Abstract
Previous studies have revealed that long intergenic non-coding RNA for kinase activation (LINK-A), a long non-coding RNA (lncRNA) promotes disease progression in triple-negative breast cancer by activating hypoxia-inducible factor 1α (HIF1α). However, the activation of HIF1α has also been demonstrated to improve diabetic nephropathy. It is therefore reasonable to expect that LINK-A may also participate in diabetic nephropathy. In the current study, the expression of LINK-A lncRNA and HIF1α was determined in renal biopsies of patients with diabetic nephropathy. LINK-A lncRNA and HIF1α expression levels were detected by reverse transcription quantitative (RT-q) PCR and ELISA in diabetic patients without complications and used as controls. Correlations between LINK-A lncRNA and HIF1α expression were analyzed using Pearson's correlation coefficient. Effects of lncRNA and HIF1α overexpression on LINK-A lncRNA expression, HIF1α expression and cell apoptosis were assessed using RT-qPCR, western blotting and a cell apoptosis assay. The results revealed that LINK-A lncRNA and HIF1α were downregulated in patients with diabetic nephropathy, as well as in diabetic patients without complications. The lowest expression of LINK-A lncRNA and HIF1α were observed in healthy controls. A positive correlation was identified between LINK-A lncRNA and HIF1α in both patients groups, but not in the control group. LINK-A lncRNA and HIF1α overexpression inhibited the apoptosis of mouse podocyte cells under a high glucose treatment. LINK-A lncRNA overexpression also promoted HIF1α expression in mouse podocyte cells, while HIF1α overexpression did not significantly affect LINK-A lncRNA expression. In conclusion, LINK-A lncRNA may activate HIF1α signaling resulting in the improvement of diabetic nephropathy treatment.
Keywords: diabetic nephropathy, long non-coding RNA, long intergenic non-coding RNA for kinase activation, hypoxia-inducible factor 1α, mouse podocyte cells, apoptosis
Introduction
Diabetes mellitus, or diabetes, is a common metabolic disorder affecting a considerable portion of the worldwide population (1). It is generally considered that the incidence of diabetes will be significantly increased in the future due to various factors, including the popularization of western-style diet structures and a sedentary lifestyle (2,3). Patients suffering from long-term diabetes usually develop complications in most major organs, an example being chronic renal failure (4,5). A previous clinical study demonstrated that >30% of patients with type 1 diabetes and 25% of patients with type 2 diabetes will develop nephropathy (6), which causes high mortality rates (7). At present, survival rates of those with the condition remain poor (8).
It has been reported that the activation of hypoxia-inducible factors (HIF) prevents diabetic nephropathy (9). In another study, it was suggested that chronic intermittent hypobaric hypoxia ameliorates rat models of diabetic nephropathy by activating HIF1 signaling (10). The current study suggests that HIF1 signaling may serve as a potential therapeutic target for diabetic nephropathy. It has been determined that HIF1 signaling participates in certain pathological changes by interacting with different long non-coding RNAs (lncRNAs) (11,12). A recent study reported LINK-A as a novel lncRNA that promotes triple negative breast cancer by interacting with HIF1α (13). Therefore, it is reasonable to hypothesize that LINK-A may also interact with HIF1α in diabetic nephropathy. The current study revealed that diabetic nephropathy may be improved via the LINK-A IncRNA mediated induction of HIF1α upregulation.
Materials and methods
Subjects and renal biopsies
A total of 102 diabetic patients with nephropathy and 466 diabetic patients without complications were treated from January 2014 to March 2018 at Kunming Medical University (Kunming, China). Form those patients, a total of 32 patients with diabetic nephropathy and 28 diabetic patients without obvious complications were enrolled in the current study. The inclusion criteria were as follows: i) Patients who had not been treated within 3 months prior to admission; ii) patients with normal major organ function except kidney function in patients with diabetic nephropathy and; iii) patients received renal biopsies to detect potential renal lesions or if renal lesions were excluded in diabetic patients without complications. The exclusion criteria were as follows: i) patients who suffered from other severe diseases or chronic diseases, (63 patients were excluded based on this parameter); ii) patients who could not fully understand experimental protocol, (6 patients were excluded based on this parameter). During the same time period (January 2014 to March 2018), 56 individuals without diabetes also received renal biopsies to confirm suspected renal lesions, the results of which revealed that renal lesions were not present in 16 of them. Among these 16 people, 14 were included in the control group and 2 cases were excluded due to individuals suffering from additional severe diseases, including one patient with Hepatitis B and another patient with heart disease. The current study received ethical approval from the Ethics Committee of Kunming Medical University (Kunming, China). Renal biopsies used within the experiment were obtained from the specimen library of Kunming Medical University (Kunming, China). All patients and their families provided their written informed consent. See Table I for basic data of 3 groups of participants.
