Abstract
Chronic kidney disease is a worldwide health crisis, while diabetic kidney disease (DKD) has become the leading cause of end-stage renal disease (ESRD). DKD is a microvascular complication and occurs in 30–40% of diabetes patients. Epidemiological investigations and clinical observations on the familial clustering and heritability in DKD have highlighted an underlying genetic susceptibility. Furthermore, DKD is a progressive and long-term diabetic complication, in which epigenetic effects and environmental factors interact with an individual’s genetic background. In recent years, researchers have undertaken genetic and epigenetic studies of DKD in order to better understand its molecular mechanisms. In this review, clinical material, research approaches and experimental designs that have been used for genetic and epigenetic studies of DKD are described. Current information from genetic and epigenetic studies of DKD and ESRD in patients with diabetes, including the approaches of genome-wide association study (GWAS) or epigenome-wide association study (EWAS) and candidate gene association analyses, are summarized. Further investigation of molecular defects in DKD with new approaches such as next generation sequencing analysis and phenome-wide association study (PheWAS) is also discussed.
Keywords: diabetic kidney disease, diabetes, end-stage renal disease, genetics, epigenetics, phenotypes
Introduction
Diabetes is a major public health problem that is approaching epidemic proportions globally. According to the latest report from the IDF, the prevalence of diabetes will increase from 425 million persons in 2017 to 629 million by 2045 (IDF 20171). Diabetic kidney disease (DKD, previously termed diabetic nephropathy, DN) is a microvascular complication and progresses gradually over many years in approximately 30–40% of individuals with T1D and T2D mellitus (Harjutsalo and Groop, 2014; Thomas et al., 2015; Barrett et al., 2017). DKD is now the main cause of chronic kidney disease (CKD) worldwide and the leading cause of end-stage-renal disease (ESRD) requiring renal replacement therapy (dialysis or transplantation). The presence of CKD is the single strongest predictor of mortality for persons with diabetes (Dousdampanis et al., 2016; Papadopoulou-Marketou et al., 2017). Pathological findings in DKD include glomerular hypertrophy, mesangial matrix expansion, reduced podocyte number, glomerulosclerosis, tubular atrophy and tubulointerstitial fibrosis. Clinical criteria used to diagnose the subjects with DKD are urine ACR higher than 300 mg/g, while microalbuminuria is diagnosed when ACR is between 30–300 mg/g (Bouhairie and McGill, 2016). Accumulating evidence has indicated that podocyte loss and epithelial dysfunction play important roles in DKD pathogenesis with further progression associated with inflammation but the exact molecular mechanisms responsible for DKD are not fully known (Badal and Danesh, 2014; Reidy et al., 2014; Gnudi et al., 2016).
Both clinical and epidemiological studies have demonstrated that there is familial aggregation of DKD in different ethnic groups, indicating that genetic factors contribute to development of the disease. Furthermore, genetic risk factors in DKD interact with the environmental factors (for example, lifestyle, diet and medication) (Freedman et al., 2007a; Murea et al., 2012; Thomas et al., 2012; Kato and Natarajan, 2014). Figure 1 is a schematic diagram representing the relationship between genetic, epigenetic and environmental factors that are involved in the development and progression of DKD. Genetic studies of DKD are mainly focused on association analyses between genomic DNA variation (for example, single nucleotide polymorphisms, SNPs, copy number variants, CNVs, and microsatellites) and clinical phenotypes of the disease (Freedman et al., 2007a; Gu and Brismar, 2012; Thomas et al., 2012; Florez, 2016). Epigenetics studies of DKD examine potentially heritable changes in gene expression that occur without variation in the original DNA nucleotide sequence (Villeneuve and Natarajan, 2010; Kato and Natarajan, 2014; Thomas, 2016; Keating et al., 2018). Therefore, epigenetic studies of DKD may provide information to help understand how environmental factors modify the expression of genes that are involved in DKD progression. Combined genetic, epigenetic and phenotypic studies together may generate information to understand new pathogenic pathways and to search for new biomarkers for early diagnosis and prediction as part of prevention programs in DKD. The results may also be useful in finding novel targets for the treatment of DKD.
FIGURE 1.
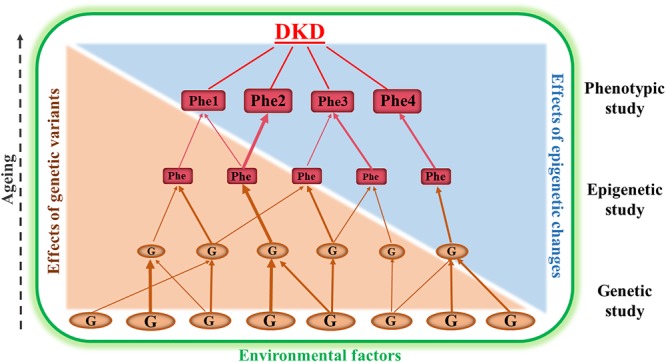
This is a schematic diagram representing the relationship between genetic, epigenetic and phenotypic studies in diabetic kidney disease (DKD). Genetic association studies are fundamentally important for identification of susceptibility or resistance genes (G). Epigenetic studies analyzing genomic DNA methylation changes, chromosome histone modification and ncRNA regulation are useful for dissecting the interaction of the genes with environmental factors. The combined data from genetic, epigenetic and phenotypic (Phe) studies may provide the opportunity for us to understand new pathways underlying the pathogenesis of DKD and to discover new biomarkers for early diagnosis and to find targets for prevention and treatment programs of this disease. The different sizes of the ‘G” and “Phe” represent the variation of genetic and phenotypic effects.
SNPs are the most common form of genomic DNA variation. The updated dbSNP database of more than 500 million reference SNPs (rs) with allele frequency data2 has provided fundamental information for genetic studies of complex diseases including, DKD. The genetic studies in DKD have implicated previously unsuspected biological pathways and subsequently improved our knowledge for understanding of the genetic basis of the disease. For most common traits studied in DKD, however, the identified genes and their SNPs only explain a fraction of associated risk, suggesting that human genomic DNA variations are only a part of underlying susceptibility to DKD. This has led to evolving interest in epigenetics to help explain some of the missing heritability of DKD. Epigenetic mechanisms mainly consist of DNA methylation, chromosome histone modification and non-coding RNA (ncRNA) regulation (Kato and Natarajan, 2014; Allis and Jenuwein, 2016). Epigenetic related ncRNAs include miRNA, siRNA, piRNA, and lncRNA (Holoch and Moazed, 2015). There are more than 30,000 identified CpG islands in the human genome. Detailed information for these CpG islands can be found in the public database3. The CpG islands are defined as stretches of DNA > 200 bp long with a GC percentage greater than 50% and an observed-to-expected CpG ratio of more than 60%. The CpG islands are often found at promoters and contain the 5′ end of the transcript, while DNA methylation occurs at 5′-cytosines of “CpG” dinucleotides4 (Cross and Bird, 1995). In DKD, the effects of DNA methylation have been studied in terms of transgenerational inheritance of the disease to explore environmental and other non-genetic factors that may influence epigenetic modifications in the genes involved in DKD (Deaton and Bird, 2011; Jones, 2012). Identification of differentially methylated CpG sites in promoters or other functional regions of genes and the analysis of the DNA methylation changes that are associated with DKD have become the most common approaches used in epigenetic studies of the disease (Villeneuve and Natarajan, 2010; Kato and Natarajan, 2014; Thomas, 2016). Furthermore, ncRNAs, particularly long ncRNAs are known to be involved in epigenetic processes. ncRNAs certainly play an important role in chromatin formation, histone modification, DNA methylation and consequently gene transcription silencing.
Genetic and epigenetic studies of DKD, initially using candidate gene approaches and more recently at genome-wide scale (known as GWAS and EWAS), have been undertaken to identify many genes conferring susceptibility or resistance to DKD. In this review, clinical phenotypes, research approaches and experimental designs that have been used for genetic and epigenetic studies of DKD are described. These research approaches and experimental designs can also be used for study of CKD. Current information from genetic and epigenetic studies of DKD is summarized. Further investigation of molecular defects in DKD with new generation sequencing analyses and phenome-wide association studies (PheWAS) are discussed.
Biological Material, Research Approaches and Study Designs Used in Genetic and Epigenetic Investigations of Diabetic Kidney Disease
Two major research approaches either at genome-wide scale or focused on candidate gene(s) have been widely used for comparative studies between cases (patients with DKD) and controls (diabetes patients without DKD). Case-control studies by recruiting large numbers of subjects can increase the statistical power of reported associations. The aim is to discover the genes presented differentially in genomic structure or genetic expression. Genome-wide or epigenome-wide association studies (GWAS or EWAS) are hypothesis-generating approaches (Rakyan et al., 2011; Do et al., 2017; Lappalainen and Greally, 2017). These study designs have benefited from rapid development of human genome research, including the creation of publicly available databases of SNPs, haplotypes and CpG islands and the rapid technical improvements in analyzing genomic variation using high-throughput techniques and high-density SNP or CpG arrays. Another approach is to focus on candidate genes and study a more limited number of genes potentially involved in the pathogenesis of DKD based upon our known knowledge or hypothesis. In genetic and epigenetic studies of DKD, DNA samples used are commonly extracted from peripheral blood samples because they are clinically accessible. Dick et al. (2014) have comparatively analyzed DNA methylation changes related to BMI by using both approaches of whole-blood DNA methylation profiling and adipose tissue specific methylation measurement. Data suggests that analysis of blood DNA methylation is worthwhile because the results can reflect the DNA methylation changes in relevant tissues for a particular phenotype. Nevertheless, there is still limited information concerning the correlation between whole blood DNA methylation profiles and kidney tissue specific DNA methylation changes in part due to the heterogeneity of cell types within the kidney. To improve the tissue specific DNA methylation analysis of kidney diseases, including DKD, it is necessary to construct biobanks of renal biopsies. Karolinska Institutet has established a biobank in KaroKidney with more than 750 renal biopsies5. The advantages and limitations of these two approaches, as well as the clinical materials and experimental design used in genetic and epigenetic studies of DKD are summarized in Table 1.
Table 1.
