Abstract
Xylem is an essential conductive tissue in vascular plants, and secondary cell wall polymers found in xylem vessel elements, such as cellulose, hemicellulose, and lignin, are promising sustainable bioresources. Thus, understanding the molecular mechanisms underlying xylem vessel element differentiation is an important step towards increasing woody biomass and crop yields. Establishing in vitro induction systems, in which vessel element differentiation is induced by phytohormonal stimuli or by overexpression of specific transcription factors, has been vital to this research. In this review, we present an overview of these in vitro induction systems, and describe two recently developed in vitro induction systems, VISUAL (Vascular cell Induction culture System Using Arabidopsis Leaves) and the KDB system. Furthermore, we discuss the potentials and limitations of each of these new in vitro induction systems for advancing our understanding of the molecular mechanisms driving xylem vessel element differentiation.
Keywords: in vitro induction system, KDB system, VISUAL, VND, xylem vessel elements
Introduction
Xylem is one of the conductive tissues found in vascular plants and is essential for transporting water and nutrients from roots to shoots. The water-conducting activity of the xylem relies on pipe-like structures consisting of cells called vessel elements. Xylem vessel elements have thick secondary cell walls (SCWs), which are composed of cellulose, hemicellulose, and lignin (Turner et al. 2007). These thick SCWs are the major source of woody biomass and represent a valuable source of renewable energy (Yang et al. 2013). Understanding the molecular basis of vessel element formation is an important step towards improving water conduction in plants to increase crop yields, and also towards controlling the quantity and quality of woody biomass.
Because of their physiological importance, xylem vessel elements have been the focus of many molecular plant biology studies. However, as xylem is embedded in the plant body, it is difficult to access xylem vessel elements for observation or molecular studies. In addition, since xylem tissues contain several kinds of cells other than vessel elements, i.e., xylem fibers and parenchyma cells, isolating vessel elements from native xylem tissues poses a challenge. To overcome these issues, several in vitro induction systems have been developed, in which vessel element differentiation can be induced by phytohormonal stimuli (Fukuda and Komamine 1980; Kondo et al. 2014, 2015, 2016; Kubo et al. 2005; Oda et al. 2005; Pesquet et al. 2010; Tan et al. 2018) or by overexpression of specific transcription factors (Oda et al. 2010; Yamaguchi et al. 2008, 2010). Using these systems, homogenous cells at the same stage of differentiation can be collected and analyzed, facilitating the identification of novel factors involved in specific stages of cell differentiation. Indeed, in vitro induction systems have already revealed many regulatory aspects of xylem vessel element differentiation, including novel key regulators of xylem vessel cell differentiation and the stage-specific regulation of the transcriptome, proteome, and metabolome (Demura et al. 2002; Endo et al. 2015; Fukuda 1997, 2004; Goué et al. 2013; Ito et al. 2006; Kawabe et al. 2018; Kondo et al. 2014, 2015, 2016; Kubo et al. 2005; Li et al. 2016; Motose et al. 2004; Noguchi et al. 2018; Oda and Fukuda 2012, 2013; Oda et al. 2010; Ohtani et al. 2016, 2018; Pesquet et al. 2010; Schuetz et al. 2014; Takenaka et al. 2018; Tan et al. 2018; Watanabe et al. 2015, 2018).
In this review, we summarize the established in vitro induction systems of xylem vessel elements and the considerable findings obtained using these systems. With an emphasis on recently established in vitro induction systems, VISUAL (Vascular cell Induction culture System Using Arabidopsis Leaves) (Kondo et al. 2014, 2015, 2016) and the KDB system (Tan et al. 2018), we discuss future directions in research examining vessel element differentiation using these systems.