Table I.
Basic data of the study groups.
| Sex | ||||
|---|---|---|---|---|
| Groups | Male, n | Female, n | Age range (years) | Average age (years) |
| Diabetic nephropathy | 17 | 15 | 23–67 | 45.9±5.2 |
| Diabetes | 15 | 13 | 25–69 | 46.4±6.7 |
| Control | 11 | 13 | 24–69 | 46.1±5.5 |
Cell culture and transfection
Mouse podocyte cells (PrimCells, LLC) were used to perform in vitro experiments. Cells were cultured in mouse podocyte primary cell culture complete medium with serum (Celprogen, Inc.) and maintained at 37°C in a 5% CO2-humidifed incubator. Full-length LINK-A lncRNA and HIF1α cDNAs were amplified via PCR using the following primer pairs: LINK-A forward, 5′-TGGAATTCAAGATGTGGGTGAG-3′ and reverse, 5′-AAGGATAATGCATTTTTATTTTAATTGAG-3′; HIF1α forward, 5′-AGTGCACAGTGCTGCCTCGTCTG-3′ and reverse, 5′-CCTGGTCCACAGAAGATGTTTA-3′. PCR amplification was performed using Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific, Inc.) using the following thermocycling conditions: Initial denaturation at 95°C for 1 min; 35 cycles at 95°C for 12 sec, 60°C for 12 sec and 70°C for 2 min. LINK-A lncRNA and HIF1α cDNA were cloned into a pEGFPC3 vector (Shanghai GenePharma Co., Ltd.) to generate LINK-A lncRNA and HIF1α expression vectors. LINK-A lncRNA and HIF1α expression vectors were used to transfect mouse podocyte cells using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.). Untransfected mouse podocyte cells were used as controls. Transfection with empty vector was used as a negative control. Following 24-h transfection, cells were collected and LINK-A lncRNA and HIF1α overexpression was confirmed by reverse transcription-quantitative (RTq)PCR.
RT-qPCR
Total RNA was extracted from renal biopsies and cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using the Reverse Transcriptase AMV kit (Sigma-Aldrich; Merck KGaA). qPCR was subsequently performed using the SuperScript III Platinum SYBR Green One-Step qPCR kit (Thermo Fisher Scientific Inc.). The following primer pairs were used for the qPCR: LINK-A IncRNA forward, 5′-TTCCCCCATTTTTCCTTTTC-3′ and reverse, 5′-CTCTGGTTGGGTGACTGGTT-3′; β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5-AGTACTTGCGCTCAGGAGG-3′. The following thermocycling conditions were as follows: Initial denaturation at 95°C for 56 sec; 40 cycles of 95°C for 12 sec and 57.6°C for 40 sec. Data was quantified using the 2−ΔΔCq method and normalized to the internal reference gene β-actin (14).
ELISA
Renal biopsies were used to measure levels of HIF1α using an ELISA kit (cat. no. EHIF1A; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Levels of HIF1α were normalized to ng/g.
Cell apoptosis assay
Cell apoptosis under 20 mM D-glucose treatments were detected via a cell apoptosis assay. Mouse podocyte cell suspensions (5×104 cells/ml) were prepared in mouse podocyte cell culture complete medium with serum (Celprogen, Inc.). Cell suspensions (10 ml) were added to each well of a six-well plate, followed by the addition of 20 mM D-glucose (Sangon Biotech Co., Ltd.). Cells were cultured for 24 h at 37°C. Cells were digested using 0.25% trypsin, collected and mixed with Mouse Podocyte Cell Culture Complete medium with serum. Following centrifugation at 1,200 × g for 3 min at 22°C, cells were stained using Annexin V-FITC (Dojindo Molecular Technologies, Inc.) and propidium iodide at 22°C for 18 min. Apoptotic cells were detected using a CytoFLEX LX flow cytometer (Beckman Coulter, Inc.) and analyzed using FCSalyzer-0.9.15 (sourceforge.net/projects/fcsalyzer/).
