Abstract
Epilepsy is the most common chronic neurological disorder, affecting ~70 million individuals worldwide. However, approximately one-third of the patients are refractory to epilepsy medication. Of note, 100% of patients with genetic epilepsy who are resistant to the traditional drug, valproic acid (VPA), are also refractory to the other anti-epileptic drugs. The aim of the present study was to compare the transcriptomes in VPA responders and non-responders, to explore the mechanism of action of VPA and identify possible biomarkers to predict VPA resistance. Thus, RNA-seq was employed for transcriptomic analysis, differentially expressed genes (DEGs) were analyzed using Cuffdiff software and the DAVID database was used to infer the functions of the DEGs. A protein-protein interaction network was obtained using STRING and visualized with Cytoscape. A total of 389 DEGs between VPA-responsive and non-responsive pediatric patients were identified. Of these genes, 227 were upregulated and 162 were downregulated. The upregulated DEGs were largely associated with cytokines, chemokines and chemokine receptor-binding factors, whereas the downregulated DEGs were associated with cation channels, iron ion binding proteins, and immunoglobulin E receptors. In the pathway analysis, the toll-like receptor signaling pathway, pathways in cancer, and cytokine-cytokine receptor interaction were mostly enriched by the DEGs. Furthermore, three modules were identified by protein-protein interaction analysis, and the potential hub genes, chemokine (C-C motif) ligand 3 and 4, chemokine (C-X-C motif) ligand 9, tumor necrosis factor-α and interleukin-1β, which are known to be closely associated with epilepsy, were identified. These specific chemokines may participate in processes associated with VPA resistance and may be potential biomarkers for monitoring the efficacy of VPA.
Keywords: RNA-seq, valproic acid, efficacy, biomarkers
Introduction
Epilepsy is the most common chronic neurological disorders, affecting ~70 million individuals worldwide (1). Furthermore, ~0.5–1% of the pediatric population suffer from epilepsy (2) and approximately one-third of these patients are refractory to epilepsy medication (3). Valproic acid (VPA) is an anti-epileptic drug recommended by the National Institute for Health and Care Excellence guidelines as the first-line therapy for absence epilepsy (4,5), and has been used for 50 years due to its efficacy and high tolerability (6,7). Previous studies have indicated that approximately one-third of patients are non-responsive to VPA (8,9), and the reason for this phenomenon remains elusive. Recently, Gesche et al (10) have demonstrated that resistance to VPA has a specificity of 100% regarding the identification of genetic generalized epileptic patients. Consequently, it is important to elucidate the mechanism underlying VPA efficacy and identify biomarkers predictive of VPA responses.
At present, two hypotheses for pharmaco-resistant epilepsy are commonly accepted, namely the multi-transporter hypothesis and the drug targets hypothesis. However, the mechanism of VPA resistance is distinct from that of other anti-epileptic drugs (AEDs). In fact, VPA is neither the substrate of multi-transporters, including P-glycoprotein, multi-drug resistant protein and breast cancer resistance protein, nor does it induce the expression of the multi-transporters in the brain (11–14). However, reported targets of VPA, including γ-aminobutyric acid receptor (15,16), sodium channels and calcium channels, appear to not be involved in VPA resistance (17–19). These results suggest that the aforementioned hypotheses hardly explain the mechanisms of VPA resistance or efficacy.
Genome-wide gene expression profiling has been increasingly used to investigate pathogenetic mechanism and identify potential biomarkers for various human diseases (20,21). RNA sequencing (RNA-seq) is a widely used method to study overall transcriptional activity and has a broad coverage. Previous studies employing the RNA-seq method led to the discovery of potential biomarkers for Alzheimer's disease and malignant glioma, including NeuroD6 and F11R, in brain tissues (22,23). Fibronectin 1 and 12 other genes implicated in oxidative phosphorylation and glycolysis/gluconeogenesis pathways in the brain were identified as candidate critical factors for temporal lobe epilepsy, with and without hippocampal sclerosis (24,25). However, few studies have addressed VPA efficacy and resistance, also due to the limited accessibility of brain tissue from VPA-treated patients.
Peripheral blood may be obtained non-invasively and is commonly used for studying biomarkers. Liew et al (26) have indicated that ~81.9% of the genes expressed in the brain were also expressed in the whole-blood microarray dataset. Borovecki et al (27) reported that the blood mRNA levels of specific target genes are associated with Huntington's disease severity and response to a histone deacetylase inhibitor. VPA is a histone deacetylase inhibitor (28,29), potentially affecting, directly or indirectly, between 2 and 5% of all genes (30). A previous study reported that 11 genes were differentially expressed after a 3-month VPA treatment (31). In the present study, the mRNA expression profile in the blood of VPA responders and non-responders was analyzed after a treatment period of ≥1 year, to identify possible biomarkers for the prediction of VPA efficacy.
Materials and methods
Patients
Subjects aged from 0 to 18 years were enrolled at the Children's Hospital of Fudan University (Shanghai, China) between July 2016 and May 2018. Each patient was evaluated according to the inclusion and exclusion criteria (32). The inclusion criteria were as follows: Pediatric patients diagnosed with epilepsy or an epileptic syndrome and administration of VPA for at least one year. The exclusion criteria were as follows: Patients with abnormal liver and kidney function, and patients who had developed infectious diseases, including upper respiratory infection and urinary tract infection, in the last three months.
Patients with a complete disappearance of seizures and a normal electroencephalogram were considered as VPA responders, while patients who continued to experience seizures were assigned to the non-responsive groups. Seizure types were identified according to the International League Against Epilepsy definition (33). Focal epileptic seizures were defined as events originating within networks limited to one hemisphere. Generalized epileptic seizures were conceptualized as originating at a certain point within, and rapidly engaging, bilaterally distributed networks (34).
RNA preparation
Blood from three VPA responders and five non-responders without seizure for 12 h was collected in PAXgene blood RNA tubes and stored at −80°C until use. PAXgene tubes were stabilized for 2 h at room temperature. After centrifugation for 10 min at 3,000–5,000 × g at 4°C by using a swing-out rotor, the supernatant was removed by pipetting. Subsequently, 4 ml of RNase-free water were added to the pellet. Total RNA was extracted according to the instructions of the PAXgene Blood RNA kit (Qiagen), and the quality and quantity of total RNA were determined using a Qubit 2.0 (Thermo Fisher Scientific, Inc.) and a Bioanalyzer 2100 (Agilent Technologies).
RNA-seq
Transcriptome sequencing libraries were prepared by using the TruSeq RNA LT V2 Sample Prep lit (Illumina, Inc.) and were qualified using the Qubit 2.0. Paired-end sequencing for 150 base pair was performed by an Illumina HiSeq 2500 instrument (Illumina, Inc.). All of the paired-end raw reads were quality-checked for low-quality bases and adapter sequences.