Development of in vitro induction systems for xylem vessel element differentiation
The first effective in vitro induction system of xylem vessel elements was developed by Fukuda and Komamine (1980) (Table 1), who established a simple and stable in vitro vessel element differentiation system using a Zinnia elegans (Zinnia) mesophyll cell culture. The induction stimuli in their system were two phytohormones, auxin and cytokinin, and ca. 50% of cultured cells differentiated into xylem vessel elements 3 days after the treatment (Fukuda and Komamine 1980). The easy preparation of Zinnia cell samples and synchronized cell differentiation made this system ideal for characterizing the temporal cytological changes that occur during differentiation, and led to the isolation of many genes involved in xylem vessel cell differentiation (Fukuda 2004; Turner et al. 2007). A comprehensive microarray analysis using this system revealed step-wise changes in gene expression associated with distinct stages of xylem vessel element differentiation (Demura and Fukuda 1993, 1994; Demura et al. 2002), demonstrating that Zinnia mesophyll cells undergo a procambium cell stage followed by a xylem precursor cell stage as they differentiate into vessel elements. In addition, this gene expression analysis identified many candidate genes hypothesized to function in xylem vessel element differentiation (Demura et al. 2002).
Table 1. In vitro xylem vessel element differentiation systems developed in herbaceous angiosperm species.
| Species | Materials | Transgenes | Inducers | Light requirementb | Final frequency of ectopic xylem vessel element differentiation (%) | Days required to reach the final frequency of ectopic xylem vessel element differentiation (d) | References |
|---|---|---|---|---|---|---|---|
| Zinnia elegans | Isolated mesophyll cell | No | Auxin and cytokinin | Dark | 50 | 3 | Fukuda and Komamine 1980 |
| Arabidopsis thaliana | Cultured cells | No | Auxin, boric acid and brassinosteroid | — | 50 | 3 | Kubo et al. 2005 |
| Arabidopsis thaliana | Cultured cells (AC-GT13)a | 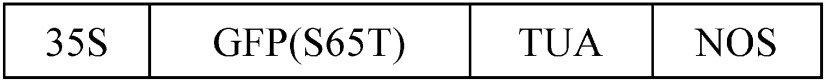 |
Removal of auxin followed by application of brassinosteroid | — | 30 | 4 | Oda et al. 2005 |
| Arabidopsis thaliana | Cultured cells | No | Auxin, cytokinin and brassinosteroid | — | 40 | 3 | Pesquet et al. 2010 |
| Arabidopsis thaliana | Cultured cells containing inducible VND6 construct | 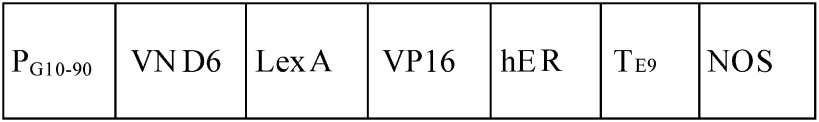 |
Estrogen and brassinosteroid | — | 80 | 2 | Oda et al. 2010; Oda and Fukuda 2012 |
| Arabidopsis thaliana | Cultured cells/plants containing inducible VND7 construct (T87) |  |
Glucocorticoid (dexamethasone, DEX) | — | 10 | 7 | Yamaguchi et al. 2010 |
| Nicotiana tabacum | Cultured cells containing inducible VND7 construct (BY-2) |  |
Glucocorticoid (dexamethasone, DEX) | — | 70 | 3 | Yamaguchi et al. 2010 |
| Arabidopsis thaliana | Leaves and cotyledons | No | Bikinin, auxin, and cytokinin | Light | ND | 3 | Kondo et al. 2014, 2015, 2016 |
| Arabidopsis thaliana | Cotyledons | No | Brassinosteroid, auxin, and cytokinin | Light | ND | 5 | Tan et al. 2018 |
aAC-GT13 is a cell suspension transformed with a vector containing a GFP(S65T)-tubulin alpha (TUA) fusion gene (Kumagai et al. 2001) that allows visualization of cortical microtubule rearrangement in living xylem cells. bLight or dark conditions are required to induce cell differentiation where indicated. –No indication about requirement of light or dark conditions. 35S and PG10–90, strong constitutive promoters; LexA, DNA-binding domain of the bacterial repressor; VP16, activation domain of herpes virus protein; hER, the carboxyl region of the human estrogen receptor; GR, hormone-binding domain of rat glucocorticoid receptor; TE9, rbcS E9 poly(A) addition sequence; NOS, terminator of nopaline synthase; GFP, green fluorescent protein; ND, not determined.