Western blotting
Protein was extracted using RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, Inc.) and protein concentration was measured using a BCA assay. After denaturing at 95°C for 10 min, 20 µg protein was subjected to 12% SDS-PAGE gel electrophoresis. Following gel transfer to PVDF membranes, 5% skimmed milk was used to block the membranes at room temperature for 1 h. Membranes were then incubated with rabbit anti-human HIF1α (1:1,650; cat. no. ab2185; Abcam) and rabbit anti-human GAPDH (1:1,400; cat. no. ab8255; Abcam) primary antibodies overnight at 4°C. Membranes were incubated the next day with IgG-horseradish peroxide conjugated secondary antibodies (goat anti-rabbit; 1:1,500; MBS435036; MyBioSource, Inc.) at room temperature for 1 h. Signals were subsequently developed by ECL (Sigma-Aldrich; Merck KGaA). Data normalization was performed using ImageJ software (v.1.60; National Institutes of Health).
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, Inc.) was used for all statistical analyses. Data were expressed as the mean ± standard deviation and analyzed using one-way analysis of variance followed by a least significant difference post-hoc test. The correlation between LINK-A lncRNA and HIF1α expression was analyzed using Pearson's correlation coefficient. Diagnostic values of LINK-A lncRNA for diabetic nephropathy was evaluated by receiver operating characteristic (ROC) curve analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
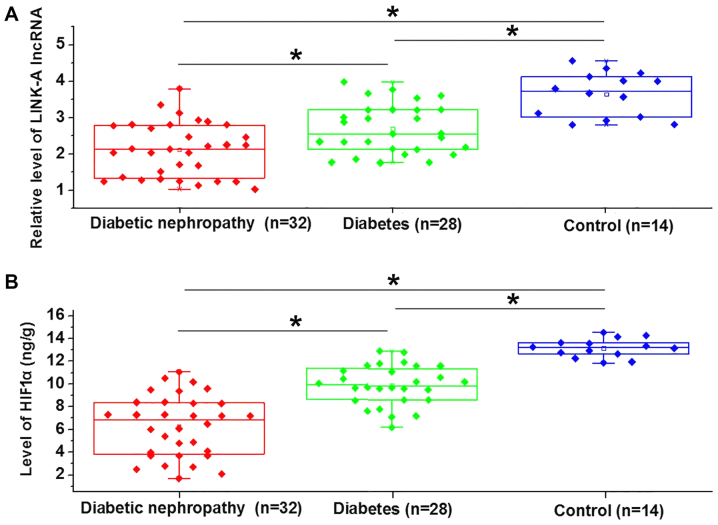
Expression of LINK-A lncRNA and HIF1α are downregulated in patients with diabetic nephropathy
LINK-A lncRNA (Fig. 1A) and HIF1α (Fig. 1B) were significantly downregulated in patients with diabetic nephropathy, with diabetic patients without complications exhibiting an intermediate expression and healthy controls exhibiting the lowest expression. This indicates that the inhibition of LINK-A lncRNA and HIF1α may contribute to diabetic nephropathy.
Figure 1.
Expression of LINK-A lncRNA and HIF1α were elevated in patients with diabetic nephropathy. Comparison of (A) LINK-A lncRNA and (B) HIF1α expression in 3 groups of participants. *P<0.05. HIF1α, hypoxia-inducible factor 1α; lncRNA, long non-coding RNA; LINK-A, long intergenic non-coding RNA for kinase activation.