Clinical material, research approaches and experimental designs used in genetic and epigenetic studies of diabetic kidney disease.
| Study | Advantage | Disadvantage | |
|---|---|---|---|
| Clinical material | Blood or saliva | Clinical accessible | Possible bias from mixed cell types |
| Kidney tissues | Gene specific methylation and expression can be analyzed | Difficult to access | |
| Renal cell lines | Intervention and mechanism study | In vitro experiment | |
| Research approach | Candidate gene DNA variation or methylation analysis | Study of candidate genes with potential biological functions | Less information on the studied genes |
| Global genomic DNA variation or methylation analyses | General information of DNA polymorphisms and methylation in genome wide scale | Analysis of repeated sequence alteration and methylation changes Lack of gene specific information | |
| Genome or epigenome-wide association studies | Numerous SNP, CNV or CpG sites methylation information in genome wide scale | Higher cost Strict validation is needed | |
| Experimental design | Case-control study | Many cohorts exist | Difficult to control genetic and environmental confounders |
| Twin study | Control for genetics | Few large cohorts | |
| Family study | Study of potential inheritance | Few large cohorts | |
| Longitudinal study | Determine causality | Time consuming |
CNV, copy-number variation; CpG sites, the regions of DNA where a cytosine nucleotide is followed by a guanine nucleotide in the linear sequence of bases along its 5′ → 3′ direction; SNP, single-nucleotide polymorphism.
Recent Data From Genetic Studies in Diabetic Kidney Disease
Considerable amounts of data from genetic studies in DKD have accumulated. A list of the genes that are reported to be associated with susceptibility or resistance to DKD are summarized in Table 2. The genes are listed in alphabetical order. Surprisingly, there are more than 150 genes. Most of them have been identified by genetic association studies employing candidate gene approaches over the past 20 years. Furthermore, a number of GWAS in DKD have been published in the last 10 years. By using GWAS approaches, approximately 33 genes have been found to be associated with the DKD, i.e., ABCG2, AFF3, AGER, APOL1, AUH, CARS, CERS2, CDCA7/SP3, CHN2, CNDP1, ELMO1, ERBB4, FRMD3, GCKR, GLRA3, KNG1, LIMK2, MMP9, NMUR2, MSRB3/HMGA2, MYH9, PVT1, RAET1L, RGMA/MCTP2, RPS12, SASH1, SCAF8/CNKSR3, SHROOM3, SLC12A3, SORBS1, TMPO, UMOD, and ZMIZ1 (Hanson et al., 2007; Sandholm et al., 2012, 2014; Maeda et al., 2013; Thameem et al., 2013; Bailey et al., 2014; Palmer et al., 2014; Guan et al., 2016; Teumer et al., 2016; Lim et al., 2017; Roden, 2017; Charmet et al., 2018; van Zuydam et al., 2018). However, most of these genes (∼80%) reportedly associated with DKD still need to be confirmed by further replication studies and detailed analysis of their functional role in DKD in experimental models. Polymorphisms in these candidate genes association with DKD studies are listed in Table 2A, while their potential biological relevance and genetic effects in DKD are briefly described. Of them, 34 genes are originally predicted by GWAS and the statistical association with DKD summarized in Table 2B.
Table 2A.
Current data from genetic association studies in diabetic kidney disease by using candidate gene approach.
| Gene symbol | Genomic DNA polymorphisms | Disease |
|---|---|---|
| ABCG2 | rs2231142 | T2D-uric acid |
| ACACB | rs2268388 | T2D-DKD |
| ACE | rs4646994 (289bp Alu I/D), rs4343, rs1799752, rs1800764, rs12449782 | T1D-DKD, T2D-DKD, T2D-ESRD |
| ADPOQ | rs266729, rs17300539, rs2241766, rs1063537, rs2241767, rs2082940 | T1D-DKD, T2D-DKD |
| ADRB2 | Arg16Gly, Gln27Glu | T2D-eGFR |
| AFF3 | rs7583877 | T1D-ESRD |
| AGER | rs2070600, rs2071288 | T2D-DKD |
| AGT | rs5050, rs4762, Met235Thr | T2D-DKD |
| AGTR1 | rs5186, +1166A/C, -106C/T, rs12695897 | T1D-DKD, T2D-ESRD |
| AGTR2 | +1675G/A, +1818A/T | T1D-DKD |
| AKR1B1 | rs759853 | T2D-DKD, T2D-ESRD |
| ALOX12 | rs14309 | T2D-DKD+CVD |
| APOE | e4 allele, e2/e3 alleles | T2D-DKD |
| APOL1 | rs136161, rs713753, rs767855, Ser342Gly, Ile384Met | T2D-ESRD |
| AUH | rs773506 | T2D-ESRD |
| BID | rs181390 | T1D-ESRD |
| CALD1 | rs3807337 | T1D-DKD |
| CARS | rs452041, rs739401 | T1D-DKD, T2D-DKD |
| CASR | rs3804594 | T2D-DKD |
| CAT | rs1001179 | T2D-ESRD |
| CERS2 | rs267734, rs267738 | T1D-DKD, T2D-DKD |
| CDH13 | rs11646213, rs3865188 | T1D-ESRD |
| CFH | rs379489 | T2D-ESRD |
| CHN2 | rs39059 | T1D-DKD |
| CNDP1 | (CTG)5, rs4892249, rs6566815, rs2346061, rs1295330, rs6566810, rs11151964, rs17817077 | T2D-dialysis, T2D-DKD, T1D-ESRD, T2D-ESRD |
| CNDP2 | rs7577, rs4892247 | T2D-ESRD |
| CYP11B2 | -344T/C | T2D-DKD |
| COQ5 | rs1167726, rs614226, rs1167725 | T1D-ESRD |
| COX6A1 | rs12310837 | T1D-ESRD |
| COX10 | rs7213412 | T1D-ESRD |
| CUBN | rs1801239 | T1D-albuminuria, T2D-ESRD |
| CYBA | rs4673, rs9932581 | T1D-ESRD, T2D-DKD |
| eNOS | -786C/T, +786T/C, +894G/T, Glu298Asp | T1D-DKD, T2D-DKD |
| ELMO1 | rs741301, rs1345365, rs11769038, rs10951509, rs1882080, rs6462776, rs6462777 | T1D-DKD, T1D-ESRD, T2D-DKD |
| ENPP1 | rs1044498, rs7754586, rs1974201 | T1D-DKD, T2D-DKD, T2D-ESRD |
| EPHX2 | rs751141 | T2D-DKD |
| EPO | rs1617640 | T1D-ESRD, T2D-DKD |
| ERBB4 | rs7588550 | T1D-DKD |
| ESR1 | rs12197043, rs11964281, rs1569788, rs9340969 | T2D-DKD |
| FNDC5 | rs16835198 | T2D-DKD |
| FRMD3 | rs1888747, rs10868025, rs942280, rs942278, rs942263, rs1535753, rs2378658, rs13288659 | T1D-ESRD, T2D-DKD |
| GAS6 | Intron 8, c.834+7G/A | T2D-DKD |
| GATC | rs2235222, rs7137953 | T1D-ESRD |
| GCK | rs730947 | T2D-ESRD |
| GCKR | rs1260326 | T2D-eGFR |
| GFPT2 | Ile147Val | T2D-DKD |
| GLRA3 | rs1564939 | T1D-AER |
| GPX1 | rs3448 | T1D-DKD |
| GREM1 | rs1129456 | T1D-DKD |
| GSTP1 | rs1695 (Ile105Val) | T2D-DKD, T2D-ESRD |
| H19-IGF2 cluster | rs2839698, rs10732516, rs201858505 | T2D-DKD |
| HIF1α | rs11549465 (Pro582Ser) | T1D-DKD, T2D-DKD |
| HO1 | -413T/A | T2D-DKD |
| HSP70 | rs2763979, rs2227956 | T2D-DKD |
| ICAM1 | rs5498 | T1D-DKD, T2D-DKD |
| IGFBP1 | rs1065780, rs3828998, rs3793344, rs4619 | T2D-DKD |
| IGF2BP2 | rs4402960 | T2D-DKD |
| IL1α | -889C/T | T2D-DKD |
| IL1β | rs16944, -511C/T | T2D-DKD |
| IL6 | -634G/C, -174G/C, rs1800796, rs1524107, rs1800795, rs1800796 | T2D-DKD |
| IL10 | -819T/C, -592A/C, -1082A/G | T2D-DKD |
| IL18 | rs360719 | T2D-DKD |
| INSR | rs2059806 | T2D-DKD |
| IRAK4 | rs4251532 | T2D-DKD |
| KCNQ1 | I/D in intron 12, rs2237897 | T2D-eGFR, T2D-DKD |
| KLRA1 | rs2168749 | T1D-ESRD |
| KNG1 | +7965C/T | T1D-DKD |
| LIMK2 | rs2106294 | T2D-ESRD |
| LTA | Thr60Asn | T1D-DKD |
| LRP2 | rs17848169 | T2D-ESRD |
| MAPRE1P2 | rs1670754 | T1D-ESRD |
| MCF2L2 | Leu359Ile | T1D-DKD |
| MGP | -138T/C | T2D-DKD |
| MME | rs3796268, rs3773885 | T1D-DKD |
| MMP12 | rs1277718, rs652438, Asn357Ser | T1D-DKD |
| MMP9 | (CA)n in promoter, rs481480, rs2032487, rs4281481, rs3752462, rs3918242 | T2D-ESRD, T2D-DKD |
| NMUR2 | rs982715, rs4958531, rs4958532, rs4958535 | T1D-DKD |
| MSC | rs9298190 | T1D-ESRD |
| MT2A | rs28366003 | T2D-DKD |
| MTHFR | rs1801133 | T1D-DKD, T2D-DKD |
| MTOR | rs7212142 | T2D-DKD |
| MyD88 | rs6853 | T2D-DKD |
| MYH9 | rs5750250 | T2D-ESRD |
| NCALD | rs1131863, +999T/A, +1298A/C, +1307A/G | T2D-DKD |
| near IRS2 | rs1411766 | T1D-DKD, T1D-ESRD, T2D-DKD |
