Abstract
Long non-coding RNA urothelial carcinoma-associated 1 (UCA1) has a role in various common types of human malignancy, including cholangiocarcinoma; however, the expression and function of UCA1 in intrahepatic cholangiocarcinoma (ICC) has remained elusive. In the present study, it was observed that UCA1 expression was significantly upregulated in ICC tissues and cell lines compared with that in the adjacent non-tumour tissues and a human intrahepatic biliary epithelial cell line, respectively. The increased expression of UCA1 was significantly associated with lymph node metastasis and clinical T-stage in ICC. Furthermore, the ICC patients with high expression of UCA1 had a shorter survival time when compared with that of patients with low UCA1 expression. Knockdown of UCA1 caused a significant decrease in ICC cell proliferation and invasion, while ectopic overexpression of UCA1 significantly promoted the proliferation and invasion of ICC cells. Furthermore, it was revealed that UCA1 directly binds to microRNA (miR)-122 to negatively regulate its expression in ICC cells. In addition, miR-122 mimics abrogated the promoting effects of UCA1 on ICC cell proliferation and invasion. In addition, an inverse correlation between miR-122 and UCA1 expression in ICC tissues was observed. In conclusion, the present study demonstrates that the lncRNA UCA1 promotes ICC cell proliferation and invasion at least in part by targeting miR-122, suggesting that the UCA1/miR-122 interaction may become a potential therapeutic target for the treatment of ICC.
Keywords: intrahepatic cholangiocarcinoma, microRNA, long non-coding RNA, proliferation, invasion
Introduction
Cholangiocarcinomas originate from cholangiocytes of small intrahepatic bile ducts or bile ductules, or of large hilar or extrahepatic bile ducts (1,2). Intrahepatic cholangiocarcinoma (ICC) is the second most common type of primary liver cancer (3,4). As ICC is the most deadly disease of the biliary tree due to its poor prognosis (4), exploration of its molecular mechanisms is urgently required for identification of novel therapeutic targets and development of effective treatment strategies.
Long non-coding RNAs (lncRNAs), a class of small non-coding RNAs of >200 nucleotides in length, comprise ~80% of non-coding RNAs and function through interaction with microRNAs (miRs) or proteins (5,6). In recent decades, a large number of lncRNAs have been identified, which have key roles in a variety of physiological and pathological processes, including differentiation, development, angiogenesis, cell proliferation, apoptosis and motility, as well as carcinogenesis (6–10). Furthermore, certain lncRNAs have been reported to be deregulated and to have key roles in ICC (11–13). For instance, upregulation of lncRNA colorectal neoplasia differentially expressed correlates with poor prognosis in ICC and promotes epithelial-mesenchymal transition (EMT) in ICC cells (11). Zhang et al (12) reported that lncRNA glucosaminyl (N-acetyl) transferase 2 (I blood group) promoted ICC cell migration, invasion and EMT through inhibition of miR-152 expression. In addition, Lv et al (13) performed a correlation analysis between lncRNA expression levels and clinicopathological characteristics, which revealed that EMP1-008, ATF3-008 and RCOR3-013 were significantly downregulated in metastatic ICC, suggesting that their downregulation may participate in ICC metastasis.
The lncRNA urothelial cancer-associated 1 (UCA1) is frequently upregulated in various common types of human cancer, and has a promoting role in tumour progression (14–16). For instance, Zhou et al (17) reported that UCA1 was upregulated in pancreatic cancer, promoted pancreatic cancer cell proliferation, invasion, migration, and inhibited cell apoptosis through the downregulation of miR-96 and the upregulation of forkhead box O3. Furthermore, the expression of UCA1 was increased in bladder cancer tissues when compared with that in normal tissues, and UCA1 promoted the migration, invasion and EMT of bladder cancer cells by inhibiting the expression of miR-143 and miR-145, while increasing the expression of high mobility group box 1, zinc finger E-box binding homeobox 1 and 2, and fascin actin-bundling protein 1 (18,19). In addition, UCA1 was upregulated in oral squamous cell carcinoma (OSCC) tissues and cell lines, as well as in cisplatin-resistant OSCC cells, promoted OSCC cell proliferation and induced cisplatin resistance through inhibition of miR-184 expression (20). Recently, UCA1 was reported to indicate an unfavourable prognosis in cholangiocarcinoma patients, and to promote cancer cell growth, migration and invasion via regulating the AKT/GSK-3β signalling pathway (21). However, the exact role of UCA1 in ICC has remained to be elucidated.
