Abstract
Homocysteine has been reported to be an independent risk factor for stroke. Scutellarin (Scu) dilates cerebral blood vessels and promotes anti-platelet aggregation; however, the mechanism by which Scu and Scu-treated exosomes protect against cerebrovascular disease is unclear. The aim of the present study was to investigate the mechanisms underlying the effects of Scu and Scu-treated exosomes on tight junction proteins in the blood-brain barrier. Rat brain microvascular endothelial cells (RBMVECs) were cultured and divided into five groups: Control, model, Scu, exosomes derived from RBMVECs and exosomes derived from RBMVECs after Scu administration. MTT, lactate dehydrogenase (LDH) and nitric oxide (NO) assays were performed to assess cell viability and injury. Reactive oxygen species (ROS) levels were detected using spectrophotometry and immunofluorescence. Western blotting and immunofluorescence were performed to measure cluster of differentiation (CD) 63, claudin 5, occludin and tight junction protein 1 (ZO1) expression. The results revealed that treatment with Scu and Scu-treated exosomes enhanced cell viability, reduced cell injury, increased NO levels, upregulated CD63, claudin 5, occludin and ZO1, and decreased LDH and ROS levels. These data suggest that Scu and Scu-treated exosomes protect homocysteine-induced RBMVECs via increased claudin 5, occludin and ZO1 expression.
Keywords: Scutellarin, exosomes, homocysteine, blood-brain barrier, stroke
Introduction
Stroke is the leading cause of mortality and disability in China and the second most common cause of mortality worldwide (1,2). It has previously been reported that homocysteine (Hcy) is an important risk factor for stroke (3,4). High levels of Hcy are automatically oxidized to produce reactive oxygen species (ROS), which may damage the cardiovascular and immune systems (5). Hcy is recognized as an independent risk factor for cerebrovascular disease, but its role in pathogenesis remains unclear.
The blood-brain barrier (BBB) is the structural basis that maintains the internal environment of the central nervous system (6). BBB destruction is an important pathological characteristic of many neurological diseases (6). Lominadze et al (7) reported that cerebrovascular disease was associated with BBB damage, while Kamath et al (8) reported that high Hcy concentrations inhibit tight junction (TJ) protein expression in cerebrovascular endothelial cells, which results in damage to the TJ structure. Lee's study revealed that high Hcy levels damaged the BBB structure and increased BBB permeability (9). Tyagi et al (10) suggested that Hcy activates matrix metalloproteinases (MMPs), whose overexpression reduces the ability of cells to connect and alters the basement membrane, resulting in destruction of the blood-brain barrier.
Scutellarin (Scu) is an active component of Erigerontis Herba, which exhibits anti-inflammatory, antioxidative and protective effect on endothelial cells (11). Erigerontis Herba has been widely used in the treatment of cardiovascular and cerebrovascular diseases (12). Yuan et al (12) reported that Scu inhibited microglial activation and alleviated the symptoms of neuroinflammation, which contributed to the clinical treatment of cerebral ischemia. Du et al (13) demonstrated that Scu activated the endothelial cGMP activated protein kinase G (PKG) pathway to protect cerebral vascular endothelial cells. Furthermore, Scu has been revealed to prevent learning and memory defects in a rat model of Alzheimer's disease via reducing oxidative stress and inflammation (14).
Exosomes are cell membrane-derived vesicles that influence cellular activity by transporting proteins, lipids and nucleic acids to target cells (15). It has been reported that exosomes are able to promote functional recovery and neurovascular plasticity following stroke (16), as well as serving an important role in the pathogenesis of Alzheimer's disease, Parkinson's disease, prion disease and other neurological diseases (17). Exosomes may therefore have potential as clinical treatments for central nervous system diseases. The aim of the present study was to investigate the expression of TJ proteins claudin 5, occludin and TJ protein 1 (ZO1) in the BBB using Hcy-induced rat brain microvascular endothelial cells (RBMVECs).
Materials and methods
Cell culture
RBMVECs (Saiqi Biological Engineering Co., Ltd., Shanghai, China) were seeded in 25-cm2 polystyrene flasks (Corning Incorporated, Corning, NY, USA) with 4.5 g/ml glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were incubated at 37°C in an atmosphere containing 5% CO2. DMEM was replaced every 48 h.