When the whole genome sequence of Arabidopsis thaliana (Arabidopsis) became available in 2000 (Arabidopsis Genome Initiative 2000), the advantage of using Arabidopsis for molecular biology studies became apparent. Several groups reported new in vitro induction systems for vessel element formation using Arabidopsis cell suspension cultures manipulated with key phytohormones, i.e., auxin, cytokinin, and brassinosteroids. Kubo et al. (2005) established an Arabidopsis cell suspension system in which brassinosteroid and boric acid induce xylem vessel element differentiation (Table 1). In this system, approximately 50% of cells differentiate into xylem vessel elements within 7 days of treatment. Oda et al. (2005) induced ectopic xylem vessel elements in Arabidopsis cell suspensions by removing auxin and applying brassinosteroid to produce a differentiation rate of ca. 30% after 4 days of culture (Oda et al. 2005). Additionally, Pesquet et al. (2010) reported that the addition of auxin, cytokinin, and brassinosteroid induces the differentiation of Arabidopsis cell cultures into xylem vessel elements at a rate of 40% after 3 days of culture (Table 1). These in vitro induction systems were key to identifying critical factors in vessel element differentiation, such as members of the VASCULAR-RELATED NAC-DOMAIN (VND) family of transcription factors that induce xylem vessel element differentiation (Kubo et al. 2005) and the microtubule-associated proteins regulating cortical microtubule alignment for SCW patterning (Oda et al. 2005; Pesquet et al. 2010).
Based on the findings by Kubo et al. (2005), transgenic Arabidopsis cell suspensions were created containing either an estrogen-inducible VND6 construct or a glucocorticoid-inducible VND7 construct (Oda et al. 2010; Oda and Fukuda 2012; Yamaguchi et al. 2008, 2010). Both systems have high rates of ectopic vessel element differentiation (ca. 80–90%) and have been used to determine the transcriptional networks downstream of VND6 and VND7 (Ohashi-Ito et al. 2010; Yamaguchi et al. 2011; Zhong et al. 2010). Moreover, the VND7-inducible system has revealed many novel cellular and molecular mechanisms involved in xylem vessel element differentiation (Endo et al. 2015; Goué et al. 2013; Kawabe et al. 2018; Li et al. 2016; Noguchi et al. 2018; Ohtani et al. 2016, 2018; Schuetz et al. 2014; Takenaka et al. 2018; Watanabe et al. 2015, 2018).
Two recently developed similar in vitro induction systems: VISUAL and the KDB system
Recently, two in vitro induction systems have been developed that can be used to examine xylem vessel element differentiation in diverse Arabidopsis mutant and reporter lines: (1) VISUAL (Vascular cell Induction culture System Using Arabidopsis Leaves) (Kondo et al. 2014, 2015, 2016) and (2) the KDB system (Tan et al. 2018) (Figure 1). In VISUAL, Arabidopsis leaf disks or excised leaves are cultured with auxin and cytokinin along with bikinin, a compound that strongly activates brassinosteroid signaling by inhibiting the BRASSINOSTEROID-INSENSITIVE 2 (BIN2) kinase, a negative regulator of brassinosteroid signaling in Arabidopsis (Kondo et al. 2014). As a result, both xylem vessel elements and phloem sieve cells can be ectopically induced in this system (Kondo et al. 2016).