Therefore, the present study aimed to determine the expression and function of UCA1 in ICC. In addition, the regulatory mechanisms of UCA1 underlying ICC progression were investigated.
Materials and methods
Clinical tissue collection
The present study was approved by the Ethics Committee of Hunan Province People's Hospital (Changsha, China). ICC tissues and their paired adjacent non-tumour tissues were collected from 66 ICC patients who received surgical resection at Hunan Province People's Hospital (Changsha, China) between May 2011 and March 2013. These patients included 35 males and 31 females, from 42–74 years old with mean of 61 years old. Written informed consent was obtained from all of these patients. These patients did not receive any treatment prior to surgery. All tissue samples were stored at −80°C until use. Follow-up after surgical resection was performed until the patients' death, which occurred mainly due to cancer recurrence and metastasis.
Cell culture and transfection
The RBE, HCCC-9810 and LICCF human ICC cell lines and a normal human intrahepatic biliary epithelial cell (HIBEC) line were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) in a cell incubator containing 5% CO2 at 37°C. For cell transfection, HCCC-9810 cells were transfected with 100 nM negative control (NC) small interfering (si)RNA (cat. no. 4457287), UCA1 siRNA (cat. no. 4390771; both Thermo Fisher Scientific, Inc.), a blank vector (cat. no. V0006) or a UCA1 expression plasmid (cat. no. P1104; both Yearthbio, Inc., Changsha, China), or co-transfected with a UCA1 expression plasmid and miR-122 mimics (cat. no. 4464066) or miR-NC mimics (cat. no. 4464058; both Thermo Fisher Scientific, Inc.) using Lipofectamine 2000™ (Thermo Fisher Scientific, Inc.). At 48 h after transfection, the cells were used for the subsequent assays.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues or cells using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total RNA was reversely transcribed into complementary DNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. qPCR was performed to examine the UCA1 and miR-122 expression by using SYBR® Premix Ex Taq™ (Takara Bio Inc., Otsu, Japan) according to the manufacturer's protocol. GAPDH was used as the internal reference for UCA1 and U6 was used as the internal reference for miR-122. The reaction conditions were 95°C for 5 min, followed with 35 cycles at 95°C for 30 sec and 60°C for 30 sec. The relative expression levels of UCA1 and miR-122 were determined using the 2−ΔΔCq method (22).
Luciferase reporter gene assay
The target miRNAs of UCA1 were predicted using RNAhybrid 2.12 (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/). The wild-type (WT) and mutant-type (MT) UCA1 luciferase reporter plasmids were obtained from Yearthbio, Inc., which contain the UCA1 sequences with or without the miR-122 binding sites. To study the targeting association between UCA1 and miR-122, Lipofectamine 2000 was used to co-transfect 9810 cells with WT or MT UCA1 luciferase reporter plasmid and miR-122 or miR-NC mimics. After transfection for 48 h, the luciferase activity was examined using the Dual Luciferase Reporter Assay System (Promega Corp., Madison, WI, USA) according to the manufacturer's protocol.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan) was used to assess cell proliferation according to the manufacturer's protocol. The transfected cells were seeded into 96-well plates (3×103 cells per well). After incubation at 37°C for 0, 24, 48 or 72 h, 10 µl CCK-8 solution was added to each well. The absorbance was measured at 450 nm using a Bio-Tek Synergy HT Multi Detection Microplate Reader (Bio-Tek, Taipei, Taiwan).
Cell invasion assay
A Transwell assay was used to study cell invasion. After transfection for 48 h, cells (105 cells per well in 200 µl serum-free DMEM) were seeded into the upper chambers of Transwell plates (Corning Inc., Corning, NY, USA). DMEM with 10% FBS was added to the lower chamber of the Transwell plates. After 24 h, non-invading cells were carefully removed from the upper chamber with cotton swabs. The filters were then stained with 0.01% crystal violet solution at room temperature for 10 min. Images of invading cells were captured under an inverted microscope.