Exosome extraction
Exosomes derived from normal RBMVECs following Scu administration (SE) and exosomes derived from control RBMVECs (CE) were extracted from cells and incubated with 100 µmol/l Scu or DMEM for 24 h, respectably. A total of 5 ml supernatant was collected from each flask and centrifuged at 3,000 × g for 15 min at room temperature to exclude cell debris. The supernatant was transferred to a new sterile centrifuge tube and the 1 ml ExoQuick-TC (Tissue Culture Media Exosome Precipitation Solution; cat. no. 170306-001; System Biosciences, Palo Alto, CA, USA) was added. The solution was vortexed overnight at 4°C. The suspension was then centrifuged at room temperature at 1,500 × g for 30 min. Exosome-containing pellets were resuspended in 500 µl PBS for intervention experiments.
Cell viability
Cells were seeded at a density of 1×104 cells/well in 96-well culture plates and incubated for 24 or 48 h at 37°C in an atmosphere containing 5% CO2. Cells were incubated with aggregated intervention medications. A total of 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each well and cells were incubated at 37°C for 4 h. The culture medium was discarded and 150 µl of dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan crystals. The number of viable cells in each well was measured at 490 nm using a microplate reader (Multiskan Mk3; Thermo Fisher Scientific, Inc.).
Experimental design
After the concentration optimization experiments by MTT assay, RBMVECs were divided into the following groups: Control, model, Scu, exosomes derived from CEs and exosomes derived from normal RBMVECs after SE. The cultured cells in all groups were pretreated with Scu (100 µmol/l), CE (40% suspension of control exosomes in 1 ml of DMEM) and SE (40% suspension of Scu exosomes in of 1 ml DMEM) for 30 min, followed by Hcy exposure (2.5 mmol/l; cat. no. Z20J8H28854; Shanghai Yuanye Bio-Technology Co., Ltd, China) for 48 h.
Measurement of lactate dehydrogenase (LDH) and nitric oxide (NO)
LDH and NO release into the culture medium from dead cells was assessed using an LDH cytotoxicity assay kit (cat. no. 20170314) and an NO detection kit (cat. no. 20170407; both Nanjing JianCheng Bioengineering Institute, Nanjing, China). Cells were lysed with 1% SDS and centrifuged at 4°C, 2,000 × g for 10 min. The supernatants from each group were assessed according to the manufacturer's protocol.
ROS detection
Intracellular ROS levels were monitored using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; cat. no. 170V; Beijing Vigorous Biotechnology Co., Ltd, China) to identify the role of ROS in Scu and Scu-treated exosomes. RBMVECs were seeded in 24 well plates at a density of 2×105 cells/well and incubated overnight at 37°C. Cells were subsequently incubated with or without intervention drugs (Scu, CE and SE) for 48 h. The cells were then treated with DCFH-DA (5 µM) at 37°C for 90 min. Fluorescence microscopy (magnification, ×400; U-SPT; Olympus Corporation, Tokyo, Japan) was used to measure the fluorescence intensity of the treated cells.
RBMVECs (1×104 in 200 µl) were seeded in quadruplicate in a 96-well plate and incubated with 2.5 mmol/l Hcy for 48 h in the presence or absence of intervention drugs (Scu, CE and SE). The cultured cells were washed three times with PBS (pH 7.4) and incubated in DMEM containing DCFH-DA (5 µM) at 37°C for 90 min. The cells were washed with prewarmed PBS and covered with 100 µl of DMEM. The fluorescence intensity (FI) of each well was measured using a microplate reader at 485/530 nm.
Detection of cluster of differentiation (CD)63, claudin-5, ZO1 and occludin by immunofluorescence
Cells were incubated with 2.5 mmol/l Hcy for 48 h in the presence or absence of intervention drugs (Scu, CE and SE). Briefly, cells were grown on cover slips, and 4% paraformaldehyde was used to fix the cells for 30 min at room temperature. Cells were subsequently blocked in 10% goat serum (Gibco; Thermo Fisher Scientific, Inc.) for 20 min at 37°C. Cells were treated with antibodies against CD63 (cat. no. o67346b; OmnimAbs, Alhambra, CA, USA), claudin-5 (cat. no. 6679g62), ZO1 (cat. no. 3268f94; both Affinity Biosciences, USA) and occludin (cat. no. o29813c; OmnimAbs) for 1 h at 37°C (1:50 dilution). Cells were subsequently incubated for 1 h at 37°C with corresponding secondary antibodies conjugated to FITC. Cells were subsequently washed three times with PBS and stained with DAPI (1 µg/ml in PBS) at 37°C in the dark. The coverslips were mounted and cells were observed under a light microscope (U-SPT; Olympus Corp., Tokyo, Japan). Data were analyzed by using Medical Image Analysis Software 16.0 (MIAS, Warrendale, WA, USA).