Analysis of differentially expressed genes (DEGs)
Low-quality fractions and Illumina universal adapters were deleted by using Trim Galore v0.4.2 with the threshold of Q<30. Fasta QC (version 0.11.5; Illumina, Inc.) was employed to assess the quality of the data. The paired-end sequencing reads were aligned to the reference genome (hg19), downloaded from the University of California Santa Cruz (UCSC) website (http://hgdownload.soe.ucsc.edu/downloads.html). Statistically significant expression changes between responders and non-responders were estimated using the Cuffilinks 2.2.1 software (http://cole-trapnell-lab.github.io/cufflinks/install/) with a threshold of 10 for NO TEST. Student's t-test was performed to calculate the P-value, while the false discovery rate controlled by the Benjamini-Hochberg procedure was used for determining the Q-value. Genes with a fold change of >1 and P<0.05 were defined as DEGs.
Validation by reverse transcription-quantitative (RT-qPCR)
Changes in the mRNA expression of specific DEGs associated with epilepsy [chemokine (C-C motif) ligand 3 and FOS], exhibiting high fold changes, were validated by qPCR in 17 samples (including 6 VPA responders and 11 non-responders). Specific primers for selected genes were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast). All samples were amplified in triplicate. qPCR amplifications were performed with the following cycling parameters: An initial hot start at 95°C for 5 min followed by 45 cycles of 95°C for 15 sec and 60°C for 30 sec. In order to normalize the qPCR results, GAPDH was included as the reference gene. The relative expression of genes was calculated based on the average quantification cycle (Cq) values across samples. Relative expression=2{-[(Cq gene of interest-Cq GAPDH of interest) non-responders]-(Cq gene of interest-Cq GAPDH of interest) responders}} (35).
Gene functions and pathways
Gene Ontology (GO) gene functions and biochemical pathways enriched by the DEGs were determined by using the web-based annotation tool DAVID v6.7 (https://david-d.ncifcrf.gov/summary.jsp) (36), providing GO terms in the categories biological process (BP), cellular component (CC), and molecular function (MF) and Kyoto Encyclopedia of Genes and Genomes pathways. P<0.05 was used as the significance threshold.
Construction and visualization of the protein-protein interaction (PPI) network
The PPI network based on the DEGs identified were constructed by using the STRING database (https://string-db.org), a pre-computed database wherein associations between proteins are assigned on the basis of high-throughput experiments, literature mining, gene fusion, co-occurrence, co-expression analysis, and also computational predictions, e.g. genomic meta-analysis. Interactions with a confidence score of 0.7 were considered for visualization by Cytoscape v3.4.0 (https://cytoscape.org/).
Network module analysis
The Molecular Complex Deletion (MCODE) plugin for Cytoscape was used to analyze the network modules (37). Densely connected regions or clusters in the co-expression network were identified using the following parameters: Degree cut-off=2, k-core=2 and max. depth=100.
Statistical analysis
DEGs analysis was performed by using Cuffilinks 2.2.1 software (cuffdiff, ttp://cole-trapnell-lab.github.io/cufflinks/install/). RT-qPCR data were analyzed using GraphPad Prism version 7.0 software (GraphPad, Inc.). Values were expressed as the mean ± standard error. Student's t-test was performed to analyze differences between VPA-responders and non-responders.
Results
Demographic data
A total of 8 pediatric patients with epilepsy were recruited, of which 3 were responders, while 5 were non-responders. All responders were males, while 3 of the non-responders were males and 2 females. The average age of the responders was 7.0±2.2 years and was not significantly different from that of the non-responsive group (4.8±1.8 years, P=0.47). Furthermore, no significant difference in the plasma VPA concentration was identified between the responsive and non-responsive groups (107.2±17.7 vs. 80.4±17.4, P=0.35; Table I).
Table I.
Demographic data.
| Demographics | Responders | Non-responders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | 1 | 2 | 3 | Median (min, max) | 1 | 2 | 3 | 4 | 5 | Median (min, max) | P-value |
| Age (Years) | 10.7 | 3 | 7.4 | 7.4 (3, 10.7) | 9.6 | 4.8 | 8 | 0.3 | 1.3 | 4.8 (0.3, 9.6) | 0.47 |
| Sex | Male | Male | Male | – | Male | Female | Male | Female | Male | – | – |
| Seizure type | Unknown | Generalized | Generalized | – | Focal | Generalized | Generalized | Generalized | Generalized | – | – |
| AEDs | VPA+TPM | VPA | VPA | – | VPA | VPA+LTG+OXC | VPA | VPA | VPA+LEV | – | – |
| Dose of VPA(mg/kg) | 33.3 | 21.5 | 18.1 | 21.5 | 29.7 | 28.6 | 31.2 | 29.1 | 36.4 | 29.7 | 0.13 |
| (18.1, 33.3) | (28.6, 36.4) | ||||||||||
| Plasma concentration | 139.1 | 77.9 | 104.6 | 104.6 | 56.7 | 62.0 | 148.2 | 57.3 | 77.7 | 62 (56.7, 148.2) | 0.35 |
| (µg/ml) | (77.9, 139.1) | ||||||||||
AEDs, antiepileptic drugs; TPM, topiramate; LTG, lamotrigine; OXC, oxcarbazepine; LEV, levetiracetam; VPA, valproic acid.