Statistical analysis
Values are expressed as the mean ± standard deviation from three independent experiments. The differences between two groups were analyzed using two-tailed Student's t-tests. The differences among more than two groups were analysed using one-way analysis of variance, followed by Tukey's post-hoc test. Kaplan-Meier survival curves were generated and differences were determined by using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulation of UCA1 is associated with ICC progression and poor prognosis
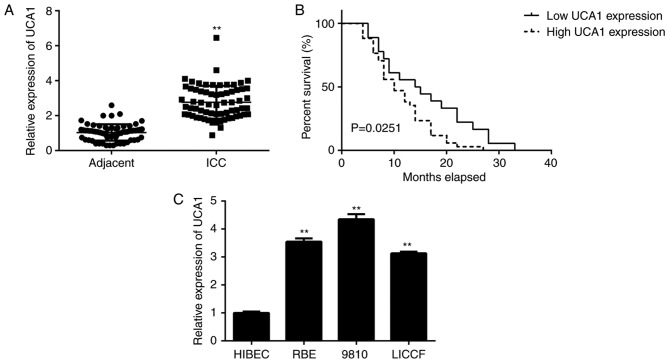
In the present study, RT-qPCR analysis indicated that the expression levels of UCA1 were significantly higher in ICC tissues when compared with those in adjacent non-tumour tissues (Fig. 1A). The ICC patients were then divided into a high and a low UCA1 expression group, based on their mean expression level. As indicated in Table I, high expression of UCA1 was significantly associated with clinical T-stage and lymph node metastasis. Furthermore, those ICC patients with a high expression of UCA1 had a significantly shorter survival when compared with that of patients with low UCA1 expression (Fig. 1B). These results suggested that upregulation of UCA1 is likely involved in ICC progression. Consistent with the data obtained with the clinical tissues, the expression of UCA1 was also higher in human ICC cell lines when compared with that in HIBECs (Fig. 1C). As 9810 cells displayed the highest expression of UCA1 among the ICC cell lines, this cell line was used in the subsequent in vitro experiments.
Figure 1.
Upregulation of UCA1 is associated with ICC progression and poor prognosis. (A) UCA1 expression is upregulated in ICC issues compared with that in paired adjacent non-tumour tissues. (B) Kaplan-Meier analysis indicated that ICC patients with high UCA1 expression had shorter survival times when compared with that of patients with low UCA1 expression. (C) UCA1 is downregulated in ICC cell lines compared with that in the normal HIBEC. **P<0.01 vs. adjacent or HIBEC. UCA1, urothelial carcinoma-associated 1; HIBEC, human intrahepatic biliary epithelial cells; ICC, intrahepatic cholangiocarcinoma.
Table I.
Association between UCA1 expression and clinicopathological characteristics of patients with intrahepatic cholangiocarcinoma.
| UCA1 expression | ||||
|---|---|---|---|---|
| Variables | Cases (n=66) | Low (n=32) | High (n=34) | P-value |
| Age (years) | 0.810 | |||
| <55 | 30 | 14 | 16 | |
| ≥55 | 36 | 18 | 18 | |
| Sex | 0.460 | |||
| Male | 35 | 15 | 20 | |
| Female | 31 | 17 | 14 | |
| Tumor focality | 0.477 | |||
| Solitary | 57 | 29 | 28 | |
| Multiple | 9 | 3 | 6 | |
| Histologic grade | 0.171 | |||
| Well-moderate | 48 | 26 | 22 | |
| Poor | 18 | 6 | 12 | |
| Lymph node metastasis | 0.034 | |||
| Present | 14 | 3 | 11 | |
| Absent | 52 | 29 | 23 | |
| Vascular invasion | 0.083 | |||
| Present | 26 | 9 | 17 | |
| Absent | 40 | 23 | 17 | |
| HBV infection | 0.477 | |||
| Present | 9 | 3 | 6 | |
| Absent | 57 | 29 | 28 | |
| Clinical T-stage | 0.027 | |||
| T1/T2 | 38 | 23 | 15 | |
| T3/T4 | 28 | 9 | 19 | |
UCA1, urothelial carcinoma-associated 1.