Western blotting
RBMVECs were harvested and lysed using phenylmethane sulfonyl fluoride-containing lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). Proteins were quantified using a BCA protein assay kit (Beyotime Institute of Biotechnology). Equal amounts of protein (30 µg) were separated by 12% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat skim milk in TBST for 1 h at room temperature followed by incubation with primary antibodies against CD63, claudin-5, ZO1, occludin and GAPDH (cat. no. 10; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C (1:1,000 dilution). The membrane was washed with TBST and incubated with horseradish peroxidase conjugated secondary antibodies for 1 h at 37°C (1:2,000 dilution; cat/no. 7074s; Cell Signaling Technology, Inc.). Proteins were detected using an ECL detection kit and Image-Lab software-5.2.1 (both Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. Significant differences between groups were determined using one-way analysis of variance followed by Bonferroni's post hoc test for multiple comparisons. Correlations between LDH, NO, ROS, CD63, claudin 5, occludin and ZO1 expression were identified using Pearson's correlation analysis. All data analyses were performed using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of Scu on Hcy-induced RBMVEC viability
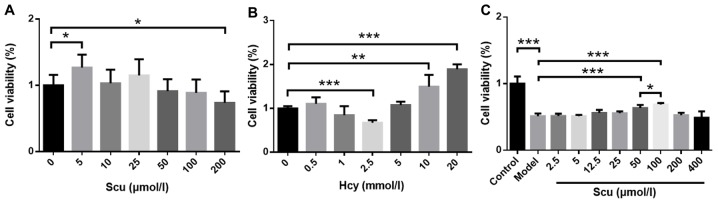
Cell viability increased in the presence of 5 µmol/l Scu (P<0.05) (Fig. 1A) and decreased in the presence of 200 µmol/l Scu (P<0.05) (Fig. 1A) compared with untreated RBMVECs. Treatment with 2.5 mmol/l Hcy significantly decreased the viability of RBMVECs compared with the control (P<0.001; Fig. 1B), while viability was increased following treatment 10–20 mmol/l Hcy (P<0.001) (Fig. 1B). The effects of 2.5–400 µmol/l Scu on the activity of RBMVECs induced with 2.5 mmol/l Hcy were then investigated. Cell viability increased significantly in the presence of 50 or 100 µmol/l Scu compared with the model group (P<0.001) (Fig. 1C). Furthermore, 100 µmol/l Scu-treated cell viability was significantly higher compared with 50 µmol/l Scu-treated cells (P<0.05) (Fig. 1C).
Figure 1.
Effects of Scu and Hcy on RBMVEC viability. RBMVEC viability following exposure to (A) 200 µmol/l Scu or (B) 2.5 mmol/l Hcy for 24 h. (C) The viability of Hcy-induced RBMVECs following treatment with 50–100 µmol/l Scu for 24 h. (A) *P<0.05 vs. 0 µmol/l Scu group; (B) **P<0.01 and ***P<0.001 vs. 0 mmol/l Hcy group; (C) *P<0.05, **P<0.01 and ***P<0.001 vs. Model group and *P<0.05 with a bar linking the 50 µmol/l Scu group and 100 µmol/l Scu group with an asterisk. n=6. Scu, Scutellarin; Hcy, homocysteine; RBMVEC, rat brain microvascular endothelial cell; Model, 2.5 mmol/l Hcy-induced RBMVECs.
Effects of pretreated exosomes on Hcy-induced RBMVEC viability
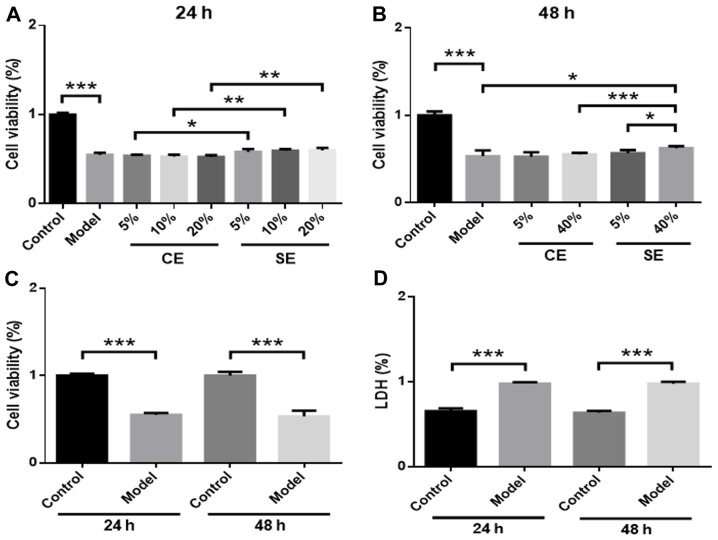
RBMVECs were incubated with different amounts of exosomes (5, 10, 20 or 40% in DMEM) in the presence of 2.5 mmol/l Hcy for 24 or 48 h. No significant changes in cell viability were observed in the 5, 10 or 20% CE and SE groups compared with the model group (Fig. 2A). However, cell viability was significantly higher in the SE group compared with the CE group at 24 h (P<0.05) (Fig. 2A). In the 40% SE group, cell viability was significantly increased compared with the model group (P<0.05) (Fig. 2B) and the 40% CE group at 48 h (P<0.001) (Fig. 2B). A dose-effect relationship was observed in the SE group, as treatment with 40% solution produced increased cell viability significantly more than the 5% solution (P<0.05) (Fig. 2B). No significant differences in cell viability or LDH levels were observed at 24 or 48 h, which suggests that the effect of Hcy in the model group was not time-dependent (Fig. 2C and D).