Comparative transcriptome profiling
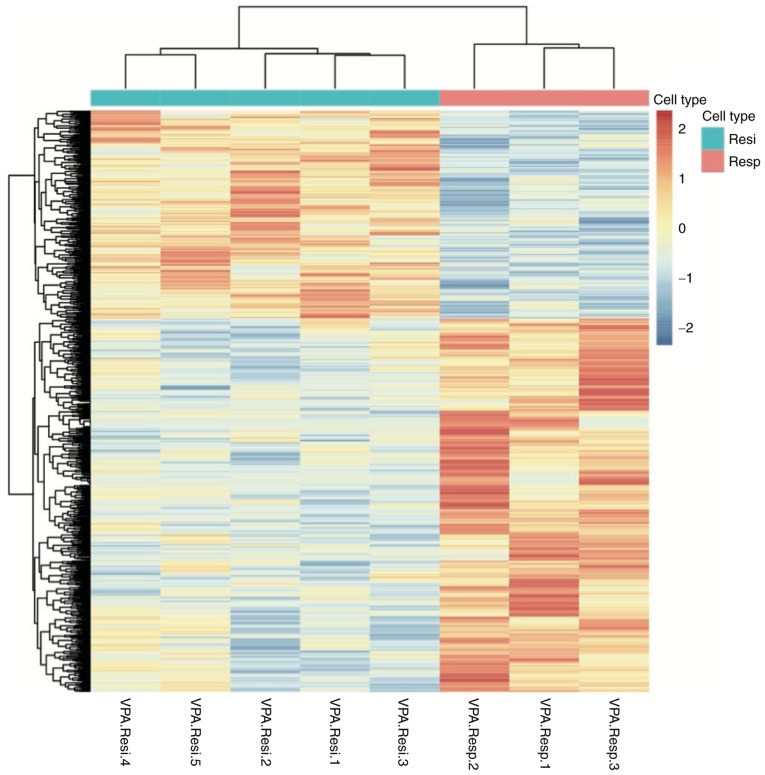
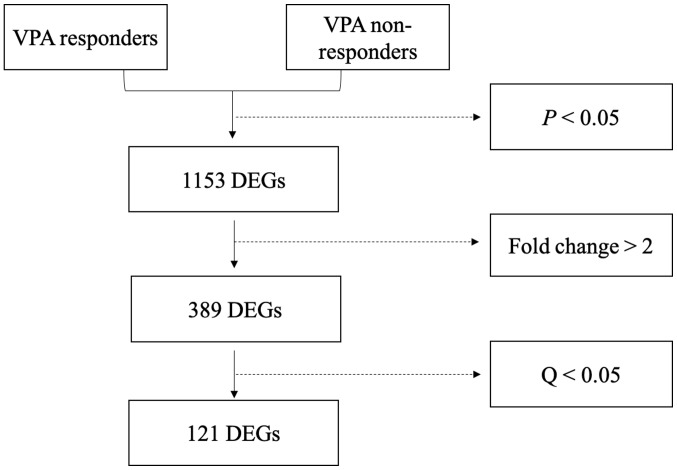
A total of 1,153 genes were differentially expressed between responders and non-responders (P<0.05; Fig. 1). Of these DEGs, 389 had a |Log 2 fold change|≥1 and comprised of 227 upregulated and 162 downregulated genes. Among the 389 DEGs, 121 variations had a Q-value of <0.05 and included 84 upregulated and 37 downregulated genes (Fig. 2). The 20 most significantly upregulated and downregulated genes are listed in Tables II and III, respectively.
Figure 1.
Heat map of differentially expressed genes between VPA responsive and non-responsive patients. The samples were clustered according to the VPA response. Blue and red color represent low and high levels of expression, respectively. VPA, valproic acid; Resp., responsive; Resi, resistant.
Figure 2.
Flow chart for the identification of differentially expressed genes. In total, 1,153 genes exhibited variations with P<0.05, and 389 of these had a fold change of >2. After P-value adjustment, 121 genes exhibited Q<0.05. VPA, valproic acid; DEGs, differentially expressed genes.
Table II.
Top 20 upregulated differentially expressed genes.
| Gene | Definition | Log2 fold change | P-value | Q-value |
|---|---|---|---|---|
| TSIX | TSIX transcript, XIST antisense RNA | 7.59 | 5.00×10−5 | 9.00×10−3 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | 5.99 | 5.00×10−5 | 9.00×10−3 |
| LILRA3 | Leukocyte immunoglobulin-like receptor subfamily a (without tm domain) member 3 | 5.96 | 5.00×10−5 | 9.00×10−3 |
| FN1 | Fibronectin 1 | 3.47 | 5.00×10−5 | 9.00×10−3 |
| GPR84 | G protein-coupled receptor 84 | 3.25 | 5.00×10−5 | 9.00×10−3 |
| PDK4 | Pyruvate dehydrogenase kinase, isozyme 4 | 2.99 | 5.00×10−5 | 9.00×10−3 |
| SEMA6B | Sema domain. Transmembrane domain (tm) and cytoplasmic domain (semaphorin) 6b | 2.74 | 5.00×10−5 | 9.00×10−3 |
| HLA-DRB5 | Major histocompatibility complex class ii dr β 5 | 2.73 | 5.00×10−5 | 9.00×10−3 |
| PTGES | Prostaglandin E synthase | 2.73 | 5.00×10−5 | 9.00×10−3 |
| MYOM2 | Myomesin 2 | 2.68 | 5.00×10−5 | 9.00×10−3 |
| CCL3 | Chemokine (C-C motif) ligand 3 | 2.60 | 5.00×10−5 | 9.00×10−3 |
| IL1B | Interleukin 1β | 2.55 | 5.00×10−5 | 9.00×10−3 |
| IFI27 | Interferon α-inducible protein 27 | 2.32 | 5.00×10−5 | 9.00×10−3 |
| HJURP | Holliday junction recognition protein | 2.17 | 5.00×10−5 | 9.00×10−3 |
| RRM2 | Ribonucleotide reductase M2 | 2.06 | 5.00×10−5 | 9.00×10−3 |
| CDCA5 | Cell division cycle associated 5 | 2.04 | 5.00×10−5 | 9.00×10−3 |
| FOLR3 | Folate receptor 3 (γ) | 1.97 | 5.00×10−5 | 9.00×10−3 |
| TNF | Tumor necrosis factor | 1.89 | 5.00×10−5 | 9.00×10−3 |
| PLK1 | Polo-like kinase 1 | 1.83 | 5.00×10−5 | 9.00×10−3 |
| SEC14L2 | SEC14-like 2 (S. cerevisiae) | 1.79 | 5.00×10−5 | 9.00×10−3 |
Table III.
Top 20 downregulated differentially expressed genes.
| Gene | Definition | Log2 fold change | P-value | Q-value |
|---|---|---|---|---|
| ARHGEF10 | Rho guanine nucleotide exchange factor 10 | −3.33 | 5.00×10−5 | 9.00×10−3 |
| KCNG1 | Potassium voltage-gated channel subfamily g member 1 | −2.63 | 5.00×10−5 | 9.00×10−3 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | −2.20 | 5.00×10−5 | 9.00×10−3 |
| PAQR8 | Progestin and adipoq receptor family member VIII | −2.06 | 5.00×10−5 | 9.00×10−3 |
| C21orf15 | Chromosome 21 open reading frame 15 | −2.06 | 5.00×10−5 | 9.00×10−3 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | −1.72 | 5.00×10−5 | 9.00×10−3 |
| HCAR2 | Hydroxycarboxylic acid receptor 2 | −1.71 | 5.00×10−5 | 9.00×10−3 |
| IL8 | Interleukin 8 | −1.67 | 5.00×10−5 | 9.00×10−3 |
| TGFA | Transforming growth factor α | −1.57 | 5.00×10−5 | 9.00×10−3 |
| HLA-DQA2 | Major histocompatibility complex class II DQ α 2 | −1.49 | 5.00×10−5 | 9.00×10−3 |
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | −1.47 | 5.00×10−5 | 9.00×10−3 |
| TNFRSF10C | Tumor necrosis factor receptor superfamily member 10c decoy without an intracellular domain | −1.46 | 5.00×10−5 | 9.00×10−3 |
| DUSP1 | Dual specificity phosphatase 1 | −1.43 | 5.00×10−5 | 9.00×10−3 |
| RTN1 | Reticulon 1 | −1.40 | 5.00×10−5 | 9.00×10−3 |
| TLR10 | Toll-like receptor 10 | −1.25 | 5.00×10−5 | 9.00×10−3 |
| KCNE3 | Potassium voltage-gated channel Isk-related family member 3 | −1.20 | 5.00×10−5 | 9.00×10−3 |
| THBD | Thrombomodulin | −1.19 | 5.00×10−5 | 9.00×10−3 |
| MYBL1 | v-myb myeloblastosis viral oncogene homolog (avian)-like 1 | −1.09 | 5.00×10−5 | 9.00×10−3 |
| ARFIP1 | ADP-ribosylation factor interacting protein 1 | −1.38 | 1.00×10−4 | 1.60×10−2 |
| FCRL5 | Fc receptor-like 5 | −1.27 | 1.00×10−4 | 1.60×10−2 |
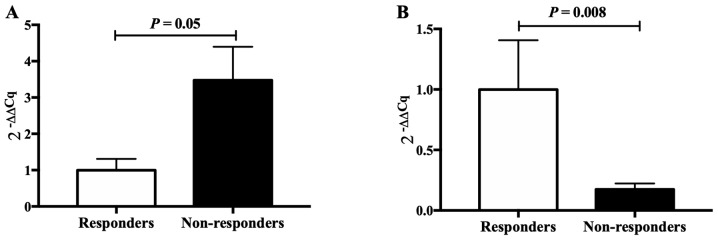
Two genes (CCL3 and FOS), closely associated with epilepsy, were selected for validation by RT-qPCR, revealing significant differences in expression between VPA responders and non-responders, in accordance with the results of the RNA-seq. This indicated that the results obtained by the RNA-seq analysis were reliable (Fig. 3).