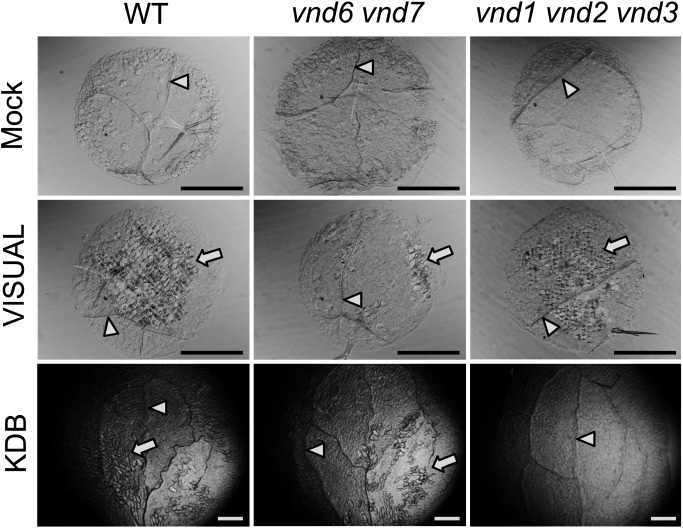
Figure 1. VISUAL and the KDB system have different dependencies on VND family genes.Ectopic xylem vessel element differentiation was induced in cotyledon leaf disks of wild type (WT), vnd6 vnd7, and vnd1 vnd2 vnd3 using VISUAL (Kondo et al. 2014, 2015, 2016) or the KDB system (Tan et al. 2018). Mock-treated leaf disks were shown as the control (upper panels). White arrowheads and arrows indicate native xylem vessels and ectopic xylem vessel elements, respectively. Scale bars, 500 µm.
Similarly, in the KDB system, the excised Arabidopsis cotyledons are treated with cytokinin (Kinetin) and auxin (2,4-D), but brassinosteroid signaling is activated by adding a brassinosteroid (Brassinolide) (Tan et al. 2018). In contrast to the VISUAL system, the KDB system induces xylem vessel element differentiation only, confirmed by the lack of SUCROSE-PROTON SYMPORTER 2 (SUC2) expression, a phloem marker gene encoding a sucrose transporter (Truernit and Sauer 1995), and of ALTERED PHLOEM DEVELOPMENT (APL) expression, a key regulator of phloem development (Bonke et al. 2003). Among the members of the VND family, VND1 through VND3 are the main contributors to the differentiation of ectopic xylem vessel elements in the excised Arabidopsis cotyledons induced by the KDB system (Tan et al. 2018). Since VISUAL can induce both xylem vessel elements and phloem sieve cells while the KDB system induces strictly vessel elements, a comparative study between these systems could provide further clues into the xylem-specific and phloem-specific molecular processes in vascular cell differentiation.
Future challenges for studies of xylem vessel element differentiation using in vitro induction systems
VISUAL and the KDB system are valuable tools for studying xylem vessel element differentiation; furthermore, the availability of Arabidopsis resources facilitates analyses of gene functions and of the molecular mechanisms underlying xylem vessel element differentiation. However, a drawback of these two in vitro systems is that cotyledon and leaf tissues contain several types of cells, and these systems lack the synchrony of cell differentiation and homogeneity of other in vitro cell suspension-based approaches, such as the systems developed by Fukuda and Komamine (1980) and Kubo et al. (2005). High-resolution analytical methods, such as single-cell/molecule-level resolution methods, are highly sensitive to such factors.
Figure 2 shows the current model of xylem vessel element differentiation, based on a combination of results from mutant analyses and in vitro induction systems (Ohashi-Ito and Fukuda 2010; Růžička et al. 2015; De Rybel et al. 2016). In vitro induction systems typically contain the following steps: 1) conversion of differentiated leaf cells to procambium-like cells, 2) cellular differentiation into xylem precursor cells, and 3) activation of VND family proteins to induce molecular events, such as SCW formation and programmed cell death (PCD), for xylem vessel element differentiation (De Rybel et al. 2016; Nakano et al. 2015; Ohashi-Ito and Fukuda 2010a; Růžička et al. 2015). Interestingly, the results of the VISUAL and KDB systems suggest that vessel elements are differentiated through different molecular pathways in these systems; despite similarities in differentiation induction stimuli (auxin, cytokinin, and brassinosteroid), the main contributors to ectopic differentiation of xylem vessel elements in VISUAL are VND6 and VND7, with low requirement for VND1 through VND3 (Kondo et al. 2015; Figure 1), among the VND family proteins including 7 family genes in Arabidopsis (Kubo et al. 2005). By contrast, the KDB system is highly dependent on VND1 through VND3, but does not require VND6 or VND7 (Tan et al. 2018; Figure 1). These results suggest the existence of several possible routes towards xylem vessel element differentiation, especially before VND protein activation (Figure 2). Which parts of these molecular pathways overlap or differ between VISUAL and the KDB system? How flexible are the molecular routes underlying xylem vessel element differentiation, and what factors regulate this flexibility? Comparative analysis among multiple in vitro induction systems will provide important insights into the molecular underpinnings of xylem vessel element differentiation.