Figure 2.
Effects of pretreated exosomes on Hcy-induced RBMVEC viability. Cells were treated with 5–20% CE or SE for (A) 24 h or (B) 48 h. (C) Cell viability and (D) LDH expression in the model and control groups was assessed at 24 and 48 h. *P<0.05, **P<0.01 and ***P<0.001 with a bar linking the groups with an asterisk. n=6. Hcy, homocysteine; RBMVEC, Rat brain microvascular endothelial cell; LDH, lactate dehydrogenase; CE, control-treated exosomes; SE, Scutellarin-treated exosomes; Model, 2.5 mmol/l Hcy-induced rat brain microvascular endothelial cells.
Effect of Scu and Scu-treated exosomes on cell viability, LDH expression and NO levels
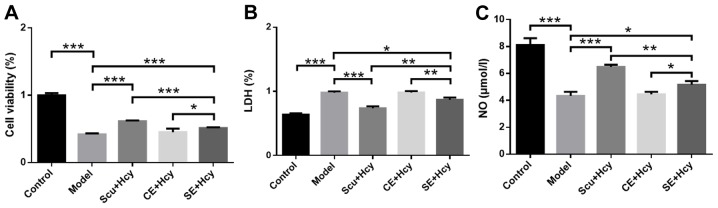
RBMVECs were incubated with 2.5 mmol/l Hcy for 48 h along with Scu and Scu-treated exosomes. Cell viability and NO levels in the Scu and SE group were significantly increased (P<0.05) (Fig. 3A) compared with the model group, while LDH levels were significantly decreased (P<0.05) (Fig. 3B). In addition, cell viability and NO levels were significantly higher in the Scu group compared with the SE group (P<0.01) (Fig. 3A and C), while LDH levels were significantly lower in the Scu group compared with the SE group (P<0.01) (Fig. 3B). Cell viability and NO levels were significantly increased (P<0.01) (Fig. 3A and C) and LDH levels were significantly decreased (P<0.05; Fig. 3B) in the SE group compared with the CE group.
Figure 3.
Effects of pretreated exosomes on LDH and NO levels. (A) Cell viability, (B) LDH expression and (C) NO levels in the Scu and SE groups. *P<0.05, **P<0.01 and ***P<0.001 with a bar linking the groups with an asteris. n=6. LDH, lactate dehydrogenase; NO, nitric oxide; Scu, Scutellarin; SE, Scu-treated exosomes; CE, control treated exosomes; Model, 2.5 mmol/l homocysteine-induced rat brain microvascular endothelial cells.
Effect of Scu and Scu-treated exosomes on ROS
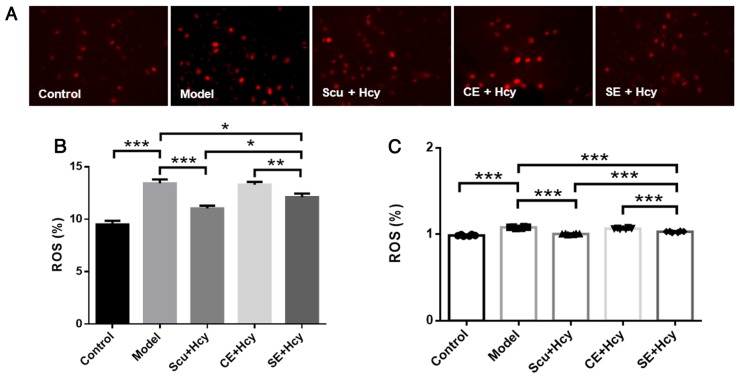
Cells were treated as described above, and ROS levels were detected using fluorescence and spectrophotometry. ROS levels were significantly decreased in the Scu and SE groups compared with the model group (P<0.05) (Fig. 4). ROS levels were reduced in the Scu and SE groups compared with the CE groups (P<0.01) (Fig. 4).