Figure 3.
The 2−∆∆Cq values of CCL3 and FOS in the valproic acid non-responsive (n=11) and responsive patients (n=6). (A) CCL3; (B) FOS. Cq, quantification cycle; CCL3, chemokine (C-C motif) ligand 3.
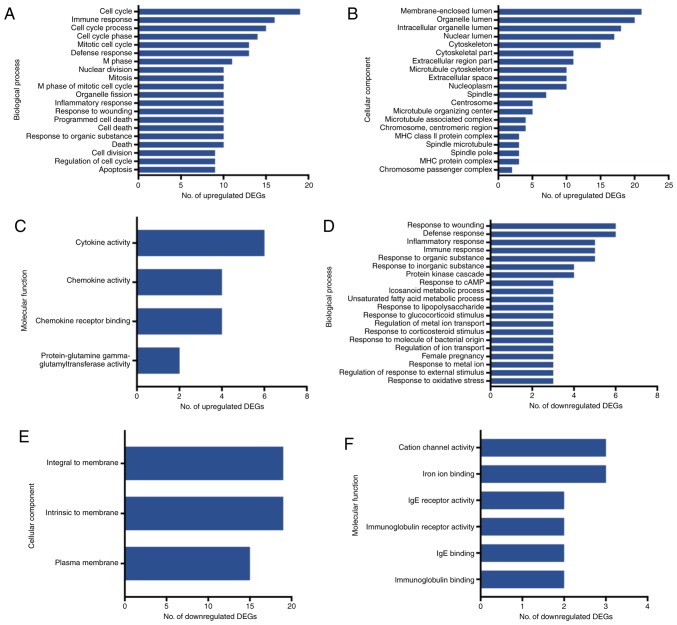
GO and pathway analysis
The GO and pathway enrichment analyses were performed for the 121 final DEGs, including 84 upregulated and 37 downregulated genes. In the GO category BP, the upregulated DEGs were significantly enriched in the GO terms ‘cell cycle’, ‘immune response’ and ‘cell cycle process’ (Fig. 4A). In the category CC, the upregulated DEGs were enriched in the GO terms ‘membrane-enclosed lumen’, ‘organelle lumen’ and ‘organelle lumen’ (Fig 4B), and in the category MF, they were enriched in the GO terms ‘cytokine activity’, ‘chemokine activity’ and ‘chemokine receptor binding’ (Fig 4C). On the other hand, the group of downregulated DEGs was highly enriched in the GO terms ‘response to wounding’, ‘defense response’ and ‘inflammatory response’ in the category BP (Fig 4D). In the category CC, the downregulated DEGs were highly enriched in the GO terms ‘integral to membrane’, ‘intrinsic to membrane’ and ‘plasma membrane’ (Fig 4E), and in the category MF, they accumulated in the GO terms ‘cation channel’, ‘iron ion binding’ and ‘IgE receptor activity’ (Fig. 4F).
Figure 4.
Gene ontology enrichment analysis for the DEGs (Q<0.05). (A-C) Terms enriched by the upregulated genes in the categories (A) biological process, (B) cellular component and (C) molecular function. (D-F) Terms enriched by the downregulated genes in the categories (D) biological process, (E) cellular component and (F) molecular function. IgE, immunoglobulin E; MHC, major histocompatibility complex; DEG, differentially expressed gene.
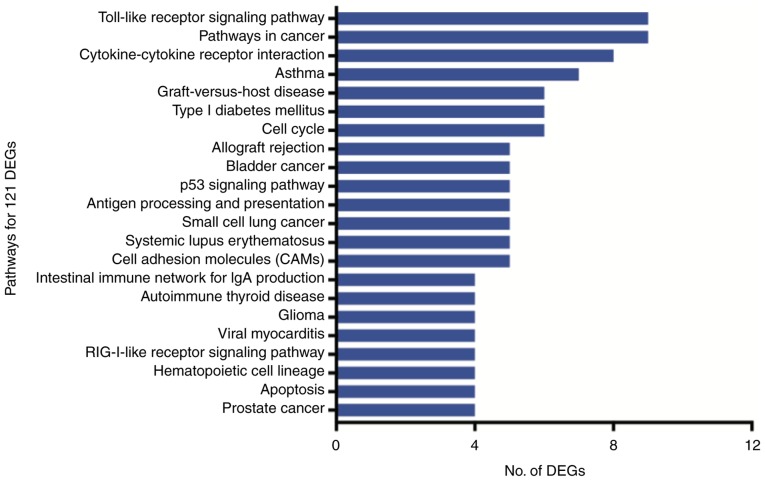
The 121 DEGs were enriched in 22 pathways (P<0.05), of which the Toll-like receptor signaling pathway, cancer pathways and cytokine-cytokine receptor interactions were the most represented (Fig. 5).
Figure 5.
Pathways enriched by the 121 DEGs (Q<0.05). DEG, differentially expressed gene; Ig, immunoglobulin; RIG, retinoic acid-inducible gene.