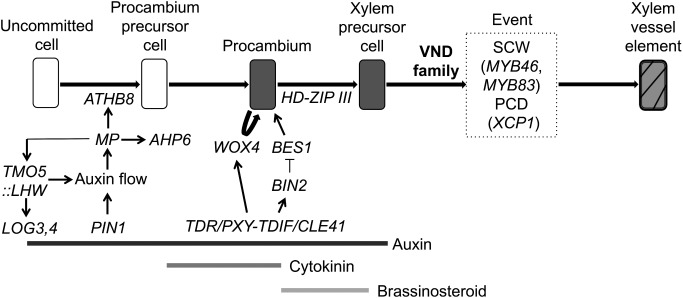
Figure 2. Proposed model of the process of xylem vessel element formation. The vascular cell formation is initiated with procambium cell development by the homeodomain-leucine zipper (HD-Zip) III gene ATHB8, which is regulated by MONOPTEROS (MP) encoding an auxin response transcription factor. For the MP expression, the regulation of auxin distribution by PIN-FORMED 1 (PIN1), an auxin efflux carrier protein, is important. MP also directly upregulates the expression of a basic helix-loop-helix (bHLH) type transcription factor gene, TARGET OF MP 5 (TMO5), as well as a key cytokinin signaling inhibitor ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN 6 (AHP6). Heterodimeric complexes of TMO5 and another bHLH protein, LONESOME HIGHWAY (LHW), promote cytokinin production by upregulating the expression of cytokinin biosynthesis genes, LONELY GUY3 (LOG3) and LOG4, which regulate cell division activity. Two regulatory pathways mediated by a mobile secreted peptide, such as TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR (TDIF)/CLE41, which is perceived by the plasma membrane receptor TDIF RECEPTOR (TDR)/PHLOEM INTERCALATED WITH XYLEM (PXY), control procambium cell activity. The first pathway results in the proliferation of cambial cells through the activity of a transcription factor, WUSCHEL HOMEOBOX RELATED 4 (WOX4), and the second pathway triggers the differentiation of cambial cells into xylem vessel elements through the kinase BRASSINOSTEROID-INSENSITIVE 2 (BIN2), which inhibits the transcription factor BRI1-EMS SUPPRESSOR 1 (BES1). After the establishment of xylem precursor cells, the VND family proteins upregulate the expression of an entire set of genes required for xylem vessel element differentiation, including those encoding SCW transcription factors (e.g., MYB46 and MYB83) and PCD (e.g., XCP1).
Concluding remarks and perspective
Over the past 40 years, research based on in vitro induction systems has elucidated many molecular processes involved in xylem vessel element differentiation. After substantial progress in xylem research that identified key regulators of each step in xylem vessel cell differentiation (Figure 2), new in vitro induction systems will expand the scope of research in the field of xylem vessel element differentiation. Emerging trends include applying high-resolution analysis to in vitro induction systems, focusing on the specific angle of molecular system for xylem vessel element differentiation (Kawabe et al. 2018; Kondo et al. 2016; Ohtani et al. 2018; Takenaka et al. 2018; Watanabe et al. 2015, 2018), and conducting trans-omics analyses using in vitro induction systems (Derbyshire et al. 2015; Li et al. 2016; Noguchi et al. 2018; Ohtani et al. 2016). Such studies will further improve our understanding of the molecular processes underlying xylem cell differentiation.
In addition to the approaches discussed above, comparative analysis of multiple types of in vitro induction systems will likely elucidate various molecular routes to vessel element differentiation in plant cells. Culture conditions in in vitro systems can be easily controlled, facilitating tests of the robustness of each molecular route. Information gleaned from these systems can be used to improve and modify xylem properties in economically important trees for biomaterial and bioenergy applications.