Figure 4.
Effect of Scu and Hcy on ROS levels. (A) Representative fluorescence microscopy images of rat brain microvascular endothelial cells (magnification, ×400; n=3). (B) Quantified results of ROS fluorescence microscopy. ROS expression was detected using fluorescence microscopy. (C) ROS expression as detected using a fluorescence microplate reader. *P<0.05, **P<0.01 and ***P<0.001 with a bar linking the groups with an asteris. n=6. Scu, Scutellarin; Hcy, homocysteine; ROS, reactive oxygen species; Model, 2.5 mmol/l Hcy-induced rat brain microvascular endothelial cells; CE, control-treated exosomes; SE, Scu-treated exosomes.
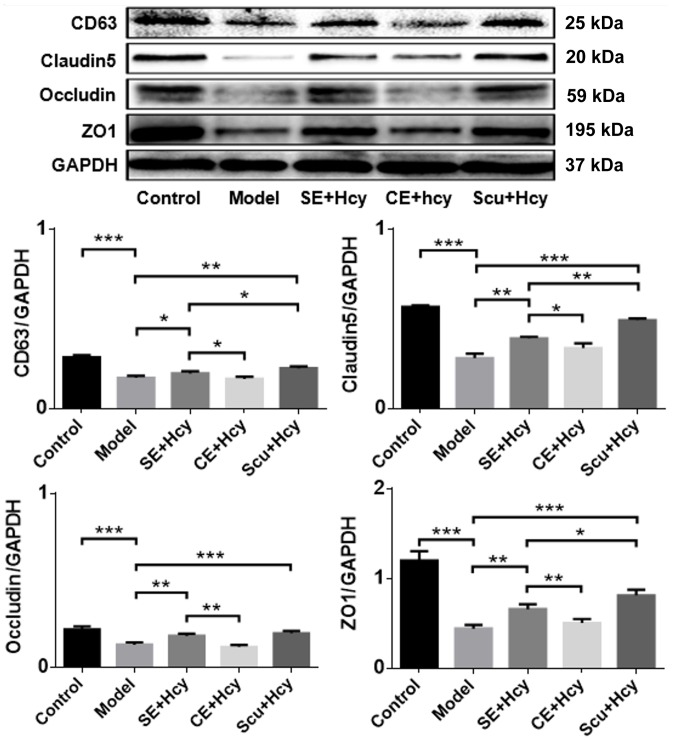
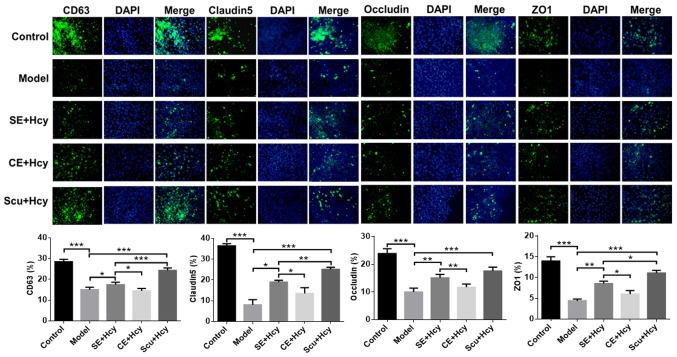
Effect of Scu and Scu-treated exosomes on CD63, claudin-5, occludin and ZO1 expression
The detailed mechanisms underlying the effect of Scu and Scu-treated exosomes were investigated. CD63, claudin-5, occludin and ZO1 expression was significantly increased in the Scu and SE groups compared with the model group (P<0.05) (Figs. 5 and 6). CD63, claudin-5, occludin and ZO1 expression was significantly increased in the Scu group compared with the SE group (P<0.05) (Figs. 5 and 6). CD63, claudin-5, occludin and ZO1 expression was significantly higher in the SE group compared with the CE group (P<0.05) (Figs. 5 and 6).
Figure 5.
Representative western blot and quantified results for CD63, claudin-5, occludin and ZO1 expression. *P<0.05, **P<0.01 and ***P<0.001 with a bar linking the groups with an asterisk. n=3. CD, cluster of differentiation; ZO1, tight junction protein 1; Model, 2.5 mmol/l Hcy-induced rat brain microvascular endothelial cells; CE, control-treated exosomes; Scu, Scutellarin; SE, Scu-treated exosomes; Hcy, homocysteine.
Figure 6.