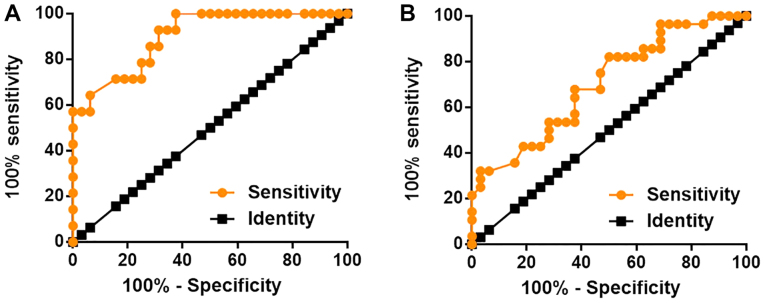
Upregulation of LINK-A lncRNA distinguished patients with diabetic nephropathy from diabetic patients without complications as well as from healthy controls
The diagnostic value of LINK-A lncRNA for diabetic nephropathy was analyzed via ROC curve analysis. With controls as references, the area under the curve (AUC) value was 0.9007 (standard error; 0.04579; 95% confidence interval; 0.8109–0.9904; Fig. 2A). With use of diabetic patients with no complications as references, the AUC was 0.7031 (standard error; 0.06672; 95% confidence interval; 0.5723–0.8339; Fig. 2B). These data may therefore indicate that LINK-A lncRNA may serve as a promising diagnostic biomarker for diabetic nephropathy.
Figure 2.
Upregulation of LINK-A lncRNA distinguished patients with diabetic nephropathy from diabetic patients without complications and healthy controls. Receiver operating characteristic curve analysis of the diagnostic value of LINK-A lncRNA for patients with diabetic nephropathy, (A) patients with diabetes but without complications and (B) with healthy controls as references. LINK-A IncRNA, LINK-A long non-coding RNA; LINK-A, long intergenic non-coding RNA for kinase activation.
Expression of LINK-A lncRNA and HIF1α are positively correlated in the 2 patient groups, but not in the control group
As presented in Fig. 3, Pearson's correlation coefficient revealed that the expression of LINK-A lncRNA and HIF1α were positively correlated in patients with diabetic nephropathy (Fig. 3A) and in patients without complications (Fig. 3B) but not in the control group (Fig. 3C). Additionally, the positive correlation between LINK-A lncRNA and HIF1α was stronger in patients with diabetic nephropathy than in diabetic patients without complications. These data indicate that LINK-A lncRNA and HIF1α may interact in diabetic nephropathy and in diabetes.
Figure 3.
Expression of LINK-A lncRNA and HIF1α were positively correlated in 2 patients groups but not in the control group. Pearson's correlation coefficient analysis revealed a significantly positive correlation in (A) patients with diabetic nephropathy, (B) patients with diabetes but without complications and (C) healthy controls. HIF1α, hypoxia-inducible factor 1α; lncRNA, long non-coding RNA; LINK-A, long intergenic non-coding RNA for kinase activation.
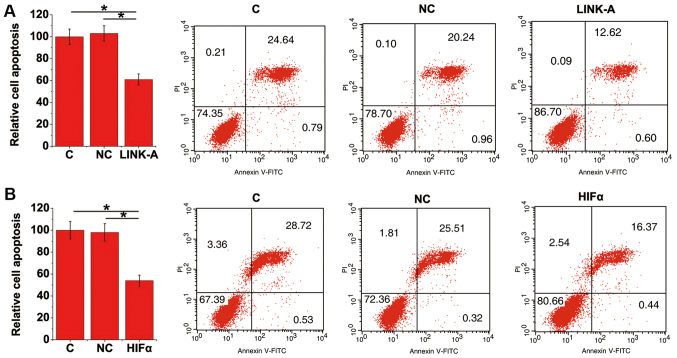
LINK-A lncRNA and HIF1α overexpression inhibits apoptosis of mouse podocyte cells under high glucose treatment
Cell apoptosis under 20 mM D-glucose treatment was detected via a cell apoptosis assay. Compared with control and negative control cells with LINK-A lncRNA overexpression (Fig. 4A) and HIF1α (Fig. 4B) exhibited significant inhibition of cell apoptosis. The overexpression of LINK-A lncRNA and HIF1α may therefore serve as a potential therapeutic target for diabetic nephropathy.
Figure 4.
