonance imaging and PET scans may also be used, while respecting their indications and objectives. Scintigraphy using technetium-99m has high sensitivity but little specificity for infection, and may give false positive results for up to one year after the primary procedure, because of bone remodeling.32 Using leukocytes marked with indium-111, accuracy of 81% has been achieved in diagnosing TKA infection.33 The AAOS has recommended that triphasic bone scintigraphy should be used in cases with a high likelihood of TKA infection following negative cultures. Tomography allows better contrast between normal and infected tissues, but the presence of artifacts caused by metal limits its use. With technical modifications, magnetic resonance imaging may be useful for making the diagnosis, particularly in cases involving the femoral implant. PET scans have shown accuracy of 77.8% in diagnosing infection, with sensitivity of 90% and specificity of 89.3%.34, 35, 36 In the investigative process, aspiration of synovial fluid from the joint is important. It should be analyzed in the laboratory to quantify the total leukocyte count and the percent of polymorphonuclear leukocytes. Counts greater than 3000 leukocytes per microliter with neutrophils counts of at least 60% are considered to be the criteria for diagnosing subacute or chronic infection. Culturing the aspirate has the objective of identifying the germ and establishing its sensitivity pattern. Use of Gram has not been indicated because of its low sensitivity and specificity.37, 38, 39 Parvizi et al.40 demonstrated that the colorimetric test for detecting leukocyte esterase in the synovial fluid is highly sensitive and specific for diagnosing TKA infection and also has the benefits of providing a result in two minutes and having low cost.
For acute cases, counts of more than 27,800 leukocytes per microliter have presented positive predictive value of 94%, while other markers have not been shown to be useful because of the normal inflammatory response of the immediate postoperative period.41
The culturing should be done for aerobic germs, anaerobic germs and fungi, which sufficient time allowed for observing the growth of all of these. Cultures on fistulous passages or swabs do not have any value.
During the surgery, at least three samples should be collected from different locations and preferably after stopping the use of antibiotics. Studies have shown sensitivity of 60% with classical laboratory culturing techniques. Sonication techniques have increased the sensitivity to 83.3%.25, 42 In cases of negative cultures before or during the operation, histological analysis can be performed, with perioperative frozen-section biopsy or repetition of joint puncture after an interval of six weeks.
If the likelihood of TKA infection is low, and provided that all the evaluations are negative, observation for three months is recommended, with reassessment at the end of this period.
Combining the clinical history, laboratory alterations and culture results guides and enables identification of the infectious condition. In around 5–10% of the cases, alterations may exist throughout the propaedeutics, but without confirmation from culturing. Berbari et al.43 reported that it was important that the treatment should be guided in accordance with the entire investigation, and not just the results from culturing. In evaluating 897 cases of periprosthetic infection, they found that 7% of the cases had false negative cultures. All of these cases underwent surgical or drug treatment with a five-year success rate of greater than 70%.
Treatment
The primary objective in treating TKA infection is to eradicate the infection. Pain relief and reestablishment of function are secondary objectives, but no less important. Through the influence of the American literature, debridement with retention (D + R) and replacement in a single procedure (1T) are used less frequently. In addition, temporary placement of a spacer containing antibiotics, followed by replacement with the definitive implant (2T)44, 45, 46 and suppression therapy (ST), has also been proposed. Segawa et al. defined four clinical phases of TKA infection that are useful for guiding the treatment: I – infection identified at the time of the procedure; II – acute postoperative infection; III – identification some years after the original procedure, coming from a distant focus; IV – chronic infection.
The surgical treatments that exist in cases of infectious conditions are D + R, 1T, 2T, resection arthroplasty, arthrodesis, amputation and ST.44 The choice of best treatment depends on the patient's condition, the condition of the implant and the germ that was isolated.
D + R is a good alternative for patients with early postoperative infectious states and acute hematogenic conditions, provided that the duration of symptoms is no more than three weeks, the skin coverage conditions are adequate, the implant is stable and an antimicrobial agent with effective action is available. It has been recommended that initial venous antibiotic therapy should be used for two to four weeks, with conversion to oral medication after this period.46, 47 Byren et al.48 demonstrated that the infection-free survival rate after D + R treatment was 82%, with a follow-up of 2.3 years. Failure was associated with arthroscopic treatment, infections in revision procedures and infection due to S. aureus. Trebse et al.49 applied a D + R protocol to a series of 24 patients with an 86% success rate over three years and defined that the factors for a good prognosis were the presence of a stable implant, absence of fistulas contiguous with the prosthetic component and duration of symptoms less than three weeks.