Immunofluorescence of CD63, claudin-5, occludin and ZO1 expression. Magnification, ×400; n=3. *P<0.05, **P<0.01 and ***P<0.001 with a bar linking the groups with an asteris. CD, cluster of differentiation; ZO1, tight junction protein 1; Scu, Scutellarin; SE, Scu-treated exosomes; Hcy, homocysteine; CE, control-treated exosomes; Model, 2.5 mmol/l Hcy-induced rat brain microvascular endothelial cells.
Correlations between experimental indicators
The association of LDH levels with NO, ROS, CD63, claudin-5, occludin and ZO1 expression was assessed using Pearson's correlation analysis. The results indicated that LDH expression was significantly negatively correlated with NO, CD63, claudin-5, occludin and ZO1 levels (P<0.05) (Table I). Furthermore, NO levels were significantly negatively correlated with ROS expression (P<0.05) (Table I), while ROS expression was significantly negatively correlated with CD63, claudin-5, occludin and ZO1 levels (P<0.05) (Table I). Significant positive correlations were observed between CD63 and claudin-5, CD63 and occludin, and CD63 and ZO1 expression (all P<0.01) (Table I). Claudin-5 expression was significantly positively correlated with occludin and ZO1 levels (P<0.01) (Table I).
Table I.
Correlation analysis.
| LDH | NO | ROS | CD63 | Claudin-5 | Occludin | ZO1 | |
|---|---|---|---|---|---|---|---|
| LDH | 1.000 | ||||||
| NO | −0.891a | 1.000 | |||||
| ROS | 0.277 | −0.889a | 1.000 | ||||
| CD63 | −0.721a | −0.327 | −0.866a | 1.000 | |||
| Claudin-5 | −0.721a | −0.327 | −0.866a | 0.999b | 1.000 | ||
| Occludin | −0.721a | −0.327 | −0.866a | 0.999b | 1.000b | 1.000 | |
| ZO1 | −0.721a | −0.327 | −0.866a | 0.999b | 1.000b | 1.000b | 1.000 |
n=6.
P<0.05
P<0.01. LDH, lactate dehydrogenase′ NO, nitric oxide; ROS, reactive oxygen species; CD, cluster of differentiation; ZO1, tight junction protein 1.
Discussion
High Hcy levels induce neuronal apoptosis and BBB destruction via peroxidation, which eventually leads to stroke (18). A recent study demonstrated that TJs close the gap between cerebral vascular endothelial cells, are involved in the blood-brain barrier, transmit intracellular signals and maintain cell growth and differentiation, thereby protecting against neurological diseases (19).
Exosomes are an important intercellular mediator in post-stroke nerve repair and injury, with beneficial effects for patient recovery (20). Scu increases blood flow, dilates blood vessels and lowers blood prophylaxis (12). However, whether high Hcy levels damage the structure and function of the BBB via TJ destruction has not previously been reported. The aim of the present study was to clarify the protective mechanisms of Scu and Scu-treated exosomes on Hcy-induced RBMVECs.
The effects of Scu and Scu-treated exosomes on Hcy-induced RBMVECs were investigated via assessing the biological activity of cells following treatment with Hcy at different concentrations and for different intervals. An LDH detection kit was used to measure changes in LDH release rate. Oxidative stress-related injury and apoptosis increase cell membrane permeability, resulting in the release of LDH (21). As such, LDH activity in the cell culture medium is proportional to cell death (22). For normal RBMVECs, 5–200 µmol/l Scu was not observed to cause significant toxicity at 24 h. However, 2.5 mmol/l Hcy was observed to be toxic to normal RBMVECs, and so this concentration was selected for subsequent experiments. It was also demonstrated that 50–100 µmol/l Scu protected RBMVECs against Hcy-induced toxicity. Normal exosomes and Scu-treated exosomes (0–20%) produced no significant effects on 2.5 mmol/l Hcy-induced RBMVECs after 24 h. However, 40% Scu-treated exosomes had a significant protective effect on cells in the model group after 48 h. Scu-treated exosomes were collected from the cell culture medium following treatment, and the exosomes concentration likely did not reach a maximum. Therefore, a higher concentration (40%) of Scu-treated exosomes and a longer exposure duration (48 h) were required to produce significant pharmacological effects. The effects of Hcy on cell viability at different time points (24 and 48 h) were investigated using MTT and LDH assays. The results indicated that Hcy-induced damage was not time-dependent in RBMVECs. As such, a treatment duration of 48 h was selected to ensure consistency across groups. Scu and Scu-treated exosomes reduced Hcy-induced cell damage in the model group, with a greater efficacy observed in the Scu group compared with the SE group in four cell injury measurements.