LINK-A lncRNA and HIF1α overexpression inhibited the apoptosis of mouse podocyte cells under a high glucose treatment. Data of the cell apoptosis assay revealed that (A) LINK-A lncRNA and (B) HIF1α overexpression inhibited apoptosis of mouse podocyte cells under a high glucose treatment. *P<0.05. lncRNA, long non-coding RNA; HIF1α, hypoxia-inducible factor 1α; C, Control; NC, negative control; LINK-A, long intergenic non-coding RNA for kinase activation.
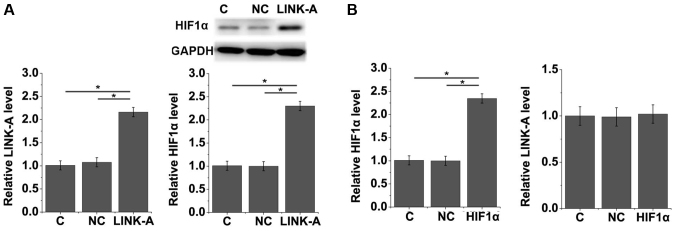
LINK-A lncRNA activates HIF1α in mouse podocyte cells
To further investigate the interactions between LINK-A lncRNA and HIF1α, LINK-A lncRNA and HIF1α expression vectors were transfected into mouse podocyte cells. The expression of LINK-A lncRNA and HIF1α was detected by RT-qPCR and Western blotting. As presented in Fig. 5A, compared with the control and negative control cells with LINK-A lncRNA overexpression exhibited significantly upregulated HIF1α expressions. In contrast, the overexpression of HIF1α did not significantly affect LINK-A lncRNA expression (Fig. 5B). Therefore, the results indicate that LINK-A lncRNA may be an upstream activator of HIF1α in mouse podocyte cells.
Figure 5.
LINK-A lncRNA activates HIF1α in mouse podocyte cells. The effect of LINK-A lncRNA overexpression on (A) HIF1α expression and HIF1α overexpression on (B) LINK-A lncRNA expression in mouse podocyte cells was assessed. *P<0.05. lncRNA, long non-coding RNA; HIF1α, hypoxia-inducible factor 1α; C, Control; NC, negative control; LINK-A, long intergenic non-coding RNA for kinase activation.
Discussion
Human materials were used in the current study to assess the role of LINK-A lncRNA in diabetic nephropathy. The results of the present study indicated that LINK-A, as a recently identified lncRNA, may participate in diabetic nephropathy by interacting with HIF1α. Data from the present study also demonstrated that LINK-A may be a potential therapeutic target for the treatment of diabetic nephropathy.
The development of diabetic nephropathy can affect the expression pattern of a large set of lncRNAs (15). Certain lncRNAs, which serve critical roles in diabetic nephropathy, are considered to be potential therapeutic targets. The present study observed the lowest expression of LINK-A lncRNA in patients with diabetic nephropathy. However, compared with the control group, significant downregulation of LINK-A lncRNA was also observed in diabetic patients without complications. Therefore, the downregulation of LINK-A lncRNA may occur in diabetes and further downregulation may be accompanied by the development of nephropathy among patients with diabetes.
The present study indicated that the downregulation of LINK-A may be used to distinguish patients with diabetic nephropathy from healthy controls and patients with diabetes but without obvious complications. These data indicate that LINK-A may serve as a potential diagnostic biomarker for the diagnosis of diabetic nephropathy. LINK-A, as a recently identified lncRNA, has an unknown expression in triple negative breast cancer (15). The expression of LINK-A may therefore be affected by other human diseases. Multiple diagnostic markers should thus be used to improve diagnostic specificity.
The interaction between LINK-A and HIF1α has been revealed to occur in triple negative breast cancer (16). The current study also observed a positive correlation between LINK-A and HIF1α in patients with diabetes but without complications and patients with diabetic nephropathy. Mouse podocyte cells were used to further confirm the interaction between LINK-A and HIF1α in the current study. Podocyte cells are critical for renal function and the number of podocyte cells have been widely used as biomarkers for renal function (17). Podocyte cell injuries are common in patients with diabetes and patients with diabetic nephropathy (18,19). In addition, podocyte cells are considered to be therapeutic targets for diabetic nephropathy (20). In the current study, the results indicated that LINK-A may be an upstream activator of HIF1α in podocyte cells. No significant correlation between LINK-A and HIF1α was observed in the patient control group, while LINK-A overexpression led to the significant upregulation of HIF1α in podocyte cells. These data suggest that overexpression techniques may not be able to fully represent the interactions between LINK-A and HIF1α in the human body.