| NOS2 | rs1137933 | T2D-DKD |
| NOS3 | rs3918188, Glu298Asp, Gly894Thr | T1D-DKD, T2D-DKD |
| NQO1 | rs1800566 | T2D-DKD |
| NPHS1 | rs35238405 | T2D-ESRD |
| NPY | Leu7Pro | T1D-DKD |
| PACRG | rs2147653, rs1408705 | T1D-ESRD |
| PAI1 | 4G/5G | T2D-DKD |
| PARK2 | rs4897081 | T2D-DKD |
| PARP1 | C410T, G1672A, Val762Ala | T2D-DKD |
| PFKFB2 | rs17258746, rs11120137 | T2D-DKD |
| PLEKHH2 | rs1368086, rs725238, rs11886047 | T1D-DKD |
| PLXDC2 | rs1571942, rs12219125 | T1D-DKD |
| PON1 | Leu55Met, Gln192Arg | T1D-DKD, T2D-ACR |
| PON2 | rs12704795 | T2D-DKD |
| PPARG | rs1805192, rs1801282 | T1D-DKD, T2D-DKD |
| PPARG2 | Pro12Ala | T2D-eGFR, T2D-DKD |
| PPARGC1A | Gly482Ser | T2D-DKD |
| PRKAA2 | rs2746342, rs10789038 | T2D-DKD |
| PROX1 | rs340841 | T2D-DKD |
| PSMD9 | rs1043307, rs14259, +460A/G, +437T/C, Glu197Gly | T2D-DKD |
| PRKCB1 | -1504C/T, -546C/T, -348A/G, -278C/T, -238C/G | T1D-DKD, T2D-eGFR |
| PTX3 | rs2305619, rs2120243 | T2D-DKD |
| PVT1 | rs2648875, rs2720709 | T2D-ESRD |
| RAGE | -429T/C, -374T/A, +2184A/G | T1D-ESRD, T2D-DKD |
| RAET1L | rs1543547 | T1D-DKD |
| RBP4 | rs3758538, rs10882278, rs7094671, rs12766992 | T2D-eGFR |
| REN | rs41317140 | T2D-DKD |
| RREB1 | rs9379084, rs41302867 | T2D-ESRD |
| TOP1MT | rs7387720, 724037 | T1D-ESRD |
| TXNRD2 | rs17745445, rs17745433, rs5992495, rs5992493 | T1D-ESRD |
| RPS12 | rs7769051 | T2D-ESRD |
| RTN1 | rs1952034, rs12431381, rs12434215 | T2D-ESRD |
| SASH1 | rs6930576 | T2D-ESRD |
| SCAF8/CNKSR3 | rs12523833 | T2D-DKD |
| SEMA6D/SLC24A5 | rs12917114 | T1D-ESRD |
| SERPINB7 | rs1720843 | T2D-DKD |
| SERPINE1 | 4G/5G polymorphism | T2D-DKD |
| SHROOM3 | rs1739721 | T2D-eGFR |
| SIK1 | rs2838302 | T1D-ESRD |
| SIRT1 | rs4746720 | T2D-DKD |
| SLC2A1 | rs3820589, HaeIII polymorphism | T1D-DKD, T2D-DKD |
| SLC2A2 | +16459C/T | T1D-DKD |
| SLC2A9 | rs11722228, rs3775948 | T2D-uric acid |
| SLC12A3 | rs11643718 | T2D-DKD, T2D-ESRD |
| SOD1 | rs2234694 | T1D-DKD |
| SOD2 | Ala9Val, Val16Ala | T1D-DKD |
| SORBS1 | rs1326934 | T1D-DKD |
| SOX2 | rs11915160 | T1D-DKD |
| SPTLC2 | rs176903 | T1D-ESRD |
| SUMO4 | rs237025 | T2D-DKD |
| SUV39H2 | rs17353856 | T1D-DKD |
| TCF7L2 | rs7903146 | T2D-DKD |
| TGFβ1 | rs1800470 | T1D-DKD, T2D-DKD |
| THP | rs12444268 | T1D-DKD |
| TMPO | rs4762495 | T1D-ESRD |
| TNFα | rs1800629, rs1800470, rs1800469, rs1800630, rs1799964 | T2D-DKD, T2D-ESRD |
| TRAF6 | rs16928973 | T2D-DKD |
| TRIB3 | rs2295490 | T2D-DKD |
| UMOD | rs12917707, rs13333226 | T2D-DKD |
| VDR | Raql variant | T2D-DKD |
| VEGF | -1499C/T, rs2010963 | T1D-DKD, T2D-DKD |
| VEGFA | rs3025021 | T1D-DKD |
| WNT4/ZBTB40 | rs12137135 | T1D-ESRD |
| ZMIZ1 | rs1749824 | T1D-ESRD |
| miRNA-146a | rs2910164 | T1D-DKD, T2D-DKD |
| miRNA-125 | rs12976445 | T2D-DKD |
Table 2B.
Current data from genetic association studies in diabetic kidney disease by using genome wide association approach.
| Gene symbol | Genomic DNA polymorphisms | P-value | Disease | References |
|---|---|---|---|---|
| ABCG8 | rs4148217 | P = 0.003 | T2D-ESRD | Nicolas et al., 2015 |
| AFF3 | rs7583877, rs7562121 | P = 1.2 × 10(-8) and <1 × 10(-6) | T1D-ESRD | Sandholm et al., 2012, 2017 |
| AGER | rs2070600, rs2071288 | P < 0.001 | T2D-DKD | Lim et al., 2017 |
| AGTR1 | rs12695897 | P = 0.032 | T2D-ESRD | Palmer et al., 2014 |
| APOL1 | rs136161, rs713753, rs767855 | P = 0.006–0.037 | T2D-ESRD | Palmer et al., 2014 |
| AUH | rs7735506 | P = 2.57 × 10(-4) | T2D-ESRD | McDonough et al., 2011 |
| BID | rs181390 | P = 0.006 | T1D-ESRD | Craig et al., 2009 |
| CARS | rs452041, rs739401 | P = 3.1 × 10(-6) | T1D-DKD, T2D-DKD | Pezzolesi et al., 2009b |
| CERS2 | rs267734, rs267738 | P = 0.0013 and 0.0015 | T1D-DKD, T2D-DKD | Shiffman et al., 2014 |
| CDCA7-SP3 | rs4972593 | P = 5 × 10(-8) | T1D-ESRD in women | Sandholm et al., 2013 |
| CHN2 | rs17157914 | P = 0.029 | T2D-ESRD | Palmer et al., 2014 |
| CNDP1 | rs4892249, rs6566815 | P = 0.0043 and 0.0076 | T2D-ESRD | Palmer et al., 2014 |
| CNTNAP2 | rs1989248 | P < 1 × 10(-6) | T1D-ESRD | Sandholm et al., 2017 |
| ELMO1 | rs741301 rs1345365, rs11769038, rs10951509, rs1882080, rs6462776, rs6462777 | P = 0.004 | T2D-DKD | Wu et al., 2013 |
| ERBB4 | rs7588550 | P = 2.1 × 10(-7) | T1D-DKD | Sandholm et al., 2012 |
| FRMD3 | rs942278, rs1888747, rs10868025, rs942280, rs942263, rs1535753, rs2378658, rs13288659 | P = 5.0 × 10(-7) | T1D-ESRD, T2D-ESRD | Pezzolesi et al., 2009a; Freedman et al., 2011 |
| GABRR1 | rs9942471 | P = 4.5 × 10(-8) | T2D-DKD | van Zuydam NR |
| GCKR | rs1260326 | P = 3.23 × 10(-3) | T2D-eGFR | Deshmukh et al., 2013 |
| GLRA3 | rs1564939 | P = 0.0013 | T1D-AER | Sandholm et al., 2018 |
| KLKB | rs4253311 | P = 5.5 × 10(-8) | Plasma renin activity | Lieb et al., 2015 |
| KNG1 | rs5030062 | P = 0.001 | Plasma renin activity | Lieb et al., 2015 |
| LIMK2 | rs2106294, rs4820043 | P = 7.49E-04 and 0.001 | T2D-ESRD | McDonough et al., 2011 |
| MMP9 | rs481480, rs2032487, rs4281481 | P = 0.038, 0.045 and 0.048 P = 0.053, 0.054 and 0.055 | T2D-ESRD T2D-DKD | Freedman et al., 2009; Cooke et al., 2012 |
| MYH9 | rs5750250, rs92280 | P = 4.3 × E(-4) P = 3 × 10(-7) | T2D-ESRD | Freedman et al., 2011; McDonough et al., 2011 |
| PTPN13 | rs61277444 | P < 1 × 10(-6) | T1D-DKD | Sandholm et al., 2017 |
| PVT1 | rs2648875, rs2720709 | P = 1.8–2.1 × (-7) | T2D-ESRD | Hanson et al., 2007 |
| RAET1L | rs1543547 | P = 1 × 10(-5) | T1D-DKD | McKnight et al., 2009 |
| RGMA-MCTP2 | rs12437854 | P = 2 × 10(-9) | T1D-ESRD | Sandholm et al., 2012 |
| RPS12 | rs9493454 | P = 8.79 × 10(-4) | T2D-ESRD | McDonough et al., 2011 |
| SHROOM3 | rs1739721 | P = 3.18 × 10(-3) | T2D-eGFR | Deshmukh et al., 2013 |
| SLC12A3 | rs11643718 | P = 0.021 | T2D-DKD, T2D-ESRD | Tanaka et al., 2003 |
| TMPO | rs4762495 | P = 0.0006 | T1D-ESRD | Craig et al., 2009 |
| UMOD | rs12917707 | P = 8.84 × 10(-4) | T2D-eGFR | Deshmukh et al., 2013 |
| ZMIZ1 | rs1749824 | P = 8.1 × 10(-5) | T1D-ESRD | Craig et al., 2009 |
Data were extracted from more than 300 references in PubMed and most studies were carryout with genetic association study of candidate gene(s). CNVs, Copy Number Variants; DKD, Diabetic Kidney Disease; eGFR, estimated Glomerular Filtration Rate; T1D, Type 1 Diabetes Mellitus; T2D, Type 2 Diabetes Mellitus; ABCG, ATP Binding Cassette Subfamily G; ACACB, Acetyl-CoA Carboxylase Beta; ACE, Angiotensin I Converting Enzyme; ADPOQ, Adiponectin; ADRB2, Adrenoceptor Beta 2; AFF3, AF4/FMR2 Family Member 3; AGER, Advanced Glycosylation End-Product Specific Receptor; AGT, Angiotensinogen; AGTR, Angiotensin II Receptor; AKR1B1, Aldo-Keto Reductase Family 1 Member B; ALOX12, Arachidonate 12-Lipoxygenase, 12S Type; ApoE, Apolipoprotein E; APOL1, Apolipoprotein L1; AUH, AU RNA Binding Methylglutaconyl-CoA Hydratase; BID, BH3 Interacting Domain Death Agonist; CALD1, Caldesmon 1; CaSR, Calcium-Sensing Receptor; CARS, Cysteinyl-TRNA Synthetase; CAT, Catalase; CERS2, Ceramide Synthase 2; CDCA7, Cell Division Cycle Associated 7; CDH13, Cadherin 13; CHN2, Chimerin 2; CNDP, Carnosine Dipeptidase; COQ5, Coenzyme Q5, Methyltransferase; COX6A1, Cytochrome C Oxidase Subunit 6A1; COX10, COX10, Heme A:Farnesyltransferase Cytochrome C Oxidase Assembly Factor; CUBN, Cubilin; CYBA, Cytochrome B-245 Alpha Chain; CYP11B2, Cytochrome P450 Family 11 Subfamily B Member 2; ELMO1, Engulfment And Cell Motility 1; eNOS, Nitric Oxide Synthase; ENPP1, Ectonucleotide