Replacement in a single procedure is a good option when there is good skin coverage, absence of comorbidities and infection not caused by multiresistant germs. Jämsen et al.50 reported that the infection eradication rates ranged from 73% to 100% over 122 months of follow-up using this strategy.
If these criteria are not all fulfilled, the best option is to replace the implant in two procedures (2T). In these cases, a mobile or rigid joint spacer made of polymethylmethacrylate (PMM) should be used. This has the objectives of keeping the soft tissues under tension, diminishing the “dead space” and enabling local release of antibiotic.51, 52 In these cases, Zimmerli et al.46 recommended that the second procedure should be performed after as short a time as possible (two to four weeks), which diminishes the costs and the duration of hospital stay. Haleem et al.51 reported that the success rate over five years of follow-up was 93.5% and over 10 years, 85%. Macheras et al.52 reported that the infection-free survival rate was 91.1% over 12.1 years of follow-up.
Despite concerns among infectologists that spacers with low release of antibiotics (which occurs after a few weeks of use) might function as sites for bacterial fixation (through formation of biofilm), there is no consensus regarding the ideal length of time for the spacer to be kept in use. Della Valle53 suggested that the minimum time should be eight weeks, provided that after the end of the initial antibiotic therapy (six weeks), the values from inflammatory tests continue to show progressive reductions over the subsequent two weeks.
Another point that is still under discussion is the mixture of antibiotics with the PMM and its concentration. There is no standardization of the quantity used. Empirically, 10% of its weight has been used, which represents 4 g of antibiotic for each unit (40 g). It is known that high doses of antibiotic may alter the mechanical properties of the spacer and make it easily breakable.54 Despite this, Anagnostakos et al.55 reported that they used high doses of antibiotics in PMM, without major clinical repercussions or side effects. Although manufactured formulations of PMM in association with gentamicin and tobramycin exist, the dosage of these antibiotics in the mixture does not reach the 10% mentioned above. When the antibiotic is mixed in on the surgical table, it is possible to add the antibiotic to the most external layer of the PMM and increase its area of contact with the bone surface (placement as a surface coating). The choice of antimicrobial agent depends on the germ to be treated and the thermoresistance of the agent, given that the polymerization reaction of the PMM when associated with barium is exothermic and may interfere with the properties of the antibiotic. Gentamicin, tobramycin and vancomycin are good alternatives as thermoresistant agents.56, 57
In addition to surgical treatment, systemic antibiotic therapy should be maintained. It has been recommended that there should be six months of treatment for patients with TKA infection who present unfavorable skin coverage conditions.45, 46 The antimicrobial agent should have bactericidal action, even against slow-growth germs or biofilm producers. Before starting any treatment, the susceptibility of the germ should be tested and alternative regimens should be discussed, given the growing levels of resistance.57, 58 A combination of rifampicin with quinolones has been used most often, with good results in vitro, in vivo and in clinical trials. Options such as linezolid, sulfamethoxazole-trimethoprim and minocycline are possible, although so far no clinical studies for validating their use have been published. The best option is to discuss the best antimicrobial therapy for each case with the hospital infection control committee.58, 59
If the patient is not in a suitable clinical condition for the new procedure, ST with long-duration antimicrobial medication becomes the best option. In these cases, the objective becomes one of controlling the acute manifestations, rather than eradication of the infection. Arthrodesis and amputation are options for immunocompromised patients and for those for whom new arthroplasty would not improve their function.59
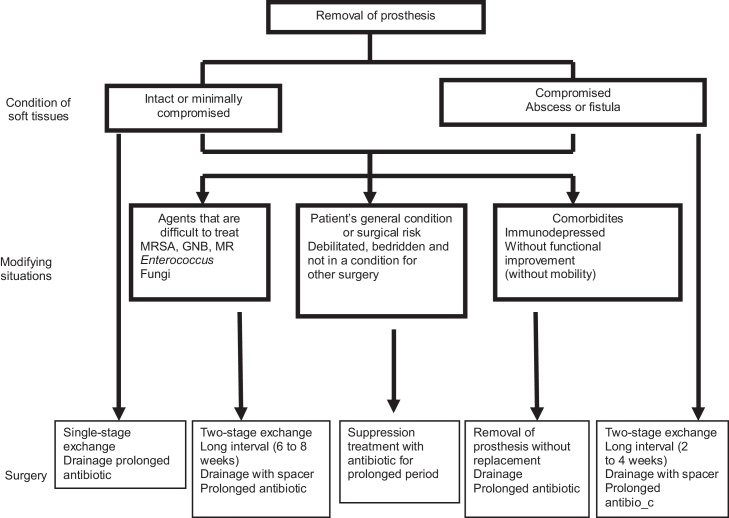
Using the AAOS and Zimmerli recommendations described in Fig. 4, Fig. 5, Giulieri et al.60 reported that the cure rate was 83%, while Trampuz et al.,61 Tsukayama et al.,62 Meehan et al.63 and Betsch et al.64 observed cure rates of 90%, 91%, 89% and 57%, respectively. Betsch et al.64 found values lower than those of the others because they had a greater number of 2T procedures, with a greater number of cases of advances disease or microorganisms of greater virulence. The risk factors for therapeutic failure were described as polymicrobial infection and infection due to Gram-negative bacilli, mycobacteria and fungi.