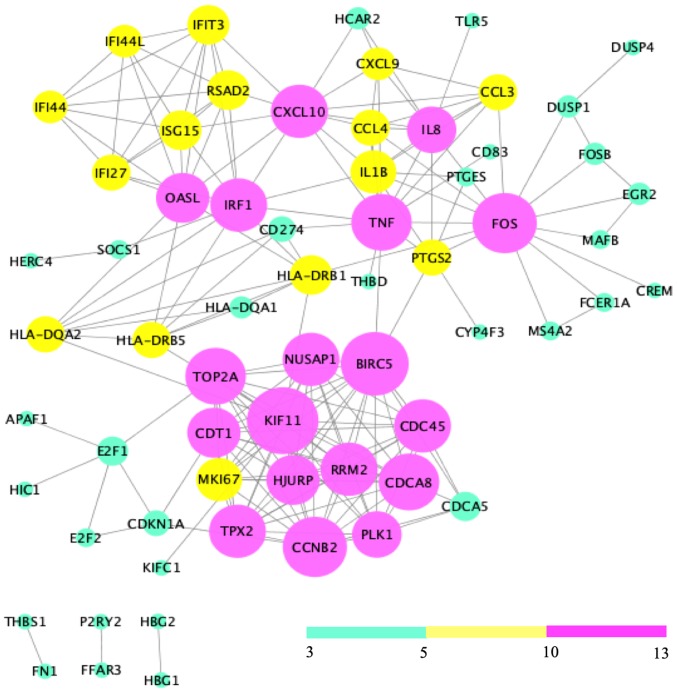
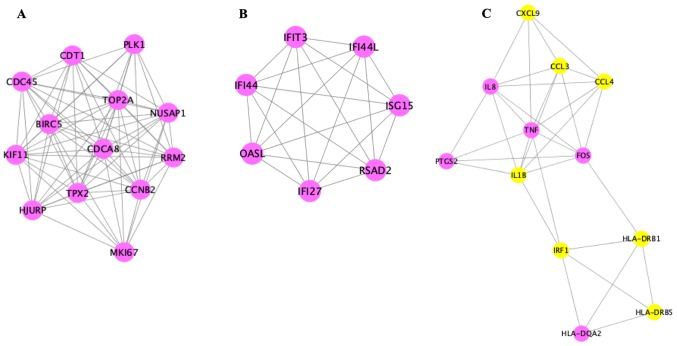
PPI network analysis and module identification
Analysis of the 121 DEGs by STRING and visualization with the Cytoscape plugin MCODE revealed 197 interactions, covering three modules (Fig. 6). Of these, module 1 contained 73 interactions with 13 nodes (CDT1, PLK1, NUSAP1, RRM2, MKI67, CCNB2, HJURP, TPX2, CDCA8, TOP2A, BIRC5, KIF11, CDC45), module 2 included 21 interactions with 7 nodes (IFIT3, IFI44L, ISG15, RSAD2, IFI27, OASL, IFI44L), and module 3 contained 32 interactions with 12 nodes [chemokine (C-X-C motif) ligand 9 (CXCL9), CCL3, CCL4, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IRF1, HLA-DRB1, HLA-DRB5, IL8, PTGS2, FOS, HLA-DQA2)] (Fig. 7).
Figure 6.
Protein-protein interaction network. Nodes with a degree of connectivity of 3–5, 5–10 and 10–13 were indicated in green, yellow and pink, respectively.
Figure 7.
The modules of the 121 DEGs (Q<0.05). (A) Module 1; (B) module 2; (C) module 3. Pink and yellow nodes represent upregulated and downregulated genes, respectively.
A literature review in PubMed confirmed that the function of CCL3, CCL4, CXCL9, TNF-α, IL-1β, and FOS is associated with epilepsy (Table IV) (38–44).
Table IV.
Functions of chemokine genes in epilepsy.
| Gene | Function | (Refs.) |
|---|---|---|
| CCL3 | Inhibition of systemic receptor leads to decrease in seizure activity | (38,39) |
| CCL4 | Inhibition of systemic receptor leads to decrease in seizure activity | (38,39) |
| CXCL9 | Immune-cell recruitment across the BBB | (38) |
| TNF | Activation of NF-κB and regulation of the process of post-seizure neurogenesis | (40,41) |
| IL-1β | Induction of spontaneously recurring seizures | (42) |
| FOS | Regulation of CA3 neuronal excitability and survival | (43,44) |
BBB, blood-brain barrier; IL, interleukin; TNF, tumor necrosis factor; CXCL9, chemokine (C-X-C motif) ligand 9; CCL3, chemokine (C-C motif) ligand 3.
Discussion
Pharmaco-resistant epilepsy remains a major clinical issue with elusive underlying mechanisms. In the present study, a total of 1,153 DEGs between VPA responders and non-responders (P<0.05) were initially identified. Of these genes, 123 upregulated and 60 downregulated genes fulfilled the criterion of P<0.001. This number was higher than that in the study of Tang et al (45), which may be accounted for by the different methods used. In the latter study, oligonucleotide microarrays were employed, containing probe sets for more than 12,000 genes and ESTs. However, previous studies have concluded that microarray platforms suffer from technical issues including cross-hybridization, nonspecific hybridization and limited range of detection of individual probes (46,47). As a result, genes with expression below or near the background level may exhibit increased variability and as such, calculated fold-changes for these genes may be difficult to detect with statistical significance. RNA-seq avoids such technical issues and exhibits a broader dynamic range. In the analysis of the current study, specific DEGs (dual specificity phosphatase 1, ribosomal protein S6 kinase A1 and aldehyde dehydrogenase 2 family member) reported by Tang et al (45) were also identified.
In the present study, most of the DEGs were implicated in the immune and inflammatory response, and associated with cytokine-cytokine receptor interactions, as also identified in epileptic patients by Floriano-Sánchez et al (32). Inflammation is increasingly recognized as an important pathogenetic factor in epilepsy. Evidence suggests the presence of all of the hallmarks of a chronic inflammatory state, i.e., infiltration of leukocytes, reactive gliosis, as well as overexpression of cytokines and their target proteins, in the brain of pharmaco-resistant epileptic patients and animal models (48). CCL3, CCL4 and CXCL9 are the chemokines that guide directional migration of leukocytes and have an important role in the inflammation of the central nervous system. Several studies have demonstrated increased mRNA and protein expression of CCL3, CCL4 and CXCL9 in the cortex and hippocampus of epileptic rats and drug-refractory patients (49–51). The present study revealed that the expression of CCL3, CCL4 and CXCL9 was higher in the blood of VPA non-responsive vs. responsive pediatric patients, which was consistent with the results obtained by Srivastava et al (52). The reason for the overexpression of CCL3, CCL4 and CXCL9 in the brain and blood of drug-refractory patients remains elusive. It has been reported that TNF-α and IL-1β induce the expression of CCL3 and CCL4 through NF-κB and activate inflammation via the mTOR signaling pathway (41,53). Of note, VPA was demonstrated to reduce the amount of leukocytes and inhibit the expression of TNF-α (54,55). The present study indicated that the mRNA levels of TNF-α, IL-1β, NF-κB and IL-1 receptor-associated kinase 2 (a regulator of TNF-α), were significantly higher in VPA non-responders than in responders, which suggested that CCL3 and CCL4 overexpression was associated with the high expression of TNF-α and IL-1β, and indicated that the transcriptional states of CCL3, CCL4, CXCL9, TNF-α and IL-1β are potential markers for monitoring the patients' resistance to VPA.