Pyrophosphatase/Phosphodiesterase 1; EPO, Erythropoietin; EPHX2, Epoxide Hydrolase 2; ERBB4, Erb-B2 Receptor Tyrosine Kinase 4; ESR1, Estrogen Receptor 1; FRMD3, FERM Domain Containing 3; FNDC5, Fibronectin Type III Domain Containing 5; GAS6, Growth Arrest Specific 6; GATC, Glutamyl-TRNA Amidotransferase Subunit C; GCK, Glucokinase; GCKR, Glucokinase Regulator; GFPT2, Glutamine-Fructose-6-Phosphate Transaminase 2; GLRA3, Glycine Receptor Alpha 3; GPX1, Glutathione Peroxidase 1; GREM1, Gremlin 1, DAN Family BMP Antagonist; GSTP1, Glutathione S-Transferase Pi 1; HIF1α, Hypoxia Inducible Factor 1 Subunit Alpha; H19, H19, Imprinted Maternally Expressed Transcript; HMGA2, High Mobility Group AT-Hook 2; HO1, Heme Oxygenase 1; HSP70, Heat Shock Protein 70; ICAM1, Intercellular Adhesion Molecule 1; IGF2, Insulin Like Growth Factor 2; IGFBP1, Insulin Like Growth Factor Binding Protein 1; IL, Interleukin; IRAK4, Interleukin 1 Receptor Associated Kinase 4; INSR, Insulin Receptor; IRS2, Insulin Receptor Substrate 2; KCNQ1, Potassium Voltage-Gated Channel Subfamily Q Member 1; KLRA1, Killer Cell Lectin Like Receptor A1; KNG1, Kininogen 1; LTA, Lymphotoxin Alpha; LIMK2, LIM Domain Kinase 2; MAPRE1P2, MAPRE1 Pseudogene 2; MCF2L2, MCF.2 Cell Line Derived Transforming Sequence-Like 2; MGP, Matrix Gla Protein; MME, Membrane Metalloendopeptidase; MMP, Matrix Metallopeptidase; MSC, Musculin; MTHFR, Methylenetetrahydrofolate Reductase; MT2A, Metallothionein 2A; MSRB3, Methionine Sulfoxide Reductase B3; MTOR, Mechanistic Target of Rapamycin Kinase; MyD88, Myeloid Differentiation Primary Response 88; MYH9, Myosin Heavy Chain 9; NCALD, Neurocalcin Delta; NOS, Nitric Oxide Synthase; NQO1, NAD(P)H Quinone Dehydrogenase 1; NPHS1, NPHS1, Nephrin; NPY, Neuropeptide Y; PACRG, Parkin Coregulated; PAI1, Plasminogen Activator Inhibitor 1; PARK2, Parkin RBR E3 Ubiquitin Protein Ligase; PFKFB2, 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 2; PLXDC2, Plexin Domain Containing 2; PLEKHH2, Pleckstrin Homology, MyTH4 and FERM Domain Containing H2; PON, Paraoxonase; PPARG, Peroxisome Proliferators-Activated Receptor Gamma; PPARGC1A, Peroxisome Proliferators-Activated Receptor Gamma Co-activator 1 alpha; PRKAA2, Protein Kinase AMP-Activated Catalytic Subunit Alpha 2; PROX1, Prospero Homeobox 1; PSMD9, Proteasome 26S Subunit, Non-ATPase 9; PRKCB1, Protein Kinase C Beta; PTX3, Pentraxin 3; PVT1, Pvt1 Oncogene; RAGE, Advanced Glycosylation End-Product Specific Receptor; RAET1L, Retinoic Acid Early Transcript 1L; RBP4, Retinol Binding Protein 4; REN, Renin; RGMA, Repulsive Guidance Molecule BMP Co-Receptor A; RREB1, Ras Responsive Element Binding Protein 1; TOP1MT, DNA Topoisomerase I Mitochondrial; RPS12, Ribosomal Protein S12; RTN1, Reticulon 1; SASH1, SAM And SH3 Domain Containing 1; SCAF8, SR-Related CTD Associated Factor 8; SEMA6D, Semaphorin 6D; SERPINB, Serpin Family; SHROOM3, Shroom Family Member 3; SIK1, Salt Inducible Kinase 1; SIRT1, Sirtuin 1; SLC2A, Solute Carrier Family 2; SLC12A3, Solute Carrier Family 12 Member 3; SOD, Superoxide Dismutase; SOX2, SRY-Box 2; SORBS1, Sorbin and SH3 Domain Containing 1; SP3, Sp3 Transcription Factor; SUMO4, Small Ubiquitin-Like Modifier 4; SUV39H2, Suppressor Of Variegation 3-9 Homolog 2; TCF7L2, Transcription Factor 7 Like 2; TGFβ1, Transforming Growth Factor Beta 1; TMPO, Thymopoietin; TNFα, Tumor Necrosis Factor alpha; THP, Tamm-Horsfall protein; TRAF6, TNF Receptor Associated Factor 6; TRIB3, Tribbles Pseudokinase 3; UMOD, Uromodulin; VEGF, Vascular Endothelial Growth Factor; VEGFA, Vascular Endothelial Growth Factor A; VDR, Vitamin D Receptor; WNT4, Wnt Family Member 4; ZBTB40, Zinc Finger and BTB Domain Containing 40; ZMIZ1, Zinc Finger MIZ-Type Containing 1.
The CNDP1 (carnosine dipeptidase 1) gene is located in chromosome 18q22.3 and contains 5-leucine (CTG) trinucleotide repeat length polymorphism (D18S880) in the coding region (Wanic et al., 2008). This trinucleotide repeat polymorphism is found to have gender specificity and to confer the susceptibility for DKD and ESRD in T2D (Albrecht et al., 2017b). Furthermore, serum carnosinase (CN-1) activity is negatively correlated with time on hemodialysis (Peters et al., 2016). In addition, several SNPs in this gene are also associated with DKD and ESRD (Janssen et al., 2005; Freedman et al., 2007b; McDonough et al., 2009; Alkhalaf et al., 2010; Mooyaart et al., 2010; Ahluwalia et al., 2011b; Chakkera et al., 2011; Kurashige et al., 2013). Interestingly, an experimental study in BTBR ob/ob mice has demonstrated that treatment with carnosine as the target of CNDP1 improves glucose metabolism and albuminuria, suggesting that carnosine may be a novel therapeutic strategy to treat patients with DKD (Albrecht et al., 2017a).
The ELMO1 (engulfment and cell motility 1) gene is located on chromosome p14.1 and encodes a member of the engulfment and cell motility protein family. The protein interacts with dedicator of cytokinesis proteins and subsequently promotes phagocytosis and cell migration. Increased expression of ELMO1 and dedicator of cytokinesis 1 may promote glioma cell invasion (Patel et al., 2010). Furthermore, several SNPs in this gene are found to be associated with DKD in both T1D and T2D (Shimazaki et al., 2005, 2006; Craig et al., 2009; Leak et al., 2009; Pezzolesi et al., 2009a; Hanson et al., 2010; Wu et al., 2013; Alberto Ramirez-Garcia et al., 2015; Bodhini et al., 2016; Hathaway et al., 2016; Mehrabzadeh et al., 2016; Sharma et al., 2016). The variants associated with DKD, however, are different in the several populations studied, suggesting the presence of allelic heterogeneity probably resulting from the diverse ancestral genetic backgrounds of the different racial groups.
The FRMD3 (FERM domain containing 3) gene is located in chromosome 9q21.32. The FRMD3 gene is expressed in adult brain, fetal skeletal muscle, thymus, ovaries, and podocytes (Ni et al., 2003). Pezzolesi et al. (2009b) have demonstrated that FRMD3 expression in kidneys of a DKD mouse model is decreased as compared with non-diabetic mice. Genetic polymorphisms in the FRMD3 gene are associated with DKD and ESRD in T1D and T2D (Freedman et al., 2011; Al-Waheeb et al., 2016). Furthermore, the members of the bone morphogenetic protein (BMP) interact with FRMD3, which implies that FRMD3 may influence the risk of DKD through regulation of the BMP pathway (Martini et al., 2013; Palmer and Freedman, 2013).
The MMP9 (matrix metallopeptidase 9) gene is located in chromosome 20q13.12. The MMP family members are involved in the breakdown of extracellular matrix (ECM) in physiological processes, such as tissue remodeling, reproduction and embryonic development, while MMP9 is the ninth member in the family. MMP9 may play an essential role in local proteolysis of the extracellular matrix and in leukocyte migration. Moreover, MMPs, including MMP9, are zinc-dependent endopeptidases and the major proteases in ECM degradation. There are common variants such as rs3918242 (-1562C/T) and microsatellites (CA)n in the promoter region and several SNPs rs481480, rs2032487, rs4281481, rs3752462 and rs3918242 are found to be associated with the susceptibility to DKD (Hirakawa et al., 2003; Nair et al., 2008; Ahluwalia et al., 2009; Freedman et al., 2011; Cooke et al., 2012; Zhang et al., 2015; Feng et al., 2016).