Hcy produces ROS via self-oxidation, which causes peroxidation of the cell membrane. O2− formed through the oxidation process combines with NO and reduces the bioavailability of NO (23). ROS also oxidizes NOS and reduces its NO production capacity (24). Changes in endothelial NO biological activity may be an important marker of vascular endothelial damage (25). NO levels were significantly decreased in the model group, which suggests that Hcy decreased NO activity via superoxide activation and induced cerebral vascular endothelial injury. NO levels in the Scu and SE groups were significantly higher compared with the group, which indicated that Scu and Scu-treated exosomes exerted protective effects on cells in the model group. However, the efficacy of Scu was greater than that of Scu-treated exosomes. ROS levels were increased in the model group, confirming that Hcy causes cell damage. ROS levels in the Scu and SE groups were significantly lower compared with the model group, suggesting that RBMVECs in the Scu and SE groups suffered less Hcy-induced damage.
Exosome-mediated communication between cells is achieved via the transfer of biomolecules from a source cell to a target cell (26). CD63 is a lysosomal membrane glycoprotein that is a surface marker protein for exosomes (27). In the present study, CD63 expression was increased in the SE group compared with the Scu group, which in turn was higher compared with the CE group These results suggest that exosomes were enriched in the SE group and provided a certain resistance to the toxic effects of Hcy. These results suggest that Scu is able to stimulate cells in the model group to secrete exosomes. However, this experiment primarily measured the pharmacological effects of Scu.
It has previously been demonstrated that Scu and Scu-treated exosomes exhibit protective effects on Hcy-induced RBMVECs, suggesting that their protective mechanism reduces Hcy-mediated damage to TJ proteins in the BBB. ZO1 is located in the subcapsular part of the TJ of epithelial cells, which are interconnected via a variety of proteins (28). Occludin and claudin form the primary chain of the linear TJ structure (29). Occludin is directly involved in TJ formation in brain microvascular endothelial cells and its expression is associated with the degree of epithelial cell closure (30). Claudin serves a role in the formation of TJs, but is not specifically expressed in TJs (31). In the present study, claudin-5, occludin and ZO1 expression was significantly reduced in the model group, suggesting that Hcy damages TJ proteins in the BBB. Claudin-5, occludin and ZO1 expression increased significantly following the administration of Scu and its exosomes, with greater efficacy in the Scu group compared with the SE group. These results suggest that Scu and its exosomes protect cells by increasing the expression of TJ proteins in the BBB.
Correlation analyses were performed to clarify the associations between experimental indicators. LDH levels were significantly and negatively correlated with NO levels, which suggests that cell damage was associated with oxidative stress. LDH expression was also significantly and negatively correlated with claudin-5, occludin and ZO1 expression, indicating that cell injury and TJ proteins are closely related. ROS levels were negatively correlated with CD63 expression, which suggests that oxidative stress was associated with decreased lysosomal glycoprotein levels. Finally, CD63 expression was significantly and positively correlated with claudin-5, occludin and ZO1 expression. Together, these results suggest that an increase in lysosomal glycoprotein levels or in exosomes is associated with the integrity of TJ proteins.
In conclusion, Scu and Scu-treated exosomes increased NO levels as well as CD63, claudin-5, occludin and ZO1 expression in Hcy-induced RBMVECs, while LDH and ROS levels were reduced. Together, these effects reduce cell damage and protect the structure and function of the BBB. However, the present study was limited by the lack of accurate identification of exosomes and the lack of in-depth exosome study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China Postdoctoral Science Foundation (grant no. 2016M592513), the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (grant no. 20181114), Guangzhou Science and Technology Project (grant no. 201508020050) and the Guangzhou Science and Technology Project (grant no. 201604020003).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ and MD conceived the study, designed the experiments, analyzed the data and prepared the manuscript. MD and MZ were major contributors in writing the manuscript. XZ, CL, MD and MZ selected the subject and obtained samples for the present study. XZ, MD and MZ performed the experiments. All authors have read and approved the manuscript.
Ethics approval and consent to participate
The Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (Guangzhou, China) approved the study protocol and all participants provided written informed consent.
Patient consent for publication
All participants provided written informed consent for publication.
Competing interests
The authors declare no conflicts of interest.