Previous reports have revealed that increased TNF-α may result in the inhibition of cytochrome P450 family 2 subfamily D member 6 (CYP2D6) and CYP2C19 expression, and may results in the enhanced expression of CYP3A4 and CYP2C9 (the enzymes responsible for VPA metabolism in the brain) (56). Furthermore, TNF-α has been reported to induce the overexpression of transporter (P-gp), which is associated with AED efficacy (57). However, Feng et al (58) indicated that there was no difference in the plasma VPA concentration between responders and non-responders, suggesting that the role of TNF-α in the efficacy of VPA may be independent of its effects on drug metabolism and P-gp.
CCL3, CCL4, IL-1β and TNF-α are able to increase the permeability of the blood-brain barrier (59,60), possibly resulting in their passage into the brain and cerebrospinal fluid (61). Furthermore, overexpression of CCL3 and TNF-α was identified to induce the influx of Ca2+ and to enhance the expression of N-methyl-D-aspartate receptor (NMDAR), leading to increased excitatory neurotransmission and contributing to the development of epileptic seizures and excitotoxicity (62,63). Previous studies have demonstrated an association between NMDAR and the efficacy of AEDs. Zellinger et al (64) indicated that blocking the glycine-binding site of the NR2B subunit of NMDAR may increase the sensitivity to AEDs. Hung et al (65) confirmed that a specific mutation (−200 T>G) of NR2B is associated with s sustained dosage of VPA. Therefore, it may be speculated that NMDAR functionally links CCL3, CCL4, IL-1β and TNF-α with VPA efficacy. However, further study is required for verification.
The protein expression of FOS, an immediate early gene and recognized biomarker of neuronal activity, is activated during spontaneous seizure (66–68). Previous studies have indicated that the expression of FOS rapidly increases 1.5 h after seizure stimulation by pentylenetetrazol and in amygdala-kindled seizures (69). However, the expression profile of FOS during seizures is complex and varies depending on the seizure type and status. FOS expression is increased in generalized seizure but not change in focal seizures. With respect to seizure status, high FOS mRNA expression was detected in rats at 1 h after the injection of kainic acid, while it tended to be low after 6 h (70). Furthermore, Madsen et al (71) identified a large increase in FOS expression at 2 h after a kindling stimulus, while after 18 h, the expression was lower than that observed upon a sham stimulation, and reached the control levels after 3 weeks. Of note, all plasma samples in the present study were collected during a non-seizure period which may account for the slightly decreased FOS expression.
In conclusion, the chemokines CCL3, CCL4, CXCL9, TNF-α and IL-1β may participate in processes associated with VPA resistance and serve as potential biomarkers for monitoring the efficacy of VPA. The study also revealed numerous critical pathways and sub-modules of potential pathogenetic relevance, deserving further investigation.
Acknowledgements
Not applicable.
Funding
The current study was supported by Important Discipline of Shanghai (grant no. 20162B0305) and the National Natural Science Foundation of China (grant nos. 81370776 and 81874325).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contribution
YW analyzed the data and drafted the manuscript. ZL designed the study and revised the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Children's Hospital of Fudan University (Shanghai, China) in 2016 (no. 136). Written informed consent was obtained from the guardians of patients prior to enrolment.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34:837–847. doi: 10.1016/j.ncl.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Lv RJ, Shao XQ, Cui T, Wang Q. Significance of MDR1 gene C3435T polymorphism in predicting childhood refractory epilepsy. Epilepsy Res. 2017;132:21–28. doi: 10.1016/j.eplepsyres.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Voll A, Hernández-Ronquillo L, Buckley S, Téllez-Zenteno JF. Predicting drug resistance in adult patients with generalized epilepsy: A case-control study. Epilepsy Behav. 2015;53:126–130. doi: 10.1016/j.yebeh.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Rakitin A, Kõks S, Haldre S. Valproate modulates glucose metabolism in patients with epilepsy after first exposure. Epilepsia. 2015;56:e172–e175. doi: 10.1111/epi.13114. [DOI] [PubMed] [Google Scholar]
- 5.Fernando-Dongas MC, Radtke RA, Vanlandingham KE, Husain AM. Characteristics of valproic acid resistant juvenile myoclonic epilepsy. Seizure. 2000;9:385–388. doi: 10.1053/seiz.2000.0432. [DOI] [PubMed] [Google Scholar]
- 6.Nevitt ST, Sudell M, Weston J, Tudur Smith C, Marson AG. Antiepileptic drug monotherapy for epilepsy: A network meta-analysis of individual participant data. Cochrane Database Syst Rev. 2017;6:CD0114121. doi: 10.1002/14651858.CD011412.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigo F, Igwe SC, Lattanzi S. Ethosuximide, sodium valproate or lamotrigine for absence seizure in children and adolescents. Cochrane Database Syst Rev. 2019;2:CD003032. doi: 10.1002/14651858.CD003032.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: A meta-analysis of published studies. Seizure. 2014;23:167–174. doi: 10.1016/j.seizure.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Trinka E, Höfler J, Zerbs A, Brigo F. Efficacy and safety of intravenous valproate for status epilepticus: A systematic review. CNS Drugs. 2014;28:623–639. doi: 10.1007/s40263-014-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gesche J, Khanevski M, Solberg C, Beier CP. Resistance to valproic acid as predictor of treatment resistance in genetic generalized epilepsies. Epilepsia. 2017;58:E64–E69. doi: 10.1111/epi.13702. [DOI] [PubMed] [Google Scholar]
- 11.Luna-Tortós C, Fedrowitz M, Löscher W. Evaluation of transport of common antiepileptic drugs by human multidrug resistance-associated proteins (MRP1, 2 and 5) that are overexpressed in pharmacoresistant epilepsy. Neuropharmacology. 2010;58:1019–1032. doi: 10.1016/j.neuropharm.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Baltes S, Fedrowitz M, Tortos CL, Potschka H, Löscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320:331–343. doi: 10.1124/jpet.106.102491. [DOI] [PubMed] [Google Scholar]