Both UMOD (uromodulin) and SLC12A3 (solute carrier family 12 member 3) genes are located in the same chromosome but in short and long arms, respectively, i.e., 16p12.3 and 16q13. SLC12A3 is also known as thiazide-sensitive sodium-chloride cotransporter in kidney distal convoluted tubules, which is important for electrolyte homeostasis. Mutations in this gene are characterized by hypokalemic alkalosis combined with hypomagnesemia, low urinary calcium, but increased renin activity. Tanaka et al. (2003) performed a GWAS in Japanese T2D subjects and reported that the SLC12A3 Arg913Gln polymorphism was associated with reduced risk of DKD. Nishiyama et al. (2005) then conducted another 10-year longitudinal study in the same population. The results confirmed that the 913Gln allele of SLC12A3 Arg913Gln polymorphism conferred a protective effect in DKD (Nishiyama et al., 2005). More recently, Abu Seman et al. (2014) performed a further genetic study of SLC12A3 polymorphisms in a Malaysian population, including the meta-analysis of the association between the SLC12A3 Arg913Gln polymorphism and DKD from all the previous studies. SLC12A3 Arg913Gln polymorphism was found to be associated with T2D (P = 0.028, OR = 0.772, 95% CI = 0.612–0.973) and DKD (P = 0.038, OR = 0.547, 95% CI = 0.308–0.973) in the Malaysian cohort. The meta-analysis confirmed the protective effects of the SLC12A3 913Gln allele in DKD (Z-value = -1.992, P = 0.046, OR = 0.792). In addition, the authors investigated the role of slc12a3 expression in the progress of DKD with db/db mice and in kidney development with zebrafish embryos. With knockdown of zebrafish ortholog, slc12a3 led to structural abnormality of kidney pronephric distal duct at 1-cell stage. Slc12a3 mRNA and protein expression levels were upregulated in kidneys of db/db mice from 6, 12, and 26 weeks at the age. The authors thus concluded that SLC12A3 is a susceptibility gene in DKD, while allele 913Gln but not allele Arg913 has a preventive effect in the disease (Abu Seman et al., 2014). This association of the SLC12A3 Arg913Gln polymorphism with DKD has been very recently replicated in a Chinese population (Zhang et al., 2018). The UMOD gene encoded glycoprotein is synthesized exclusively in renal tubular cells and released into urine. Furthermore, UMOD may prevent urinary tract infection and inhibit formation of liquid containing supersaturated salts and subsequent formation of salt crystals. SNPs rs4293393 and rs1297707 in the UMOD gene are found to be associated with the susceptibility to DKD in T2D (Ahluwalia et al., 2011a; Prudente et al., 2017; van Zuydam et al., 2018).
The Human Genome Project has revealed that there are more than twenty thousand protein coding genes, and probably more than one million of RNA genes6. Genetic association studies of RNA gene polymorphisms with DKD are very limited. Up to date, only two SNPs, i.e., rs2910164 and rs12976445 in the genes for miRNA-146a and miRNA-125 have been found to be associated with DKD in T1D and T2D (Li et al., 2014; Kaidonis et al., 2016). Further investigation of RNA genetic variation conferring susceptibility to DKD needs to be undertaken.
Current Information From Epigenetic Studies in Diabetic Kidney Disease
Similar to genetic association studies, epigenome-wide (EWAS) and candidate gene DNA methylation analyses have been used for epigenetic studies of DKD. Current information from epigenetic studies in DKD are represented in Table 3. An EWAS suggested that several genes, including SLC22A12, TRPM6, AQP9, HP, AGTX, and HYAL2, may have epigenetic effects in DKD (VanderJagt et al., 2015). Interestingly, SLC22A12 encodes for urate anion transporter 1 (URAT1), which is a kidney-specific urate transporter that transports urate across the apical membrane of the proximal tubule in kidneys. Loss-of-function SLC22A12 mutations are associated with renal hypouricaemia and affected persons can develop exercise-induced acute kidney injury and are at increased risk of developing urate stones (Lee et al., 2008). TRPM6 is a member of transient receptor potential superfamily of cation channels. This gene is widely expressed in the body, including kidneys along the nephron. The TRPM6 channels are mainly located in the renal distal convoluted tubule, the site of active transcellular calcium and magnesium transport in the kidney (Felsenfeld et al., 2015). As described previously, several studies have implicated UMOD genetic polymorphisms in the susceptibility to DKD (Ahluwalia et al., 2011a; Prudente et al., 2017; van Zuydam et al., 2018). A recent study has demonstrated that UMOD regulates renal magnesium homeostasis through TRPM6 (Nie et al., 2018). Furthermore, analyses of the candidate genes such as IGFBP1 and MTHFR have also provided evidence that DNA methylation changes in these genes may be involved in the pathogenesis of DKD (Gu et al., 2013, 2014; Yang et al., 2016). Combining and analyzing data from genetic and epigenetic studies together may help understand some of the pathophysiology in DKD.
Table 3.
Current information from epigenetic studies in diabetic kidney disease.
| Analysis | Gene symbol/ Target | Material and methods | Results | References |
|---|---|---|---|---|
| DNA methylation | AKR1B1, TIMP-2 | T2DM-DKD | Hypomethylation of the genes are associated with albuminuria | Aldemir et al., 2017 |
| AKR1B1, IGF1, SLC12A3 | T2DM-DKD and ESRD | Those genes implicated in DKD based upon the inter-individual epigenetic differences | Sapienza et al., 2011 | |
| CTGF | T2DM-DKD Glomerular and mesangial cells | Hypomethylation through the decreased Dnmt3a binding in the gene promoter | Zhang et al., 2014 | |
| IGFBP1 | T1DM-DKD | Hypermethylation | Gu et al., 2014 | |
| IL13RA1, IL15, EDG3, INHA | Hemodialyzed patients with DKD | Hypermethylation | Korabecna et al., 2013 | |
| MTHFR | Diabetic complications, including DKD | Hypermethylation | Dos Santos Nunes et al., 2018 | |
| MTHFR | T2DM-DKD | Demethylation | Yang et al., 2016 | |
| MIOX | Human and mouse | Hypomethylation | Sharma et al., 2017 | |
| PIK3C2B | Glomeruli in DKD | Up-regulated with methylation in glomeruli | Wang et al., 2018 | |
| POLR2G, DDB1, ZNF230 | Down-regulated with methylation in glomeruli | |||
| SLC30A8 | T2DM-DKD | Hypermethylation | Seman et al., 2015 | |
| SLC22A12, TRPM6, AQP9, HP, AGXT, HYAL2 | Pre-diabetes and T2DM-DN | Hypermethylation found in 174 of 694 CpG sites | VanderJagt et al., 2015 | |
| TAMM41, PMPCB, TSFM, AUH | T1DM-DKD | DNA methylation changes in these genes and influence with mitochondrial function | Swan et al., 2015 | |
| UNC13B | T1DM-DKD | An intronic polymorphism rs13293564 in the gene is associated with DKD DNA methylation levels in 19 CpG sites are changed | Bell et al., 2010 | |
| KLF4 | Glomerular podocytes in human and mouse | DNA methylation levels in the promoters of genes encoding mesenchymal markers are increased | Hayashi et al., 2014 | |
| aPC | Podocytes | aPC epigenetically controls p66(Shc) expression | Bock et al., 2013 | |
| egfr | Cultured proximal tubule (normal rat kidney) cells | Inhibition of histone deacetylase in eGFR | Gilbert et al., 2011 | |
| pxr | db/db mice and proximal tubular cells | Demethylation of DNA | Watanabe et al., 2018 | |
| dnmt1 | db/db mice | Hypomethylation | Zhang et al., 2017 | |
| agt, abcc4, cyp4a10, glut5 | db/m mouse | Hypomethylation | Marumo et al., 2015 | |
| kif20b, cldn18, slco1a1 | Hypomethylation | |||
| sglt2, pck1, g6pc, hnf4a | db/db mice | Demethylated in the proximal tubules | Marumo et al., 2015 | |
| tgfb1, tet2 | db/db mice | Decreased DNA methylation | Yang et al., 2018 | |
| Histone modification | MTHFR | T2D with DN | MTHFR regulates histone modification rs1801133 C677T in the gene is associated with DN | Zhou et al., 2015 |
| TGFB1 | Glomerular and mesangial cells | TGF-β1 increases expression of the H3K4 methyltransferase SET7/9 | Sun et al., 2010 | |
| 12/15-LO | Glomerular and mesangial cells | Up-regulation of histone lysine modifications | Yuan et al., 2016 | |
| h3k9/14ac, at1r | Glomerular and mesangial cells db/db mice | Losartan attenuated increased H3K9/14Ac at RAGE, PAI-1 and MCP-1 promoters, while the chromatin state at these genes are mediated in part by AT1R | Reddy et al., 2014 | |
| h3k9, h3k23 | db/db and C57BL/6 mice | Acetylation | Sayyed et al., 2010 | |
| h3k4 in serine 10 | Demethylation and phosphorylation | |||
| h3k9/14ac | db/+ mice | Losartan reversed permissive epigenetic changes in renal glomeruli | Reddy et al., 2014 | |
| set7/9 | db/db mice | Induced histone modification and mcp-1 expression | Chen et al., 2014 | |
| xbp1 | db/db mice | XBP1s-mediated of histone SET7/9 and consequently decreased MCP-1 expression | Chen et al., 2014 | |
| opn/h3k27me3 | Sur1-E1506K mice | Histone modification with opn | Cai et al., 2016 | |
| txnip, h3k9ac, h3k4me3, h3k4me1, h3k27me3 | Sur1-E1506K mice | Histone acetylation changes | De Marinis et al., 2016 | |
| egfr | Cultured proximal tubule (normal rat kidney) cells | Inhibition of histone deacetylase in eGFR | Gilbert et al., 2011 | |
| grp78/histone h4 | Diabetic rats | Acetylation changes | Sun et al., 2016 | |
| mfn2 | Diabetic rats | Histone acetylation at collagen IV promoter | Mi et al., 2016 | |
| h3 and hsp-27, map kinase p28 | Sprague-Dawley rats | Dephosphorylation and acetylation of h3 | Tikoo et al., 2008 | |
| Non-coding RNA dysregulation | miR-9-3, miR34a, miR-137 | DKD and diabetic retinopathy | DNA methylation changes | Dos Santos Nunes et al., 2018 |
| miR-199b-5p, klotho | T2DM-DKD and STZ mice | Increased serum klotho levels are mediated by miR-199b-5p | Kang and Xu, 2016 | |
| microRNA Let-7a-3 | T2DM with DKD | DNA methylation levels in the promoter are increased by targeting UHRF1 | Peng et al., 2015 | |
| microRNA 1207-5P | Glomerular and mesangial cells | This PVT1-derived microRNA is upregulated by glucose and TGF-β1 | Alvarez et al., 2013 | |
| creb1, miR-10a | HFD/STZ mice | This microRNA regulate epigenetic modification by targeting creb1 | Shan et al., 2016 |
DKD, Diabetic Kidney Disease; T1D, Type 1 Diabetes; T2DM, Type 2 Diabetes. The genes predicted by epigenome-wide association analysis are shown in bold, while genes from rodent studies are shown in lower case. AKR1B1, Aldo-Keto Reductase family 1, member B1; aPC, activated Protein C; AQP9, Aquaporin; AT1R, Angiotensin II Receptor type 1; AUH, AU RNA binding protein/enoyl-CoA hydratase; EGFR, epidermal growth factor receptor; CTGF, Connective Tissue Growth Factor; DDB1, Damage Specific DNA Binding Protein 1; EDG3, Endothelial Differentiation G-protein coupled receptor 3; DNMT1, DNA methyltransferase 1; HFD, High Fat Diet; IGF1, Insulin like Growth Factor 1; IGFBP1, Insulin-like Growth Factor Binding Protein 1; IL13RA1, interleukin 13 receptor subunit alpha 1; IL15, Interleukin 15; INHA, Inhibin alpha; KLF4, Kkruppel-like factor 4; MTHFR, Methylenetetrahydrofolate Reductase; MIOX, Myo-Inositol Oxygenase; PIK3C2B, Phosphatidylinositol-4-Phosphate 3-Kinase Catalytic Subunit Type 2 Beta; PMPCB, Peptidase, Mitochondrial Processing beta subunit; POLR2G, RNA Polymerase II Subunit G; SLC12A3, Solute Carrier family 12 member 3; SLC22A12, Solute Carrier family 22 member 12; SLC30A8, Solute Carrier family 30 member 8; TAMM41, TAM41 Mitochondrial translocator assembly and maintenance homolog; tet2, tet methylcytosine dioxygenase 2; TIMP2, TIMP metallopeptidase inhibitor 2; TRPM6, Transient Receptor Potential cation channel subfamily M member 6; TSFM, Ts translation elongation Factor, Mitochondrial; UHRF1, Ubiquitin like with PHD and Ring Finger domains 1; UNC13B, Unc-13 homolog B member 3; XBP1, X-Box Binding Protein 1; ZNF230, Zinc Finger Protein 230; 12/15-LO, 12/15-lipoxygenase; TGFB1, Transforming Growth Factor Beta 1.