Fig. 4.
Algorithm for treating acute or subacute prosthetic infection.
Fig. 5.
Algorithm for treating infection that is not qualified for debridement + retention.
With better comprehension of the pathogenesis of the disease and development of new diagnostic and investigative techniques, better treatment and management of TKA infection will be achieved, with fewer complications and morbidity–mortality.
Final remarks
After TKA infection has been diagnosed, its treatment should be individualized but generally involves a combination of systemic antibiotic therapy with surgical treatment. Replacement of the implant in one or two stages is the preferred procedure. Debridement with retention of the prosthesis is an option in acute cases that have stable implants and present germs that are sensitive to the antimicrobial agents.
Conflicts of interest
The authors declare that there were no conflicts of interest.
Footnotes
Study conducted at the Hospital Madre Teresa, Belo Horizonte, MG, Brazil.
References
- 1.Mahomed N.N., Barrett J., Katz J.N., Baron J.A., Wright J., Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87:1222–1228. doi: 10.2106/JBJS.D.02546. [DOI] [PubMed] [Google Scholar]
- 2.Wilson M.G., Kelley K., Thornhill T.S. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]
- 3.Windsor R.E., Bono J.V. Infected total knee replacements. J Am Acad Orthop Surg. 1994;2:44–53. doi: 10.5435/00124635-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hanssen A.D., Rand J.A. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 5.Bozic K.J., Kurtz S.M., Lau E., Ong K., Chiu V., Vail T.P. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz S.M., Ong K.L., Lau E., Bozic K.J., Berry D., Parvizi J. Prosthetic joint infection risk after TKA in the medicare population. Clin Orthop Relat Res. 2010;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berbari E.F., Hanssen A.D., Duffy M.C., Steckelberg J.M., Ilstrup D.M., Harmsen W.S. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 8.Ranawat C.S., Flynn W.F., Jr., Saddler S., Hansraj K.K., Maynard M.J. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop Relat Res. 1993;286:94–102. [PubMed] [Google Scholar]
- 9.Ritter M.A., Berend M.E., Meding J.B., Keating E.M., Faris P.M., Crites B.M. Long-term followup of anatomic graduated components posterior cruciate-retaining total knee replacement. Clin Orthop Relat Res. 2001;388:51–57. doi: 10.1097/00003086-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Fisman D.N., Reilly D.T., Karchmer A.W., Goldie S.J. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–430. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 12.Masterson E.L., Masri B.A., Duncan C.P. Treatment of infection at the site of total hip replacement. Instr Course Lect. 1998;47:297–306. [PubMed] [Google Scholar]
- 13.Sculco T.P. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–873. [PubMed] [Google Scholar]
- 14.Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Waldvogel F.A., Bisno A.L., editors. Infections associated with indwelling medical devices. 3rd ed. American Society for Microbiology; Washington: 2000. pp. 173–209. [Google Scholar]
- 15.Segawa H., Tsukayama D.T., Kyle R.F., Becker D.A., Gustilo R.B. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Shirtliff M.E., Mader J.T. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH consensus conference: total hip replacement. NIH consensus development panel on total hip replacement. JAMA. 1995;273:1950–1956. [PubMed] [Google Scholar]
- 18.Lidgren L. Joint prosthetic infections: a success story. Acta Orthop Scand. 2001;72:553–556. doi: 10.1080/000164701317268969. [DOI] [PubMed] [Google Scholar]