References
- 1.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, et al. Rapid health transition in China, 1990–2010: Findings from the global burden of disease study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: A meta-analysis of prospective observational studies. Nutr Metab Cardiovasc Dis. 2014;24:1158–1165. doi: 10.1016/j.numecd.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y, Li J, Fu J, Huang X, Cheng X, et al. Homocysteine and stroke risk: Modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke. 2017;48:1183–1190. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]
- 5.Boldyrev AA. Molecular mechanisms of homocysteine toxicity. Biochemistry (Mosc) 2009;74:589–598. doi: 10.1134/S0006297909060017. [DOI] [PubMed] [Google Scholar]
- 6.Wallin A, Sjögren M, Edman A, Blennow K, Regland B. Symptoms, vascular risk factors and blood-brain barrier function in relation to CT white-matter changes in dementia. Eur Ncuro1. 2000;44:229–235. doi: 10.1159/000008242. [DOI] [PubMed] [Google Scholar]
- 7.Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocy-steine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1206–H1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Kim JM, Kim HJ, Lee I, Chang N. Folic acid supplementation can reduce the endothelial damage in rat brain microvasculature due to hyperhomocysteinemia. J Nutr. 2005;135:544–548. doi: 10.1093/jn/135.3.544. [DOI] [PubMed] [Google Scholar]
- 10.Tyagi SC, Lominadze D, Roberts AM. Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem Biophys. 2005;43:37–44. doi: 10.1385/CBB:43:1:037. [DOI] [PubMed] [Google Scholar]
- 11.Mo J, Yang R, Li F, Zhang X, He B, Zhang Y, Chen P, Shen Z. Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation. Phytomedicine. 2018;42:66–74. doi: 10.1016/j.phymed.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Fang M, Wu CY, Ling EA. Scutellarin as a potential therapeutic agent for microglia-mediated neuroinflammation in cerebral ischemia. Neuromolecular Med. 2016;18:264–273. doi: 10.1007/s12017-016-8394-x. [DOI] [PubMed] [Google Scholar]
- 13.Du X, Chen C, Zhang M, Cai D, Sun J, Yang J, Hu N, Ma C, Zhang L, Zhang J, Yang W. Scutellarin reduces endothelium dysfunction through the PKG-I pathway. Evid Based Complement Alternat Med. 2015;2015:430271. doi: 10.1155/2015/430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo LL, Guan ZZ, Huang Y, Wang YL, Shi JS. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol. 2013;65:579–584. doi: 10.1016/j.etp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I. Exosomes as reconfigurable therapeutic systems. Trends Mol Med. 2017;23:636–650. doi: 10.1016/j.molmed.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janas AM, Sapoń K, Janas T, Stowell MH, Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta. 2016;1858:1139–1151. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Lehotsky J, Petras M, Kovalska M, Tothova B, Drgova A, Kaplan P. Mechanisms involved in the ischemic tolerance in brain: Effect of the homocysteine. Cell Mol Neurobiol. 2015;35:7–15. doi: 10.1007/s10571-014-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CY, Fang M, Karthikeyan A, Yuan Y, Ling EA. Scutellarin attenuates microglia-mediated neuroinflammation and promotes astrogliosis in cerebral ischemia-a therapeutic consideration. Curr Med Chem. 2017;24:718–727. doi: 10.2174/0929867324666161118142045. [DOI] [PubMed] [Google Scholar]
- 20.Corrigan F, Mander KA, Leonard AV, Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation. 2016;13:264. doi: 10.1186/s12974-016-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, Yang LJ, Sun XB. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Ju B, Yan Y, Xu H, Wu S, Zhu D, Cao D, Hu J. Neuroprotective effects of phenylethanoid glycosides in an in vitro model of Alzheimer's disease. Exp Ther Med. 2017;13:2423–2428. doi: 10.3892/etm.2017.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JS, Bojovic D, Chen Y, Lindgren CA. Homocysteine sensitizes the mouse neuromuscular junction to oxidative stress by nitric oxide. Neuroreport. 2018;29:1030–1035. doi: 10.1097/WNR.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Cui L, Joseph J, Jiang B, Pimental D, Handy DE, Liao R, Loscalzoa J. Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol. 2012;52:753–760. doi: 10.1016/j.yjmcc.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sydow K, Hornig B, Arakawa N, Bode-Böger SM, Tsikas D, Münzel T, Böger RH. Endothclial dysfunction in patients with peripheral artcrial disease and chronic hyperhomocys-teinemia:potential role of ADMA. Vasc Med. 2004;9:93–101. doi: 10.1191/1358863x04vm538oa. [DOI] [PubMed] [Google Scholar]
- 26.Stremersch S, Vandenbroucke RE, Van Wonterghem E, Hendrix A, De Smedt SC, Raemdonck K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release. 2016;232:51–61. doi: 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HG, Grizzle WE. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44:130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li Q, Pan CS, Yan L, Hu BH, Liu YY, Yang L, Huang P, Zhao SY, Wang CS, et al. Bushen huoxue attenuates diabetes-induced cognitive impairment by improvement of cerebral microcirculation: Involvement of RhoA/ROCK/moesin and src signaling pathways. Front Physiol. 2018;9:527. doi: 10.3389/fphys.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.