The present study only performed in vitro experiments. The in vivo functions of LINK-A and HIF1α in diabetic nephropathy are therefore unclear and future studies should be performed with animal models to support the conclusions drawn from the current study.
LINK-A overexpression inhibited the apoptosis of mouse podocyte cells under high glucose treatment in the current study. Therefore, LINK-A overexpression may serve as a therapeutic target for diabetic nephropathy. The present study is limited by the small sample size due to the difficulties in collecting renal biopsies. In the future, greater sample sizes should be used to further support results.
In conclusion, the current study revealed that LINK-A and HIF1α were upregulated in patients with diabetic nephropathy. LINK-A may also upregulate HIF1α to improve diabetic nephropathy and therefore may be a useful therapeutic target for potential treatments and therapies.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JY and LL designed the study and performed all of the experiments. SH, ZZ and WF analyzed data. LL interpreted data and prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Kunming Medical University (Kunming, China). All patients and healthy volunteers provided written informed consent prior to their inclusion within the study.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Kunming Medical University (Kunming, China). All patients and healthy volunteers provided written informed consent prior to their inclusion within the study.
Patient consent for publication
All patients provided informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 12(10) 2016:616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 2.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowley WR, Bezold C. Creating public awareness: State 2025 diabetes forecasts. Popul Health Manag. 2012;15:194–200. doi: 10.1089/pop.2011.0053. [DOI] [PubMed] [Google Scholar]
- 4.Ahlqvist E, Van Zuydam NR, Groop LC, McCarthy MI. The genetics of diabetic complications. Nat Rev Nephrol. 2015;11:277–287. doi: 10.1038/nrneph.2015.37. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 37(Supplement 1) 2014:S14–S80. doi: 10.2337/dc14-S014. [DOI] [Google Scholar]
- 6.Navarro-González JF, Jarque A, Muros M, Mora C, García J. Tumor necrosis factor-alpha as a therapeutic target for diabetic nephropathy. Cytokine Growth Factor Rev. 2009;20:165–173. doi: 10.1016/j.cytogfr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Lei L, Mao Y, Meng D, Zhang X, Cui L, Huo Y, Wang Y. Percentage of circulating CD8+ T lymphocytes is associated with albuminuria in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:27–30. doi: 10.1055/s-0033-1358666. [DOI] [PubMed] [Google Scholar]
- 8.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 9.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian YM, Guan Y, Li N, Ma HJ, Zhang L, Wang S, Zhang Y. Chronic intermittent hypobaric hypoxia ameliorates diabetic nephropathy through enhancing HIF1 signaling in rats. Diabetes Res Clin Pract. 2016;118:90–97. doi: 10.1016/j.diabres.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Hong Q, Li O, Zheng W, Xiao WZ, Zhang L, Wu D, Cai GY, He JC, Chen XM. LncRNA HOTAIR regulates HIF-1α/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis. 2017;8:e2772. doi: 10.1038/cddis.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Xiang S, Gu H, Jin L, Thorne RF, Zhang XD, Wu M. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci U S A. 2018;115:E1465–E1474. doi: 10.1073/pnas.1711257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin A, Li C, Xing Z, Hu Q, Liang K, Han L, Wang C, Hawke DH, Wang S, Zhang Y, et al. The LINK-A lncRNA activates normoxic HIF1α signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Mothods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Dong C, Qian X, Huang S, Feng Y, Ye X, Miao H, You Q, Lu Y, Ding D. Microarray analysis of long noncoding RNA expression patterns in diabetic nephropathy. J Diabetes Complications. 2017;31:569–576. doi: 10.1016/j.jdiacomp.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Long J, Danesh FR. Values and limitations of targeting lncRNAs in diabetic nephropathy. Diabetes. 2018;67:552–553. doi: 10.2337/dbi17-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Fufaa GD, Weil EJ, Lemley KV, Knowler WC, Brosius FC 3rd, Yee B, Mauer M, Nelson RG. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol. 2016;11:254–261. doi: 10.2215/CJN.05760515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6:3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015;26:1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.