- 19.Malinzak R.A., Ritter M.A., Berend M.E., Meding J.B., Olberding E.M., Davis K.E. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(Suppl. 6):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Marchant M.H., Jr., Viens N.A., Cook C., Vail T.P., Bolognesi M.P. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91:1621–1629. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe G., Wouters P., Weekers F., Verwaest C., Bruyninckx F., Schetz M. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 22.Winiarsky R., Barth P., Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80:1770–1774. doi: 10.2106/00004623-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Galat D.D., McGovern S.C., Larson D.R., Harrington J.R., Hanssen A.D., Clarke H.D. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91:48–54. doi: 10.2106/JBJS.G.01371. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J., Ghanem E., Joshi A., Sharkey P.F., Hozack W.J., Rothman R.H. Does excessive anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(Suppl. 2):24–28. doi: 10.1016/j.arth.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Trampuz A., Widmer A.F. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 26.Schafroth M., Zimmerli W., Brunazzi M., Ochsner P.E. Infections. In: Ochsner P.E., editor. Total hip replacement. Springer-Verlag; Berlin: 2003. pp. 65–90. [Google Scholar]
- 27.Maderazo E.G., Judson S., Pasternak H. Late infections of total joint prostheses. A review and recommendations for prevention. Clin Orthop Relat Res. 1988;229:131–142. [PubMed] [Google Scholar]
- 28.Carvalho Junior L.H., Santos R.L., Mendonça C.J.A., Campos C.T., Andrade M.A.P. Avaliação da variação da temperatura cutânea, proteína C reativa e velocidade de hemossedimentação na artroplastia total do joelho primária, isenta de complicações. Acta Ortop Bras. 2006;14:161–164. [Google Scholar]
- 29.Piper K.E., Fernandez-Sampedro M., Steckelberg K.E., Mandrekar J.N., Karau M.J., Steckelberg J.M. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5:e9358. doi: 10.1371/journal.pone.0009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berbari E., Mabry T., Tsaras G., Spangehl M., Erwin P.J., Murad M.H. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102–2109. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 31.Tigges S., Stiles R.G., Roberson J.R. Appearance of septic hip prostheses on plain radiographs. Am J Roentgenol. 1994;163:377–380. doi: 10.2214/ajr.163.2.8037035. [DOI] [PubMed] [Google Scholar]
- 32.Smith S.L., Wastie M.L., Forster I. Radionuclide bone scintigraphy in the detection of significant complications after total knee joint replacement. Clin Radiol. 2001;56:221–224. doi: 10.1053/crad.2000.0620. [DOI] [PubMed] [Google Scholar]
- 33.Hain S.F., O’Doherty M.J., Smith M.A. Functional imaging and the orthopaedic surgeon. J Bone Joint Surg Br. 2002;84:315–321. doi: 10.1302/0301-620x.84b3.12369. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang H., Duarte P.S., Pourdehnad M., Maes A., Van Acker F., Shnier D. The promising role of 18F-FDG PET in detecting infected lower limb prosthesis implants. J Nucl Med. 2001;42:44–48. [PubMed] [Google Scholar]
- 35.Ivanćević V., Perka C., Hasart O., Sandrock D., Munz D.L. Imaging of low-grade bone infection with a technetium-99m labelled monoclonal anti-NCA-90 Fab’ fragment in patients with previous joint surgery. Eur J Nucl Med Mol Imaging. 2002;29:547–551. doi: 10.1007/s00259-001-0744-7. Erratum in: Eur J Nucl Med Mol Imaging 2002;29(6):835. [DOI] [PubMed] [Google Scholar]
- 36.Larikka M.J., Ahonen A.K., Junila J.A., Niemelä O., Hämäläinen M.M., Syrjälä H.P. Improved method for detecting knee replacement infections based on extended combined 99mTc-white blood cell/bone imaging. Nucl Med Commun. 2001;22:1145–1150. doi: 10.1097/00006231-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Ghanem E., Parvizi J., Burnett R.S., Sharkey P.F., Keshavarzi N., Aggarwal A. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 38.Schinsky M.F., Della Valle C.J., Sporer S.M., Paprosky W.G. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 39.Duff G.P., Lachiewicz P.F., Kelley S.S. Aspiration of the knee joint before revision arthroplasty. Clin Orthop Relat Res. 1996;331:132–139. doi: 10.1097/00003086-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Parvizi J., Jacovides C., Antoci V., Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93:2242–2248. doi: 10.2106/JBJS.J.01413. [DOI] [PubMed] [Google Scholar]
- 41.Bedair H., Ting N., Jacovides C., Saxena A., Moric M., Parvizi J. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469:34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holinka J., Bauer L., Hirschl A.M., Graninger W., Windhager R., Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 43.Berbari E.F., Marculescu C., Sia I., Lahr B.D., Hanssen A.D., Steckelberg J.M. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;1–45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 44.Westrich G.H., Salvati E.A., Brause B. Postoperative infection. In: Bono J.V., McCarty J.C., Thornhill T.S., Bierbaum B.E., Turner R.H., editors. Revision total hip arthroplasty. Springer-Verlag; New York: 1999. pp. 371–390. [Google Scholar]