Promoting effects of UCA1 on ICC cell proliferation and invasion
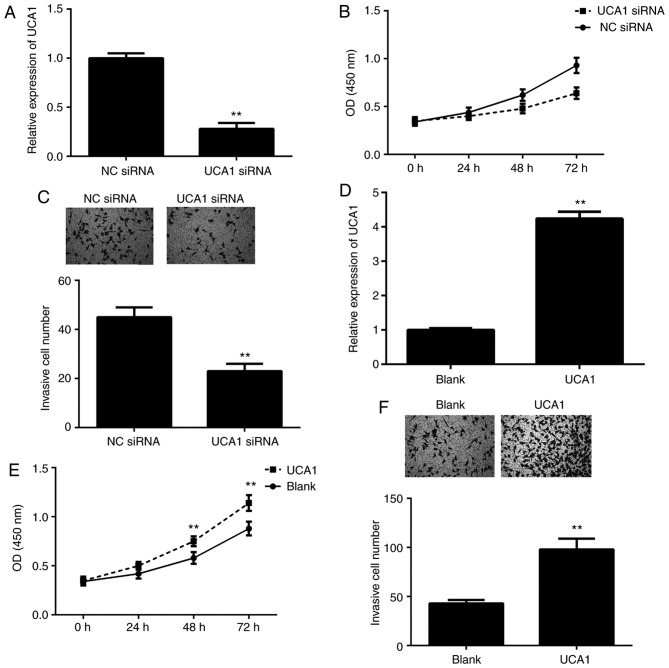
To further clarify the function of UCA1 in ICC, 9810 cells were transfected with UCA1 siRNA or NC siRNA. RT-qPCR indicated that the expression of UCA1 was significantly downregulated in the UCA1 siRNA group compared with that in the NC siRNA group (Fig. 2A). A CCK-8 assay and a Transwell assay were then performed to assess cell proliferation and invasion. As presented in Fig. 2B and C, knockdown of UCA1 led to a significant decrease in 9810 cell proliferation and invasion. To further confirm these results, 9810 cells were transfected with UCA1 plasmid or blank vector. After transfection, the expression levels of UCA1 were significantly upregulated in the UCA1 group when compared with those in the blank group (Fig. 2D). Furthermore, overexpression of UCA1 markedly promoted the proliferation and invasion of 9810 cells (Fig. 2E and F). These results suggested that UCA1 promotes ICC cell proliferation and invasion.
Figure 2.
Positive effects of UCA1 on ICC cell proliferation and invasion. (A) UCA1 is downregulated in 9810 cells transfected with UCA1 siRNA compared with that in cells transfected with NC siRNA. (B) The proliferation and (C) invasion of 9810 cells in the UCA1 siRNA group were downregulated compared with those in the NC siRNA group. Magnification, ×200. (D) UCA1 was upregulated in 9810 cells transfected with UCA1 expression plasmid compared with that in cells transfected with blank vector. (E) The proliferation and (F) invasion of 9810 cells were upregulated in the UCA1 group compared with those in the blank group. Magnification, ×200. **P<0.01 vs. NC siRNA or blank. UCA1, urothelial carcinoma-associated 1; ICC, intrahepatic cholangiocarcinoma; siRNA, small interfering RNA; NC, negative control; OD, optical density.
UCA1 directly targets miR-122 in ICC cells
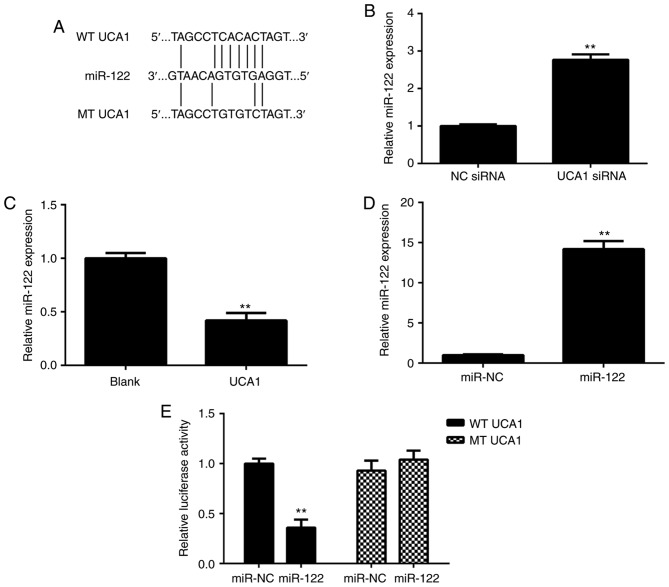
As lncRNAs generally act as ligands for pools of target miRNAs, miRanda software (http://www.micro-RNA.org/) was used to predict potential UCA1-miR interactions. As presented in Fig. 3A, miR-122 had a potential binding site for UCA1. The effects of UCA1 upregulation or downregulation on the miR-122 expression in 9810 cells were then studied. As presented in Fig. 3B and C, ectopic overexpression of UCA1 significantly reduced the expression of miR-122 in 9810 cells, while knockdown of UCA1 markedly promoted the miR-122 expression in 9810 cells. Next, 9810 cells were transfected with the miR-NC or miR-122 mimics. After transfection, RT-qPCR confirmed that the expression of miR-122 was significantly increased in the miR-122 group compared with that in the miR-NC group (Fig. 3D). These results suggested