ncRNAs regulate gene expression at the post-transcriptional level and are involved in chromatin histone modification. Most of studies concerning histone modification and ncRNA dysregulation have been performed in diabetic animal models, while a few studies have been undertaken in subjects with DKD (Table 3). Reddy et al. (2014) have analyzed histone modification profiles in genes associated with DKD pathology and the modified regulation of these genes following treatment with the angiotensin II type 1 receptor (AT1R) blocker losartan. The data indicate that losartan attenuates key parameters of DKD and modifies gene expression, and reverses some epigenetic changes in db/db mice. Losartan also attenuates increased H3K9/14Ac at RAGE, PAI-1, and MCP-1 promoters in mesangial cells cultured under diabetic conditions (Reddy et al., 2014). In a recent study of subjects of T2D and diabetic complications (including DKD) (Dos Santos Nunes et al., 2018) the methylation profiles of miR gene were compared and related to the presence of diabetic complications. Results indicated that miRs can modulate the expression of a variety of genes and methylation changes of miR-9-3, miR-34a, and miR-137 were found to be associated with diabetic complications (Dos Santos Nunes et al., 2018). These two studies provide evidence suggesting that therapies targeting epigenetic regulators might be beneficial in the treatment of DKD.
Summary and Perspectives
Researchers have made major efforts to undertake well powered genetic and epigenetic studies in DKD to help understand its pathogenesis. The data, however, need to be confirmed by several strategies, for instance, replication studies could be performed with better selection of subjects with similar genetic background to limit influences from migration; intermarriage; cultural preferences; coupled with further investigation of DNA variation and methylation changes in RNA regulation genes and biological experiments to determine functional impact of these variants. Furthermore, new technologies for DNA and ncRNA sequencing analysis such as third generation sequencing and a PheWAS approach have recently been developed.
New Generation Sequencing
DNA sequencing analysis is used for determining the accurate order of nucleotides along chromosomes and genomes. Second-generation sequencing, commonly known as next-generation sequencing (NGS), has presently become popular in DNA sequencing analysis because NGS can enable a massively-paralleled approach capable of producing large numbers of reads at high coverages along the genome and therefore dramatically reduce the cost of DNA sequencing analysis (Treangen and Salzberg, 2011; Gu et al., 2018; Mone et al., 2018). Today, third-generation sequencing (often called as long-read sequencing) is a new generation sequencing method, which works by reading the nucleotide sequences at single molecule level in contrast to the first and second generations of DNA sequencing (van Dijk et al., 2018). Moreover, it is necessary to develop the molecular instruments for whole genome sequencing to make this new generation sequencing commercially available. The advanced sequencing technologies will improve genetic and epigenetic studies in DKD in the near future.
ncRNA Genetic and Epigenetic Studies
In the human genome, RNA genes are much more abundant than protein coding genes, while ncRNAs mainly include miRNAs and lncRNAs. Both forms of ncRNAs have been found to be involved in chromatin histone modifications, and subsequently can have epigenetic effects on the target genes. Therefore, identification of RNA genetic variation and investigation of biological alteration of these RNA genes should be included in research plans. Kato has very recently pointed out a hypothesis that transforming growth factor-β (TGF1β) may play an important role in early stage development of DKD, while some miRNAs and lncRNAs regulate the key molecules in the TGF1β pathway. These ncRNAs may be served as biomarkers for predicting the potential targets for prevention and treatment in DKD (Kato, 2018). Furthermore, Smyth et al. (2018) have compared Sanger sequencing and NGS to validate the five top ranked miRNAs that are predicted to be associated with DKD by EWAS. This study suggests that targeted NGS may offer a more cost-effective and sensitive approach and implied that the methylated miR-329-2, in which region SNP rs10132943 is located, and miR-429 where SNPs rs7521584 and rs112695918 exist, are associated with DKD (Smyth et al., 2018). Although these two studies are preliminary, they may be good examples to help direct further DKD research.
Phenome-Wide Association Study (PheWAS)
PheWAS is a new approach to analyze many phenotypes in comparison with a single genetic variant. This approach was originally described using electronic medical record (EMR) data from EMR-linked with a DNA biobank and also can be combined with GWAS and EWAS. Therefore, PheWAS has become a powerful tool to investigate the impact of genetic variation on drug response among many individuals and may expand our knowledge of new drug targets and effects (Pendergrass and Ritchie, 2015; Denny et al., 2016; Roden, 2017). Clearly, combined with GWAS and EWAS, PheWAS will provide us with the possibility to discover the associations with drug effects, including therapeutic response and side effect profiles in DKD (Hebbring, 2014).
Taken together, application of these advanced studies in DKD will be very useful not only for evaluating current data from genetic and epigenetic studies but also for generating new knowledge for dissecting the complexity of this disease.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ACR
albumin-to-creatinine ratio
- ADA
American Diabetes Association
- BMI
body mass index
- CNV
copy number variant
- DKD
diabetic kidney disease
- ESRD
end-stage renal disease
- EWAS
epigenome-wide association study
- GFR
glomerular filtration rate
- GWAS
genome-wide association study
- IDF
International Diabetes Federation
- IHME
Institute for Health Metrics and Evaluation
- LD
Linkage disequilibrium
- PheWAS
phenome-wide association study
- SNP
single nucleotide polymorphism
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- UAE
urinary albumin excretion
Funding. The study was supported by the Start Grant from China Pharmaceutical University.
References
- Abu Seman N., He B., Ojala J. R., Wan Mohamud W. N., Östenson C. G., Brismar K., et al. (2014). Genetic and biological effects of sodium-chloride cotransporter (SLC12A3) in diabetic nephropathy. Am. J. Nephrol. 40 408–416. 10.1159/000368916 [DOI] [PubMed] [Google Scholar]
- Ahluwalia T. S., Khullar M., Ahuja M., Kohli H. S., Bhansali A., Mohan V., et al. (2009). Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One 4:e5168. 10.1371/journal.pone.0005168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia T. S., Lindholm E., Groop L., Melander O. (2011a). Uromodulin gene variant is associated with type 2 diabetic nephropathy. J. Hypertens. 29 1731–1734. 10.1097/HJH.0b013e328349de25 [DOI] [PubMed] [Google Scholar]
- Ahluwalia T. S., Lindholm E., Groop L. C. (2011b). Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia 54 2295–2302. 10.1007/s00125-011-2178-5 [DOI] [PubMed] [Google Scholar]
- Alberto Ramirez-Garcia S., Charles-Niño C., Mazariegos-Rubí M., Rosalba Topete-González L., Topete-González R., Javier Flores-Alvarado L., et al. (2015). Association of the ELMO1 gene (snp rs1345365) with development of type 2 diabetes mellitus in the Mexican mestizo population. Invest. Clin. 56 341–355. [PubMed] [Google Scholar]
- Albrecht T., Schilperoort M., Zhang S., Braun J. D., Qiu J., Rodriguez A., et al. (2017a). Carnosine attenuates the development of both type 2 diabetes and diabetic nephropathy in BTBR ob/ob mice. Sci. Rep. 7:44492. 10.1038/srep44492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht T., Zhang S., Braun J. D., Xia L., Rodriquez A., Qiu J. (2017b). The CNDP1 (CTG)(5) polymorphism is associated with biopsy-proven diabetic nephropathy, time on hemodialysis, and diabetes duration. J. Diabetes Res. 2017:9506730. 10.1155/2017/9506730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldemir O., Turgut F., Gokce C. (2017). The association between methylation levels of targeted genes and albuminuria in patients with early diabetic kidney disease. Ren. Fail. 39 597–601. 10.1080/0886022X.2017.1358180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalaf A., Bakker S. J., Bilo H. J., Gans R. O., Navis G. J., Postmus D., et al. (2010). A polymorphism in the gene encoding carnosinase (CNDP1) as a predictor of mortality and progression from nephropathy to end-stage renal disease in type 1 diabetes mellitus. Diabetologia 53 2562–2568. 10.1007/s00125-010-1863-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C. D., Jenuwein T. (2016). The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17 487–500. 10.1038/nrg.2016.59 [DOI] [PubMed] [Google Scholar]
- Alvarez M. L., Khosroheidari M., Eddy E., Kiefer J. (2013). Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS One 8:e77468. 10.1371/journal.pone.0077468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waheeb S., Alwohhaib M., Abdelghani A., Al-Sharrah S., Al-Shafey E., Al-Sahow A., et al. (2016). Evaluation of associations between single nucleotide polymorphisms in the FRMD3 and CARS genes and diabetic nephropathy in a Kuwaiti population. Genet. Mol. Res. 15:gmr7619. 10.4238/gmr.15017619 [DOI] [PubMed] [Google Scholar]