- 45.Langlais F. Can we improve the results of revision arthroplasty for infected total hip replacement? J Bone Joint Surg Br. 2003;85:637–640. [PubMed] [Google Scholar]
- 46.Zimmerli W., Trampuz A., Ochsner P.E. Prosthetic-joint infections. N Engl J Med. 2004;14–351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerli W., Widmer A.F., Blatter M., Frei R., Ochsner P.E. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;20–279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 48.Byren I., Bejon P., Atkins B.L., Angus B., Masters S., McLardy-Smith P. One hundred and twelve infected arthroplasties treated with Dair (debridement, antibiotics, and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63:1264–1271. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trebse R., Pisot V., Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br. 2005;87:249–256. doi: 10.1302/0301-620x.87b2.15618. [DOI] [PubMed] [Google Scholar]
- 50.Jämsen E., Stogiannidis I., Malmivaara A., Pajamäki J., Puolakka T., Konttinen Y.T. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haleem A.A., Berry D.J., Hanssen A.D. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 52.Macheras G.A., Kateros K., Galanakos S.P., Koutsostathis S.D., Kontou E., Papadakis S.A. The long-term results of a two-stage protocol for revision of an infected total knee replacement. J Bone Joint Surg Br. 2011;93:1487–1492. doi: 10.1302/0301-620X.93B11.27319. [DOI] [PubMed] [Google Scholar]
- 53.Della Valle C.J. Comunicação Pessoal. 14° Congresso Brasileiro de Cirurgia do Joelho; Mata de São João, Bahia; 2012. [Google Scholar]
- 54.Kelm J., Regitz T., Schmitt E., Jung W., Anagnostakos K. In vivo and in vitro studies of antibiotic release from and bacterial growth inhibition by antibiotic-impregnated polymethylmethacrylate hip spacers. Antimicrob Agents Chemother. 2006;50:332–335. doi: 10.1128/AAC.50.1.332-335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anagnostakos K., Wilmes P., Schmitt E., Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80:193–197. doi: 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Springer B.D., Lee G.C., Osmon D., Haidukewych G.J., Hanssen A.D., Jacofsky D.J. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47–51. doi: 10.1097/01.blo.0000144476.43661.10. [DOI] [PubMed] [Google Scholar]
- 57.Anderl J.N., Zahller J., Roe F., Stewart P.S. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47:1251–1256. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwank S., Rajacic Z., Zimmerli W., Blaser J. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob Agents Chemother. 1998;42:895–898. doi: 10.1128/aac.42.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osmon D.R., Berbari E.F. Outpatient intravenous antimicrobial therapy for the practicing orthopaedic surgeon. Clin Orthop Relat Res. 2002;403:80–86. doi: 10.1097/00003086-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Giulieri S.G., Graber P., Ochsner P.E., Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32:222–228. doi: 10.1007/s15010-004-4020-1. Erratum in: Infection. 2004;32(5):309. [DOI] [PubMed] [Google Scholar]
- 61.Trampuz A., Cattelan C., Flückiger U., Frei R., Zimmerli W. Treatment outcome of prosthetic joint infection: ten year cohort study (1994–2003) [abstract K-883]. Program and abstracts of the 45th interscience conference on antimicrobial agents and chemotherapy; Washington, DC; Washington: American Society for Microbiology; 2005. p. 47. [Google Scholar]
- 62.Tsukayama D.T., Estrada R., Gustilo R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Meehan A.M., Osmon D.R., Duffy M.C., Hanssen A.D., Keating M.R. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis. Clin Infect Dis. 2003;1–36:845–849. doi: 10.1086/368182. [DOI] [PubMed] [Google Scholar]
- 64.Betsch B.Y., Eggli S., Siebenrock K.A., Täuber M.G., Mühlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis. 2008;15–46:1221–1226. doi: 10.1086/529436. [DOI] [PubMed] [Google Scholar]