that transfection with the miR-122 mimics effectively upregulated miR-122 expression in 9810 cells. To verify the predicted direct binding of miR-122 with UCA1, the WT or MT UCA1 luciferase reporter gene plasmids with or without the binding sites with miR-122, respectively, were purchased (Fig. 3A). A luciferase reporter gene assay was then performed in 9810 cells. As presented in Fig. 3E, transfection with the miR-122 mimics caused a significant decrease in the luciferase activity of cells transfected with the WT UCA1 luciferase reporter plasmid; however, the miR-122 mimics had no effect on the luciferase activity of cells transfected with the MT UCA1 luciferase reporter plasmid. Therefore, it was proven that UCA1 directly targets miR-122 in ICC cells.
Figure 3.
UCA1 directly targets miR-122 in ICC cells. (A) The WT and MT UCA1 binding sites of miR-122 are indicated. (B) UCA1 overexpression significantly reduces the expression of miR-122 in 9810 cells. (C) Knockdown of UCA1 markedly promotes miR-122 expression in 9810 cells. (D) Transfection with miR-122 mimics increases the miR-122 levels in 9810 cells. (E) Transfection with miR-122 mimics causes a significant decrease in the luciferase activity of WT UCA1 luciferase reporter plasmid but has no effect on the luciferase activity of the MT UCA1 luciferase reporter plasmid. **P<0.01 vs. blank, siRNA NC or miR-NC. UCA1, urothelial carcinoma-associated 1; WT, wild-type; MT, mutant type; ICC, intrahepatic cholangiocarcinoma; siRNA, small interfering RNA; NC, negative control; miR, microRNA.
miR-122 functions as a downstream effector in the UCA1-mediated ICC cell proliferation and invasion
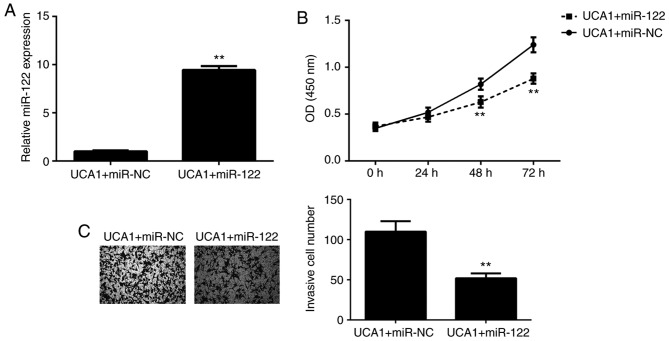
As UCA1 was proven to directly target miR-122 and negatively regulate its expression in ICC cells, it was then investigated whether miR-122 functions as a downstream effector in the UCA1-mediated proliferation and invasion of ICC cells. 9810 cells were co-transfected with UCA1 plasmid and either miR-122 mimics or miR-NC mimics. After transfection, the miR-122 levels were significantly increased in the UCA1+miR-122 group when compared with those in the UCA1+miR-NC group (Fig. 4A). Next, a CCK-8 assay and a Transwell assay were performed to assess cell proliferation and invasion, respectively. As presented in Fig. 4B and C, respectively, the proliferation and invasion of 9810 cells were significantly downregulated in the UCA1+miR-122 group when compared with those in the UCA1+miR-NC group. These results suggested that overexpression of UCA1 promotes the proliferation and invasion of 9810 cells via inhibition of miR-122 expression. Therefore, miR-122 functioned as a downstream effector in the UCA1-mediated ICC cell proliferation and invasion.