- Badal S. S., Danesh F. R. (2014). New insights into molecular mechanisms of diabetic kidney disease. Am. J. Kidney Dis. 63(2 Suppl. 2) S63–S83. 10.1053/j.ajkd.2013.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. N. C., Palmer N. D., Ng M. C. Y., Bonomo J. A., Hicks P. J., Hester J. M., et al. (2014). Analysis of coding variants identified from exome sequencing resources for association with diabetic and non-diabetic nephropathy in African Americans. Hum. Genet. 133 769–779. 10.1007/s00439-013-1415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. J., Liu Z., Khamaisi M., King G. L., Klein R., Klein B. E. K., et al. (2017). Diabetic microvascular disease: an endocrine society scientific statement. J. Clin. Endocrinol. Metab. 102 4343–4410. 10.1210/jc.2017-01922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. G., Teschendorff A. E., Rakyan V. K., Maxwell A. P., Beck S., Savage D. A. (2010). Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genomics 3:33. 10.1186/1755-8794-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock F., Shahzad K., Wang H., Stoyanov S., Wolter J., Dong W., et al. (2013). Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc. Natl. Acad. Sci. U.S.A. 110 648–653. 10.1073/pnas.1218667110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhini D., Chidambaram M., Liju S., Revathi B., Laasya D., Sathish N., et al. (2016). Association of rs11643718 SLC12A3 and rs741301 ELMO1 variants with diabetic nephropathy in south Indian population. Ann. Hum. Genet. 80 336–341. 10.1111/ahg.12174 [DOI] [PubMed] [Google Scholar]
- Bouhairie V. E., McGill J. B. (2016). Diabetic kidney disease. Mol. Med. 113 390–394. [PMC free article] [PubMed] [Google Scholar]
- Cai M., Bompada P., Atac D., Laakso M., Groop L., De Marinis Y. (2016). Epigenetic regulation of glucose-stimulated osteopontin (OPN) expression in diabetic kidney. Biochem. Biophys. Res. Commun. 469 108–113. 10.1016/j.bbrc.2015.11.079 [DOI] [PubMed] [Google Scholar]
- Chakkera H. A., Hanson R. L., Kobes S., Millis M. P., Nelson R. G., Knowler W. C., et al. (2011). Association of variants in the carnosine peptidase 1 gene (CNDP1) with diabetic nephropathy in American Indians. Mol. Genet. Metab. 103 185–190. 10.1016/j.ymgme.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmet R., Duffy S., Keshavarzi S., Gyorgy B., Marre M., Rossing P., et al. (2018). Novel risk genes identified in a genome-wide association study for coronary artery disease in patients with type 1 diabetes. Cardiovasc. Diabetol. 17:61. 10.1186/s12933-018-0705-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Guo Y., Zeng W., Huang L., Pang Q., Nie L., et al. (2014). ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Renal Physiol. 306 F916–F925. 10.1152/ajprenal.00697.2012 [DOI] [PubMed] [Google Scholar]
- Cooke J. N., Bostrom M. A., Hicks P. J., Ng M. C., Hellwege J. N., Comeau M. E., et al. (2012). Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol. Dial. Transplant. 27 1505–1511. 10.1093/ndt/gfr522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D. W., Millis M. P., DiStefano J. K. (2009). Genome-wide SNP genotyping study using pooled DNA to identify candidate markers mediating susceptibility to end-stage renal disease attributed to Type 1 diabetes. Diabet. Med. 26 1090–1098. 10.1111/j.1464-5491.2009.02846.x [DOI] [PubMed] [Google Scholar]
- Cross S. H., Bird A. P. (1995). CpG islands and genes. Curr. Opin. Genet. Dev. 5 309–314. [DOI] [PubMed] [Google Scholar]
- De Marinis Y., Cai M., Bompada P., Atac D., Kotova O., Johansson M. E., et al. (2016). Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int. 89 342–353. 10.1016/j.kint.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Deaton A. M., Bird A. (2011). CpG islands and the regulation of transcription. Genes Dev. 25 1010–1022. 10.1101/gad.2037511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny J. C., Bastarache L., Roden D. M. (2016). Phenome-wide association studies as a tool to advance precision medicine. Annu. Rev. Genomics Hum. Genet. 17 353–373. 10.1146/annurev-genom-090314-024956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh H. A., Palmer C. N., Morris A. D., Colhoun H. M. (2013). Investigation of known estimated glomerular filtration rate loci in patients with type 2 diabetes. Diabet. Med. 30 1230–1235. 10.1111/dme.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick K. J., Nelson C. P., Tsaprouni L., Sandling J. K., Aïssi D., Wahl S., et al. (2014). DNA methylation and body-mass index: a genome-wide analysis. Lancet 383 1990–1998. 10.1016/S0140-6736(13)62674-4 [DOI] [PubMed] [Google Scholar]
- Do C., Shearer A., Suzuki M., Terry M. B., Gelernter J., Greally J. M., et al. (2017). Genetic-epigenetic interactions in cis: a major focus in the post-GWAS era. Genome Biol. 18:120. 10.1186/s13059-017-1250-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Nunes M. K., Silva A. S., Wanderley de Queiroga Evangelista I., Modesto Filho J., Alves Pegado Gomes C. N., Ferreira do Nascimento R. A., et al. (2018). Analysis of the DNA methylation profiles of miR-9-3, miR-34a, and miR-137 promoters in patients with diabetic retinopathy and nephropathy. J. Diabetes Complications 32 593–601. 10.1016/j.jdiacomp.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Dousdampanis P., Trigka K., Mouzaki A. (2016). Tregs and kidney: from diabetic nephropathy to renal transplantation. World J. Transplant. 6 556–563. 10.5500/wjt.v6.i3.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld A. J., Levine B. S., Rodriguez M. (2015). Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin. Dial. 28 564–577. 10.1111/sdi.12411 [DOI] [PubMed] [Google Scholar]
- Feng S., Ye G., Bai S., Wei H., Liao X., Li L. (2016). Matrix metalloproteinase-9 -1562C/T gene polymorphism is associated with diabetic nephropathy. Biomed Res. Int. 2016:1627143. 10.1155/2016/1627143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez J. C. (2016). Genetics of diabetic kidney disease. Semin. Nephrol. 36 474–480. [DOI] [PubMed] [Google Scholar]
- Freedman B. I., Bostrom M., Daeihagh P., Bowden D. W. (2007a). Genetic factors in diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2 1306–1316. [DOI] [PubMed] [Google Scholar]
- Freedman B. I., Hicks P. J., Bostrom M. A., Comeau M. E., Divers J., Bleyer A. J.et al. (2009). Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol. Dial. Transplant. 24 3366–3371. 10.1093/ndt/gfp316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B. I., Hicks P. J., Sale M. M., Pierson E. D., Langefeld C. D., Rich S. S., et al. (2007b). A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol. Dial. Transplant. 22 1131–1135. 10.1093/ndt/gfl717 [DOI] [PubMed] [Google Scholar]
- Freedman B. I., Langefeld C. D., Lu L., Divers J., Comeau M. E., Kopp J. B., et al. (2011). Differential effects of MYH9 and APOL1 risk variants on FRMD3 association with diabetic ESRD in African Americans. PLoS Genet. 7:e1002150. 10.1371/journal.pgen.1002150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. E., Huang Q., Thai K., Advani S. L., Lee K., Yuen D. A., et al. (2011). Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int. 79 1312–1321. 10.1038/ki.2011.39 [DOI] [PubMed] [Google Scholar]
- Gnudi L., Coward R. J. M., Long D. A. (2016). Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol. Metab. 27 820–830. 10.1016/j.tem.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Gu H. F., Brismar K. (2012). Genetic association studies in diabetic nephropathy. Curr. Diabetes Rev. 8 336–344. 10.2174/157339912802083522 [DOI] [PubMed] [Google Scholar]
- Gu T., Falhammar H., Gu H. F., Brismar K. (2014). Epigenetic analyses of the insulin-like growth factor binding protein 1 gene in type 1 diabetes and diabetic nephropathy. Clin. Epigenetics 6:10. 10.1186/1868-7083-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T., Gu H. F., Hilding A., Sjöholm L. K., Ostenson C. G., Ekström T. J., et al. (2013). Increased DNA methylation levels of the insulin-like growth factor binding protein 1 gene are associated with type 2 diabetes in Swedish men. Clin. Epigenetics 5:21. 10.1186/1868-7083-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Miller S., Chiu C. Y. (2018). Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M., Ma J., Keaton J. M., Dimitrov L., Mudgal P., Stromberg M., et al. (2016). Association of kidney structure-related gene variants with type 2 diabetes-attributed end-stage kidney disease in African Americans. Hum. Genet. 135 1251–1262. 10.1007/s00439-016-1714-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. L., Craig D. W., Millis M. P., Yeatts K. A., Kobes S., Pearson J. V., et al. (2007). Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes 56 975–983. 10.2337/db06-1072 [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Millis M. P., Young N. J., Kobes S., Nelson R. G., Knowler W. C., et al. (2010). ELMO1 variants and susceptibility to diabetic nephropathy in American Indians. Mol. Genet. Metab. 101 383–390. 10.1016/j.ymgme.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjutsalo V., Groop P. H. (2014). Epidemiology and risk factors for diabetic kidney disease. Adv. Chronic Kidney Dis. 21 260–266. 10.1053/j.ackd.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Hathaway C. K., Chang A. S., Grant R., Kim H. S., Madden V. J., Bagnell C. R., Jr., et al. (2016). High Elmo1 expression aggravates and low Elmo1 expression prevents diabetic nephropathy. Proc. Natl. Acad. Sci. U.S.A. 113 2218–2222. 10.1073/pnas.1600511113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Sasamura H., Nakamura M., Azegami T., Oguchi H., Sakamaki Y., et al. (2014). KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J. Clin. Invest. 124 2523–2537. 10.1172/JCI69557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbring S. J. (2014). The challenges, advantages and future of phenome-wide association studies. Immunology 141 157–165. 10.1111/imm.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S., Lange E. M., Colicigno C. J., Freedman B. I., Rich S. S., Bowden D. W. (2003). Evaluation of genetic variation and association in the matrix metalloproteinase 9 (MMP9) gene in ESRD patients. Am. J. Kidney Dis. 42 133–142. 10.1016/s0272-6386(03)00416-5 [DOI] [PubMed] [Google Scholar]
- Holoch D., Moazed D. (2015). RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 16 71–84. 10.1038/nrg3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B., Hohenadel D., Brinkkoetter P., Peters V., Rind N., Fischer C., et al. (2005). Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54 2320–2327. 10.2337/diabetes.54.8.2320 [DOI] [PubMed] [Google Scholar]