Acknowledgments
We thank Dr. Yusuke Saijo and Dr. Keiji Nakajima (Nara Institute of Science and Technology) and Dr. Yuki Kondo (University of Tokyo) for critical discussions. This work was supported in part by the Japan Society for the Promotion of Science (KAKENHI Grant Number 25291062 and 18H02466 to T.D.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research on Innovative Areas “The Plant Cell Wall as Information Processing System” Grant Number 25114520 and 15H01235 to M.O., 24114002 to T.D., “Plant-Structure Optimization Strategy” Grant Number 18H05484 and 18H05489 to M.O. and T.D.), and the Exploratory Research for Advanced Technology (ERATO) from Japan Science and Technology Agency (JST) (Grant Number JPMJER1602 to M.O.).
Abbreviations
- APL
ALTERED PHLOEM DEVELOPMENT
- PCD
programmed cell death
- SUC2
SUCROSE-PROTON SYMPORTER 2
- SCW
secondary cell wall
- VISUAL
vascular cell induction culture system using Arabidopsis leaves
- VND
VASCULAR-RELATED NAC-DOMAIN
References
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mahonen AP, Hauser MT, Helariutta Y (2003) APL regulates vascular tissue identity in Arabidopsis. Nature 426: 181–186 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Mahonen AP, Helariutta Y, Weijers D (2016) Plant vascular development: From early specification to differentiation. Nat Rev Mol Cell Biol 17: 30–40 [DOI] [PubMed] [Google Scholar]
- Demura T, Fukuda H (1993) Molecular cloning and characterization of cDNAs associated with tracheary element differentiation in cultured Zinnia cells. Plant Physiol 103: 815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Fukuda H (1994) Novel vascular cell-specific genes whose expression is regulated temporally and spatially during vascular system development. Plant Cell 6: 967–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, Minami A, Nagata-Hiwatashi M, Nakamura K, Okamura Y, et al. (2002) Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc Natl Acad Sci USA 99: 15794–15799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire P, Ménard D, Green P, Saalbach G, Buschmann H, Lloyd CW, Pesquet E (2015) Proteomic analysis of microtubule interacting proteins over the course of xylem tracheary element formation in Arabidopsis. Plant Cell 27: 2709–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Yamaguchi M, Tamura T, Nakano Y, Nishikubo N, Yoneda A, Kato K, Kubo M, Kajita S, Katayama Y, et al. (2015) Multiple classes of transcription factors regulate the expression of VASCULAR-RELATED NAC-DOMAIN7, a master switch of xylem vessel differentiation. Plant Cell Physiol 56: 242–254 [DOI] [PubMed] [Google Scholar]
- Fukuda H (1997) Tracheary element differentiation. Plant Cell 9: 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5: 379–391 [DOI] [PubMed] [Google Scholar]
- Fukuda H, Komamine A (1980) Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated form the mesophyll of Zinnia elegans. Plant Physiol 65: 57–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goué N, Mortimer JC, Nakano Y, Zhang Z, Josserand M, Ohtani M, Paul D, Kakegawa K, Demura T (2013) Secondary cell wall characterization in a BY-2 inductive system. Plant Cell Tiss Org 115: 223–232 [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- Kawabe H, Ohtani M, Kurata T, Sakamoto T, Demura T (2018) Protein S-nitrosylation regulates xylem vessel cell differentiation in Arabidopsis. Plant Cell Physiol 59: 17–29 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fujita T, Sugiyama M, Fukuda H (2015) A novel system for xylem cell differentiation in Arabidopsis thaliana. Mol Plant 8: 612–621 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Nurani AM, Saito C, Ichihashi Y, Saito M, Yamazaki K, Mitsuda N, Ohme-Takagi M, Fukuda H (2016) Vascular cell induction culture system using Arabidopsis leaves (VISUAL) reveals the sequential differentiation of sieve element-like cells. Plant Cell 28: 1250–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Ito T, Nakagami H, Hirakawa Y, Saito M, Tamaki T, Shirasu K, Fukuda H (2014) Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signaling. Nat Commun 5: 3504. [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai F, Yoneda A, Tomida T, Sano T, Nagata T, Hasezawa S (2001) Fate of nascent microtubules organized at the M/G1 interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP-tubulin: Time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant Cell Physiol 42: 723–732 [DOI] [PubMed] [Google Scholar]