Figure 4.
miR-122 functions as a downstream effector in the UCA1-mediated proliferation and invasion of intrahepatic cholangiocarcinoma cells. UCA1-transfected 9810 cells were transfected with miR-122 or miR-NC mimics. (A) miR-122 was upregulated in the UCA1+miR-122 group when compared with that in the UCA1+miR-NC group. (B) The cell proliferation and (C) invasion were also upregulated in the UCA1+miR-122 group when compared with those in the UCA1+miR-NC group. Magnification, ×200. **P<0.01 vs. UCA1+miR-NC. UCA1, urothelial carcinoma-associated 1; NC, negative control; miR, microRNA; OD, optical density.
miR-122 is downregulated in ICC, with an inverse correlation to the expression of UCA1
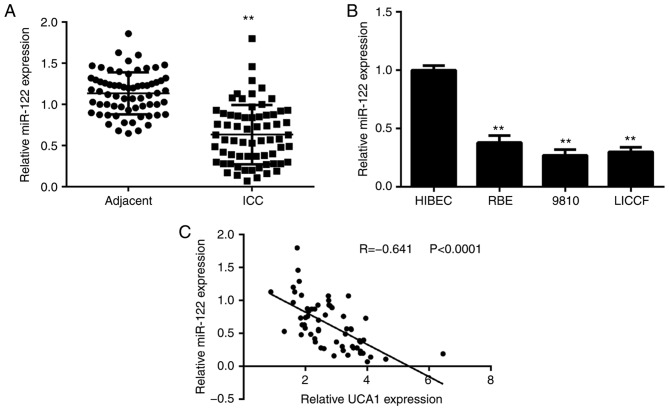
Finally, the expression levels of miR-122 were determined in ICC tissues and cell lines. As presented in Fig. 5A, the expression of miR-122 was significantly reduced in ICC tissues when compared with that in adjacent non-tumour tissues. Consistently, the expression of miR-122 was also downregulated in ICC cell lines when compared with that in HIBECs (Fig. 5B). Of note, an inverse correlation was observed between the UCA1 and miR-122 expression in ICC tissues (Fig. 5C). Therefore, the reduced expression of miR-122 may at least in part be due to the increased expression of UCA1 in ICC tissues.
Figure 5.
miR-122 is downregulated in ICC and inversely correlated to the expression of UCA1. (A) miR-122 is downregulated in ICC issues compared with that in matched adjacent non-tumour tissues. (B) miR-22 is downregulated in ICC cell lines compared with that in HIBEC cells. **P<0.01 vs. adjacent or HIBEC. (C) An inverse correlation between the UCA1 and miR-122 expression in ICC tissues was identified. UCA1, urothelial carcinoma-associated 1; ICC, intrahepatic cholangiocarcinoma; miR, microRNA; HIBEC, human intrahepatic biliary epithelial cells.
Discussion
In the present study, UCA1 was observed to be significantly upregulated in ICC tissues and cell lines, when compared with that in the adjacent non-tumour tissues and HIBECs, respectively. The increased expression of UCA1 was significantly associated with lymph node metastasis and clinical T-stage in ICC. Furthermore, the ICC patients with high expression of UCA1 had shorter survival times when compared with those with low UCA1 expression. Knockdown of UCA1 caused a significant decrease in ICC cell proliferation and invasion, and overexpression of UCA1 significantly promoted the proliferation and invasion of ICC cells. Furthermore, UCA1 was confirmed to directly bind to miR-122, and the expression of miR-122 was negatively regulated by UCA1 in ICC cells. Of note, miR-122 mimics inhibited the promoting effects of UCA1 on ICC cell proliferation and invasion. In addition, an inverse correlation between miR-122 and UCA1 expression in ICC tissues was observed.