- 13.Moerman L, Wyffels L, Slaets D, Raedt R, Boon P, De Vos F. Antiepileptic drugs modulate P-glycoproteins in the brain: A mice study with (11)C-desmethylloperamide. Epilepsy Res. 2011;94:18–25. doi: 10.1016/j.eplepsyres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Römermann K, Helmer R, Löscher W. The antiepileptic drug lamotrigine is a substrate of mouse and human breast cancer resistance protein (ABCG2) Neuropharmacology. 2015;93:7–14. doi: 10.1016/j.neuropharm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Cao Y, Long H, Long L, Xu L, Liu Z, Zhang Y, Xiao B. ABCB1, ABCC2, SCN1A, SCN2A, GABRA1 gene polymorphisms and drug resistant epilepsy in the Chinese Han population. Pharmazie. 2015;70:416–420. [PubMed] [Google Scholar]
- 16.Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K, Banerjee M. GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenet Genomics. 2013;23:605–610. doi: 10.1097/FPC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 17.Singh E, Pillai KK, Mehndiratta M. Characterization of a lamotrigine-resistant kindled model of epilepsy in mice: Evaluation of drug resistance mechanisms. Basic Clin Pharmacol Toxicol. 2014;115:373–378. doi: 10.1111/bcpt.12238. [DOI] [PubMed] [Google Scholar]
- 18.Glauser TA, Holland K, O'Brien VP, Keddache M, Martin L, Clark PO, Cnaan A, Dlugos D, Hirtz DG6 Shinnar S, et al. Pharmacogenetics of antiepileptic drug efficacy in childhood absence epilepsy. Ann Neurol. 2017;81:444–453. doi: 10.1002/ana.24886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv N, Qu J, Long H, Zhou L, Cao Y, Long L, Liu Z, Xiao B. Association study between polymorphisms in the CACNA1A, CACNA1C, and CACNA1H genes and drug-resistant epilepsy in the Chinese Han population. Seizure. 2015;30:64–69. doi: 10.1016/j.seizure.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Mou P, Chen Z, Jiang L, Cheng J, Wei R. PTX3: A potential biomarker in thyroid associated ophthalmopathy. Biomed Res Int. 2018;2018:5961974. doi: 10.1155/2018/5961974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters WR, Maggioli MF, Palmer MV, Thacker TC, McGill JL, Vordermeier HM, Berney-Meyer L, Jacobs WR, Jr, Larsen MH. Interleukin-17A as a Biomarker for bovine tuberculosis. Clin Vaccine Immunol. 2015;23:168–180. doi: 10.1128/CVI.00637-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh J, Yamamoto Y, Asahina N, Kitano S, Kino Y. RNA-Seq data mining: Downregulation of NeuroD6 serves as a possible biomarker for alzheimer's disease brains. Dis Markers. 2014;2014:123165. doi: 10.1155/2014/123165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pong WW, Walker J, Wylie T, Magrini V, Luo J, Emnett RJ, Choi J, Cooper ML, Griffith M, Griffith OL, et al. F11R is a novel monocyte prognostic biomarker for malignant glioma. PLoS One. 2013;8:e77571. doi: 10.1371/journal.pone.0077571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixit AB, Banerjee J, Srivastava A, Tripathi M, Sarkar C, Kakkar A, Jain M, Chandra PS. RNA-seq analysis of hippocampal tissues reveals novel candidate genes for drug refractory epilepsy in patients with MTLE-HS. Genomics. 2016;107:178–188. doi: 10.1016/j.ygeno.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Griffin NG, Wang Y, Hulette CM, Halvorsen M, Cronin KD, Walley NM, Haglund MM, Radtke RA, Skene JH, Sinha SR, Heinzen EL. Differential gene expression in dentate granule cells in mesial temporal lobe epilepsy with and without hippocampal sclerosis. Epilepsia. 2016;57:376–385. doi: 10.1111/epi.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liew CC, Ma J, Tang HC, Zheng R, Dempsey AA. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J Lab Clin Med. 2006;147:126–132. doi: 10.1016/j.lab.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Borovecki F, Lovrecic L, Zhou J, Jeong H, Then F, Rosas HD, Hersch SM, Hogarth P, Bouzou B, Jensen RV, Krainc D. Genome-wide expression profiling of human blood reveals biomarkers for Huntington's disease. Proc Natl Acad Sci USA. 2005;102:11023–11028. doi: 10.1073/pnas.0504921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jergil M, Forsberg M, Salter H, Stockling K, Gustafson AL, Dencker L, Stigson M. Short-time gene expression response to valproic acid and valproic acid analogs in mouse embryonic stem cells. Toxicol Sci. 2011;121:328–342. doi: 10.1093/toxsci/kfr070. [DOI] [PubMed] [Google Scholar]
- 29.Dozawa M, Kono H, Sato Y, Ito Y, Tanaka H, Ohshima T. Valproic acid, a histone deacetylase inhibitor, regulates cell proliferation in the adult zebrafish optic tectum. Dev Dyn. 2014;243:1401–1415. doi: 10.1002/dvdy.24173. [DOI] [PubMed] [Google Scholar]
- 30.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- 31.Rakitin A, Kõks S, Reimann E, Prans E, Haldre S. Changes in the peripheral blood gene expression profile induced by 3 months of valproate treatment in patients with newly diagnosed epilepsy. Front Neurol. 2015;6:188. doi: 10.3389/fneur.2015.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floriano-Sánchez E, Brindis F, Ortega-Cuellar D, Ignacio-Mejía I, Moreno-Arriola E, Romero-Morelos P, Ceballos-Vasquez E, Córdova-Espinoza MG, Arregoitia-Sarabia CK, Sandoval-Pacheco R, et al. Differential gene expression profile induced by valproic acid (VPA) in pediatric epileptic patients. Genes (Basel) 2018;9(pii):E328. doi: 10.3390/genes9070328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 34.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID gene functional classification tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahma R, Gurumayum S, Naorem LD, Muthaiyan M, Gopal J, Venkatesan A. Identification of hub genes and pathways in Zika Virus infection using RNA-Seq Data: A network-based computational approach. Viral Immunol. 2018;31:321–332. doi: 10.1089/vim.2017.0116. [DOI] [PubMed] [Google Scholar]
- 38.Kan AA, de Jager W, de Wit M, Heijnen C, van Zuiden M, Ferrier C, van Rijen P, Gosselaar P, Hessel E, van Nieuwenhuizen O, de Graan PN. Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. J Neuroinflammation. 2012;9:207. doi: 10.1186/1742-2094-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louboutin JP, Chekmasova A, Marusich E, Agrawal L, Strayer DS. Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB J. 2011;25:737–753. doi: 10.1096/fj.10-161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. Prog Brain Res. 2001;132:555–565. doi: 10.1016/S0079-6123(01)32103-9. [DOI] [PubMed] [Google Scholar]
- 41.Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 43.Jin W, Zhang J, Lou D, Chavkin C, Xu M. C-fos-deficient mouse hippocampal CA3 pyramidal neurons exhibit both enhanced basal and kainic acid-induced excitability. Neurosci Lett. 2002;331:151–154. doi: 10.1016/S0304-3940(02)00872-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat Genet. 2002;30:416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]