- Li Z, Omranian N, Neumetzler L, Wang T, Herter T, Usadel B, Demura T, Giavalisco P, Nikoloski Z, Persson S (2016) A transcriptional and metabolic framework for secondary wall formation in Arabidopsis. Plant Physiol 172: 1334–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motose H, Sugiyama M, Fukuda H (2004) A proteoglycan mediates inductive interaction during plant vascular development. Nature 429: 873–878 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Yamaguchi M, Endo H, Rejab NA, Ohtani M (2015) NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, Fujiwara M, Sano R, Nakano Y, Fukao Y, Ohtani M, Demura T (2018) Proteomic analysis of xylem vessel cell differentiation in VND7-inducible tobacco BY-2 cells by two-dimensional gel electrophoresis. Plant Biotechnol 35: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2012) Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337: 1333–1336 [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2013) Rho pf plant GTPase signaling regulates the behavior of Arabidopsis kinesin-13A to establish secondary cell wall patterns. Plant Cell 25: 4439–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Mimura T, Hasezawa S (2005) Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol 137: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Iida Y, Kondo Y, Fukuda H (2010) Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol 20: 1197–1202 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H (2010) Transcriptional regulation of vascular cell fates. Curr Opin Plant Biol 13: 670–676 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Oda Y, Fukuda H (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22: 3461–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Kawabe H, Demura T (2018) Evidence that thiol-based redox state is critical for xylem vessel cell differentiation. Plant Signal Behav 13: e1428512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Morisaki K, Sawada Y, Sano R, Uy AL, Yamamoto A, Kurata T, Nakano Y, Suzuki S, Matsuda M, et al. (2016) Primary metabolism during biosynthesis of secondary wall polymers of protoxylem vessel elements. Plant Physiol 172: 1612–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesquet E, Korolev AV, Calder G, Lloyd CW (2010) The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr Biol 20: 744–749 [DOI] [PubMed] [Google Scholar]
- Růžička K, Ursache R, Hejátko J, Helariutta Y (2015) Xylem development from the cradle to the grave. New Phytol 207: 519–535 [DOI] [PubMed] [Google Scholar]
- Schuetz M, Benske A, Smith RA, Watanabe Y, Tobimatsu Y, Ralph J, Demura T, Ellis B, Samuels LA (2014) Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol 166: 798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka Y, Watanabe Y, Schuetz M, Unda F, Hill JL Jr, Phookaew P, Yoneda A, Mansfield SD, Samuels L, Ohtani M, et al. (2018) Patterned deposition of xylan and lignin is independent from the secondary wall cellulose of Arabidopsis xylem vessels. Plant Cell 30: 2663–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TT, Endo H, Sano R, Kurata T, Yamaguchi M, Ohtani M, Demura T (2018) Transcription factors VND1-VND3 contribute to cotyledon xylem vessel formation. Plant Physiol 176: 773–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: Evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Turner S, Gallois P, Brown D (2007) Tracheary element differentiation. Annu Rev Plant Biol 58: 407–433 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Meents MJ, McDonnell MJ, Barkwil S, Sampathkumar A, Cartwright HN, Demura T, Ehrhardt DE, Samuels AL, Mansfield SD (2015) Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 350: 198–203 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Schneider R, Barkwill S, Gonzales-Vigil E, Hill JL Jr, Samuels AL, Persson S, Mansfield SD (2018) Cellulose synthase complexes display distinct dynamic behaviors during xylem transdifferentiation. Proc Natl Acad Sci USA 115: E6366–E6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55: 652–664 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Goue N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama Y, Kakegawa K, et al. (2010) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Mitsuda N, Ohtani M, Ohme-Takagi M, Kato K, Demura T (2011) VASCULAR-RELATED NAC-DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J 66: 579–590 [DOI] [PubMed] [Google Scholar]
- Yang F, Mitra P, Zhang L, Prak L, Verhertbruggen Y, Kim JS, Sun L, Zheng K, Tang K, Auer M, et al. (2013) Engineering secondary cell wall deposition in plants. Plant Biotechnol J 11: 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3: 1087–1103 [DOI] [PubMed] [Google Scholar]