Several studies have focused on the function of lncRNAs in ICC. For instance, Wang et al (2) performed a lncRNA microarray using ICC tissues and paired adjacent non-tumour tissues, and identified 2,773 lncRNAs that were significantly upregulated and 2,392 lncRNAs that were downregulated in ICC tissues. They then observed a positive correlation between 4 lncRNA-mRNA pairs in ICC tissues, including RNA43085 and sulfatase 1, RNA47504 and lysine demethylase 8, RNA58630 and proprotein convertase subtilisin/kexin type 6, and RNA40057 and cytochrome P450 family 2 subfamily D member 6 (CYP2D6) (2). Furthermore, the ICC patients with high CYP2D6 and RNA40057 expression had a better prognosis (2). These results suggested that certain lncRNAs may become promising diagnostic and prognostic biomarkers for ICC. Ma et al (23) reported that the lncRNA carbamoyl-phosphate synthase 1-intronic transcript 1 was upregulated in ICC tissues, and its upregulation was associated with poor liver function and shorter survival time of ICC patients. In addition, Zeng et al (24) indicated that the lncRNA taurine up-regulated 1 (TUG1) was upregulated in ICC, which correlated with ICC progression and poor prognosis, and inhibition of TUG1 expression reduced ICC cell proliferation, migration and invasion in vitro and tumour growth in vivo. However, the function of lncRNA UCA1 in ICC has not been previously reported, to the best of our knowledge. The results of the present study indicated that UCA1 was significantly upregulated in ICC tissues and cell lines. Furthermore, high expression of UCA1 was identified to be associated with clinical T-stage and lymph node metastasis, as well as shorter survival times of ICC patients. These results suggested that the increased expression of UCA1 may contribute to the malignant progression of ICC. To further study the role of UCA1 in ICC, two common ICC cell lines were used to perform in vitro experiments. Knockdown of UCA1 inhibited ICC cell proliferation and invasion, and overexpression of UCA1 promoted the proliferation and invasion of ICC cells. These results further suggested that the lncRNA UCA1 may participate in ICC growth and metastasis.
As several cellular responses function through interaction with miRs, the present study subsequently focused on the downstream miRs of UCA1 in ICC. miRs, another type of non-coding small RNA with 22–25 nucleotides, are important regulators for gene expression, and have key roles during cancer development and progression. A Bioinformatics analysis suggested that miR-122 has a potential binding site with UCA1, which was confirmed in the ICC cell lines by using a luciferase reporter gene assay. A previous study reported that miR-122 is significantly downregulated in ICC tissues (25). In line with this, a downregulation of miR-122 in ICC tissues and cell lines was also observed in the present study. Furthermore, an inverse correlation between the UCA1 and miR-122 expression in ICC tissues was observed. As ectopic overexpression of UCA1 significantly reduced the miR-122 expression and knockdown of UCA1 markedly promoted miR-122 expression in ICC cells, it was suggested that the downregulation of miR-122 in ICC may be due to the upregulation of UCA1. Furthermore, miR-122 mimics impaired the effects of UCA1 upregulation on ICC cell proliferation and invasion, suggesting that miR-122 functions as a downstream effector in the UCA1-mediated ICC cell proliferation and invasion.
In addition to ICC, the association between UCA1 and miR-122 has been reported in glioma and breast cancer cells (26). Sun et al (26) indicated that UCA1 targeted miR-122 to promote glioma cell proliferation, migration and invasion. Zhou et al (27) reported that histocompatibility minor 13 regulated the UCA1-mediated invasion of breast cancer cells through UCA1 decay and decreasing the interaction between UCA1 and miR-122. Therefore, the present study expands on the understanding of the function of the UCA1/miR-122 interaction in human cancers.