- 45.Tang Y, Glauser TA, Gilbert DL, Hershey AD, Privitera MD, Ficker DM, Szaflarski JP, Sharp FR. Valproic acid blood genomic expression patterns in children with epilepsy-a pilot study. Acta Neurol Scand. 2004;109:159–168. doi: 10.1046/j.1600-0404.2003.00253.x. [DOI] [PubMed] [Google Scholar]
- 46.Minnier J, Pennock ND, Guo Q, Schedin P, abd Harrington CA. RNA-Seq and expression arrays: Selection guidelines for genome-wide expression profiling. Methods Mol Bio. 2018;1783:7–33. doi: 10.1007/978-1-4939-7834-2_2. [DOI] [PubMed] [Google Scholar]
- 47.Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One. 2014;9:e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker L, Sills GJ. Inflammation and epilepsy: The foundations for a new therapeutic approach in epilepsy? Epilepsy Curr. 2012;12:8–12. doi: 10.5698/1535-7511-12.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzik-Kornacka A, Sliwa A, Plucinska G, Lukasiuk K. Status epilepticus evokes prolonged increase in the expression of CCL3 and CCL4 mRNA and protein in the rat brain. Acta Neurobiol Exp (Wars) 2011;71:193–207. doi: 10.55782/ane-2011-1840. [DOI] [PubMed] [Google Scholar]
- 50.Owens GC, Huynh MN, Chang JW, McArthur DL, Hickey MJ, Vinters HV, Mathern GW, Kruse CA. Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflammation. 2013;10:56. doi: 10.1186/1742-2094-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arisi GM, Foresti ML, Katki K, Shapiro LA. Increased CCL2, CCL3, CCL5, and IL-1β cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures. J Neuroinflammation. 2015;12:129. doi: 10.1186/s12974-015-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava A, Dixit AB, Paul D, Tripathi M, Sarkar C, Chandra PS, Banerjee J. Comparative analysis of cytokine/chemokine regulatory networks in patients with hippocampal sclerosis (HS) and focal cortical dysplasia (FCD) Sci Rep. 2017;7:15904. doi: 10.1038/s41598-017-16041-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saber S, Mahmoud AAA, Helal NS, El-Ahwany E, Abdelghany RH. Renin-angiotensin system inhibition ameliorates CCl4-induced liver fibrosis in mice through the inactivation of nuclear transcription factor kappa B. Can J Physiol Pharmacol. 2018;96:569–576. doi: 10.1139/cjpp-2017-0728. [DOI] [PubMed] [Google Scholar]
- 54.Guenther S, Bauer S, Hagge M, Knake S, Olmes DG, Tackenberg B, Rosenow F, Hamer HM. Chronic valproate or levetiracetam treatment does not influence cytokine levels in humans. Seizure. 2014;23:666–669. doi: 10.1016/j.seizure.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res. 2000;857:246–251. doi: 10.1016/S0006-8993(99)02439-7. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh C, Hossain M, Solanki J, Najm IM, Marchi N, Janigro D. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia. 2017;58:576–585. doi: 10.1111/epi.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee NY, Riechmann P, Kang YS. The changes of P-glycoprotein activity by interferon-γ and tumor necrosis factor-α in primary and immortalized human brain microvascular endothelial cells. Blomol Ther (Seoul) 2012;20:293–298. doi: 10.4062/biomolther.2012.20.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng W, Mei S, Zhu L, Yu Y, Yang W, Gao B, Wu X, Zhao Z, Feng F. Effects of UGT2B7, SCN1A and CYP3A4 on the therapeutic response of sodium valproate treatment in children with generalized seizures. Seizure. 2018;58:96–100. doi: 10.1016/j.seizure.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Mantle JL, Lee KH. A differentiating neural stem cell-derived astrocytic population mitigates the inflammatory effects of TNF-α and IL-6 in an iPSC-based blood-brain barrier model. Neurobiol Dis. 2018;119:113–120. doi: 10.1016/j.nbd.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 60.Alluri H, Grimsley M, Anasooya Shaji C, Varghese KP, Zhang SL, Peddaboina C, Robinson B, Beeram MR, Huang JH, Tharakan B. Attenuation of blood-brain barrier breakdown and hyperpermeability by calpain inhibition. J Biol Chem. 2016;291:26958–26969. doi: 10.1074/jbc.M116.735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2015;86:820–822. doi: 10.1136/jnnp-2014-309388. [DOI] [PubMed] [Google Scholar]
- 62.Kuijpers M, van Gassen KL, de Graan PN, Gruol D. Chronic exposure to the chemokine CCL3 enhances neuronal network activity in rat hippocampal cultures. J Neuroimmunol. 2010;229:73–80. doi: 10.1016/j.jneuroim.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anaparti V, Pascoe CD, Jha A, Mahood TH, Ilarraza 3, Unruh H, Moqbel R, Halayko AJ. Tumor necrosis factor regulates NMDA receptor-mediated airway smooth muscle contractile function and airway responsiveness. Am J Physiol Lung Cell Mol Physiol. 2016;311:L467–480. doi: 10.1152/ajplung.00382.2015. [DOI] [PubMed] [Google Scholar]
- 64.Zellinger C, Salvamoser JD, Soerensen J, van Vliet EA, Aronica E, Gorter J, Potschka H. Pre-treatment with the NMDA receptor glycine-binding site antagonist L-701,324 improves pharmacosensitivity in a mouse kindling model. Epilepsy Res. 2014;108:634–643. doi: 10.1016/j.eplepsyres.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Hung CC, Ho JL, Chang WL, Tai JJ, Hsieh TJ, Hsieh YW, Liou HH. Association of genetic variants in six candidate genes with valproic acid therapy optimization. Pharmacogenomics. 2011;12:1107–1117. doi: 10.2217/pgs.11.64. [DOI] [PubMed] [Google Scholar]
- 66.Gautier NM, Glasscock E. Spontaneous seizures in Kcna1-null mice lacking voltage-gated Kv1.1 channels activate Fos expression in select limbic circuits. J Neurochem. 2015;135:157–164. doi: 10.1111/jnc.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Taracha E, Skórzewska A, Lehner M, Bidziński A, Hamed A, Wisłowska-Stanek A, et al. Mapping of c-Fos expression in the rat brain during the evolution of pentylenetetrazol-kindled seizures. Epilepsy Behav. 2009;16:216–224. doi: 10.1016/j.yebeh.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 68.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology. 2008;33:1884–1895. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 69.Deransart C Lê BT, Marescaux C, Depaulis A. Role of the subthalamo-nigral input in the control of amygdala-kindled seizures in the rat. Brain Res. 1998;807:78–83. doi: 10.1016/S0006-8993(98)00745-8. [DOI] [PubMed] [Google Scholar]
- 70.Bozzi Y, Vallone D, Borrelli E. Neuroprotective role of dopamine against hippocampal cell death. J Neurosci. 2000;20:8643–8649. doi: 10.1523/JNEUROSCI.20-22-08643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madsen TM, Bolwig TG, Mikkelsen JD. Differential regulation of c-Fos and FosB in the rat brain after amygdala kindling. Cell Mol Neurobiol. 2006;26:87–100. doi: 10.1007/s10571-006-9202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.