In conclusion, the present study demonstrates for the first time that lncRNA UCA1 promotes the proliferation and invasion of ICC cells through targeting miR-122 and thus suggests that the UCA1/miR-22 interaction may be used as a potential therapeutic target for the treatment of ICC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
WY collected clinical tissues. OL, PY and CG performed the clinical analyses and cell experiments. OL and CP designed the study and wrote the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hunan Province People's Hospital (Changsha, China). Written informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, Fiel MI, Schwartz M, Thung SN. Intrahepatic cholangiocarcinoma: New insights in pathology. Semin Liver Dis. 2011;31:49–60. doi: 10.1055/s-0031-1272839. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Xie H, Ling Q, Lu D, Lv Z, Zhuang R, Liu Z, Wei X, Zhou L, Xu X, Zheng S. Coding-noncoding gene expression in intrahepatic cholangiocarcinoma. Transl Res. 2016;168:107–121. doi: 10.1016/j.trsl.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JB. Molecular pathogenesis of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2015;22:101–113. doi: 10.1002/jhbp.155. [DOI] [PubMed] [Google Scholar]
- 4.Deng G, Zhu L, Huang F, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. SALL4 is a novel therapeutic target in intrahepatic cholangiocarcinoma. Oncotarget. 2015;6:27416–27426. doi: 10.18632/oncotarget.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018;17:61. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolle MA, Pichler M. The role of long non-coding RNAs in osteosarcoma. Noncoding RNA. 2018;4(pii):E7. doi: 10.3390/ncrna4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S, Kong D, Chen Q, Ping Y, Pang D. Oncogenic long noncoding RNA landscape in breast cancer. Mol Cancer. 2017;16:129. doi: 10.1186/s12943-017-0696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan X. Long noncoding RNA central of glucose homeostasis. J Cell Biochem. 2016;117:1061–1065. doi: 10.1002/jcb.25427. [DOI] [PubMed] [Google Scholar]
- 9.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DH, Chu ET, Spektor R, Soloway PD. Long non-coding RNA regulation of reproduction and development. Mol Reprod Dev. 2015;82:932–956. doi: 10.1002/mrd.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia XL, Xue D, Xiang TH, Xu HY, Song DK, Cheng PG, Wang JQ. Overexpression of long non-coding RNA CRNDE facilitates epithelial-mesenchymal transition and correlates with poor prognosis in intrahepatic cholangiocarcinoma. Oncol Lett. 2018;15:4105–4112. doi: 10.3892/ol.2018.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Xiao J, Chai Y, Du YY, Liu Z, Huang K, Zhou X, Zhou W. LncRNA-CCAT1 promotes migration, invasion, and EMT in intrahepatic cholangiocarcinoma through suppressing miR-152. Dig Dis Sci. 2017;62:3050–3058. doi: 10.1007/s10620-017-4759-8. [DOI] [PubMed] [Google Scholar]
- 13.Lv L, Wei M, Lin P, Chen Z, Gong P, Quan Z, Tang Z. Integrated mRNA and lncRNA expression profiling for exploring metastatic biomarkers of human intrahepatic cholangiocarcinoma. Am J Cancer Res. 2017;7:688–699. [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, Zang W, Zhao G. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol. 2016;33:88. doi: 10.1007/s12032-016-0804-2. [DOI] [PubMed] [Google Scholar]
- 15.Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HM, Lu JH, Chen WY, Gu AQ. Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int J Clin Exp Med. 2015;8:11824–11830. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu Y, Qian H, Dai T. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. IUBMB Life. 2018;70:276–290. doi: 10.1002/iub.1699. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, Chen J, Li H, Yang Y, Yun H, Yang S, Mao X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol Lett. 2017;14:5556–5562. doi: 10.3892/ol.2017.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue M, Pang H, Li X, Li H, Pan J, Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Z, Zhao J, Xie W, Sun Q, Wang H, Qiao B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 2017;6:2897–2908. doi: 10.1002/cam4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Yao Y, Leng K, Li Z, Qin W, Zhong X, Kang P, Wan M, Jiang X, Cui Y. Long non-coding RNA UCA1 indicates an unfavorable prognosis and promotes tumorigenesis via regulating AKT/GSK-3β signaling pathway in cholangiocarcinoma. Oncotarget. 2017;8:96203–96214. doi: 10.18632/oncotarget.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Ma SL, Li AJ, Hu ZY, Shang FS, Wu MC. Co-expression of the carbamoyl-phosphate synthase 1 gene and its long non-coding RNA correlates with poor prognosis of patients with intrahepatic cholangiocarcinoma. Mol Med Rep. 2015;12:7915–7926. doi: 10.3892/mmr.2015.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng B, Ye H, Chen J, Cheng D, Cai C, Chen G, Chen X, Xin H, Tang C, Zeng J. LncRNA TUG1 sponges miR-145 to promote cancer progression and regulate glutamine metabolism via Sirt3/GDH axis. Oncotarget. 2017;8:113650–113661. doi: 10.18632/oncotarget.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52:297–303. doi: 10.1002/mc.21864. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Jin JG, Mi WY, Zhang SR, Meng Q, Zhang ST. Long noncoding RNA UCA1 targets miR-122 to promote proliferation, migration, and invasion of glioma cells. Oncol Res. 2018;26:103–110. doi: 10.3727/096504017X14934860122864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Meng X, Chen S, Li W, Li D, Singer R, Gu W. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. 2018;20:32. doi: 10.1186/s13058-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.