- Jones P. A. (2012). Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13 484–492. 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- Kaidonis G., Gillies M. C., Abhary S., Liu E., Essex R. W., Chang J. H., et al. (2016). A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol. 53 643–650. 10.1007/s00592-016-0850-4 [DOI] [PubMed] [Google Scholar]
- Kang W. L., Xu G. S. (2016). Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci. Rep. 6:19979. 10.1038/srep19979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. (2018). Noncoding RNAs as therapeutic targets in early stage diabetic kidney disease. Kidney Res. Clin. Pract. 37 197–209. 10.23876/j.krcp.2018.37.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Natarajan R. (2014). Diabetic nephropathy–emerging epigenetic mechanisms. Nat. Rev. Nephrol. 10 517–530. 10.1038/nrneph.2014.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating S. T., van Diepen J. A., Riksen N. P., El-Osta A. (2018). Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia 61 6–20. 10.1007/s00125-017-4490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korabecna M., Pazourkova E., Horinek A., Mokrejsova M., Tesar V. (2013). Methylation status of immune response genes promoters in cell-free DNA differs in hemodialyzed patients with diabetic nephropathy according to the intensity of anemia therapy. Blood Purif. 36 280–286. 10.1159/000356094 [DOI] [PubMed] [Google Scholar]
- Kurashige M., Imamura M., Araki S., Suzuki D., Babazono T., Uzu T., et al. (2013). The influence of a single nucleotide polymorphism within CNDP1 on susceptibility to diabetic nephropathy in Japanese women with type 2 diabetes. PLoS One 8:e54064. 10.1371/journal.pone.0054064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen T., Greally J. M. (2017). Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet. 18 441–451. 10.1038/nrg.2017.32 [DOI] [PubMed] [Google Scholar]
- Leak T. S., Perlegas P. S., Smith S. G., Keene K. L., Hicks P. J., Langefeld C. D., et al. (2009). Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann. Hum. Genet. 73 152–159. 10.1111/j.1469-1809.2008.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Choi H. J., Lee B. H., Kang H. K., Chin H. J., Yoon H. J., et al. (2008). Prevalence of hypouricaemia and SLC22A12 mutations in healthy Korean subjects. Nephrology 13 661–666. 10.1111/j.1440-1797.2008.01029.x [DOI] [PubMed] [Google Scholar]
- Li S. Y., Huang P. H., Yang A. H., Tarng D. C., Yang W. C., Lin C. C., et al. (2014). Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int. 86 358–369. 10.1038/ki.2014.67 [DOI] [PubMed] [Google Scholar]
- Lieb W., Chen M. H., Teumer A., de Boer R. A., Lin H., Fox E. R. (2015). EchoGen consortium. Genome-wide meta-analyses of plasma renin activity and concentration reveal association with the kininogen 1 and prekallikrein genes. Circ. Cardiovasc. Genet. 8 131–140. 10.1093/hmg/ddr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. C., Dorajoo R., Zhang X., Wang L., Ang S. F., Tan C. S. H., et al. (2017). Genetic variants in the receptor for advanced glycation end products (RAGE) gene were associated with circulating soluble RAGE level but not with renal function among Asians with type 2 diabetes: a genome-wide association study. Nephrol. Dial. Transplant. 32 1697–1704. 10.1093/ndt/gfw263 [DOI] [PubMed] [Google Scholar]
- Maeda S., Imamura M., Kurashige M., Araki S., Suzuki D., Babazono T., et al. (2013). Replication study for the association of 3 SNP loci identified in a genome-wide association study for diabetic nephropathy in European type 1 diabetes with diabetic nephropathy in Japanese patients with type 2 diabetes. Clin. Exp. Nephrol. 17 866–871. 10.1007/s10157-013-0797-5 [DOI] [PubMed] [Google Scholar]
- Martini S., Nair V., Patel S. R., Eichinger F., Nelson R. G., Weil E. J., et al. (2013). From single nucleotide polymorphism to transcriptional mechanism: a model for FRMD3 in diabetic nephropathy. Diabetes 62 2605–2612. 10.2337/db12-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo T., Yagi S., Kawarazaki W., Nishimoto M., Ayuzawa N., Watanabe A., et al. (2015). Diabetes induces aberrant DNA methylation in the proximal tubules of the kidney. J. Am. Soc. Nephrol. 26 2388–2397. 10.1681/ASN.2014070665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough C. W., Hicks P. J., Lu L., Langefeld C. D., Freedman B. I., Bowden D. W. (2009). The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum. Genet. 126 265–275. 10.1007/s00439-009-0667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough C. W., Palmer N. D., Hicks P. J., Roh B. H., An S. S., Cooke J. N., et al. (2011). A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 79 563–572. 10.1038/ki.2010.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Currie D., Patterson C. C., Maxwell A. P., Fogarty D. G. Warren, 3/UK GoKinD Study Group. (2009). Targeted genome-wide investigation identifies novel SNPs associated with diabetic nephropathy. Hugo J. 3 77–82. 10.1007/s11568-010-9133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabzadeh M., Pasalar P., Karimi M., Abdollahi M., Daneshpour M., Asadolahpour E., et al. (2016). Association between ELMO1 gene polymorphisms and diabetic nephropathy in an Iranian population. J. Diabetes Metab. Disord. 15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi X., Tang W., Chen X., Liu F., Tang X. (2016). Mitofusin 2 attenuates the histone acetylation at collagen IV promoter in diabetic nephropathy. J. Mol. Endocrinol. 57 233–249. 10.1530/JME-16-0031 [DOI] [PubMed] [Google Scholar]
- Mone F., Quinlan-Jones E., Kilby M. D. (2018). Clinical utility of exome sequencing in the prenatal diagnosis of congenital anomalies: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 231 19–24. 10.1016/j.ejogrb.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Mooyaart A. L., Zutinic A., Bakker S. J., Grootendorst D. C., Kleefstra N., van Valkengoed I. G., et al. (2010). Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes 59 1555–1559. 10.2337/db09-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murea M., Ma L., Freedman B. I. (2012). Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. 9 6–22. 10.1900/RDS.2012.9.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Phillips A. O., Norton N., Spurlock G., Williams H. J., Craig K. J., et al. (2008). Further evidence for the association of MMP9 with nephropathy in type 2 diabetes and application of DNA pooling technology to candidate gene screening. J. Nephrol. 21 400–405. [PubMed] [Google Scholar]
- Ni X., Ji C., Cao G., Cheng H., Guo L., Gu S., et al. (2003). Molecular cloning and characterization of the protein 4.1O gene, a novel member of the protein 4.1 family with focal expression in ovary. J. Hum. Genet. 48 101–106. 10.1007/s100380300015 [DOI] [PubMed] [Google Scholar]
- Nicolas A., Fatima S., Lamri A., Bellili-Muñoz N., Halimi J. M., Saulnier P. J., et al. (2015). ABCG8 polymorphisms and renal disease in type 2 diabetic patients. Metabolism 64 713–719. 10.1016/j.metabol.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Nie M., Bal M. S., Liu J., Yang Z., Rivera C., Wu X. R., et al. (2018). Uromodulin regulates renal magnesium homeostasis through the ion channel transient receptor potential melastatin 6 (TRPM6). J. Biol. Chem. 293 16488–16502. 10.1074/jbc.RA118.003950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Tanaka Y., Nakajima K., Mokubo A., Atsumi Y., Matsuoka K., et al. (2005). Polymorphism of the solute carrier family 12 (sodium/chloride transporters) member 3, SLC12A3, gene at exon 23 (+78G/A: Arg913Gln) is associated with elevation of urinary albumin excretion in Japanese patients with type 2 diabetes: a 10-year longitudinal study. Diabetologia 48 1335–1338. 10.1007/s00125-005-1785-4 [DOI] [PubMed] [Google Scholar]
- Palmer N. D., Freedman B. I. (2013). Diabetic nephropathy: FRMD3 in diabetic nephropathy–guilt by association. Nat. Rev. Nephrol. 9 313–314. 10.1038/nrneph.2013.81 [DOI] [PubMed] [Google Scholar]
- Palmer N. D., Ng M. C., Hicks P. J., Mudgal P., Langefeld C. D., Freedman B. I., et al. (2014). Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS One 9:e88273. 10.1371/journal.pone.0088273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou-Marketou N., Chrousos G. P., Kanaka-Gantenbein C. (2017). Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab. Res. Rev. 33:e2841. 10.1002/dmrr.2841 [DOI] [PubMed] [Google Scholar]
- Patel M., Margaron Y., Fradet N., Yang Q., Wilkes B., Bouvier M., et al. (2010). An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr. Biol. 20 2021–2027. 10.1016/j.cub.2010.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass S. A., Ritchie M. D. (2015). Phenome-wide association studies: leveraging comprehensive phenotypic and genotypic data for discovery. Curr. Genet. Med. Rep. 3 92–100. 10.1007/s40142-015-0067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R., Liu H., Peng H., Zhou J., Zha H., Chen X., et al. (2015). Promoter hypermethylation of let-7a-3 is relevant to its down-expression in diabetic nephropathy by targeting UHRF1. Gene 570 57–63. 10.1016/j.gene.2015.05.073 [DOI] [PubMed] [Google Scholar]
- Peters V., Kebbewar M., Janssen B., Hoffmann G. F., Möller K., Wygoda S. (2016). CNDP1 genotype and renal survival in pediatric nephropathies. J. Pediatr. Endocrinol. Metab. 29 827–833. 10.1515/jpem-2015-0262 [DOI] [PubMed] [Google Scholar]
- Pezzolesi M. G., Katavetin P., Kure M., Poznik G. D., Skupien J., Mychaleckyj J. C., et al. (2009a). Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes 58 2698–2702. 10.2337/db09-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzolesi M. G., Poznik G. D., Mychaleckyj J. C., Paterson A. D., Barati M. T., Klein J. B. (2009b). Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente S., Di Paola R., Copetti M., Lucchesi D., Lamacchia O., Pezzilli S. (2017). The rs12917707 polymorphism at the UMOD locus and glomerular filtration rate in individuals with type 2 diabetes: evidence of heterogeneity across two different European populations. Nephrol. Dial. Transplant. 32 1718–1722. 10.1093/ndt/gfw262 [DOI] [PubMed] [Google Scholar]
- Rakyan V. K., Down T. A., Balding D. J., Beck S. (2011). Epigenome-wide association studies for common human diseases. Nat. Rev. Genet. 12 529–541. 10.1038/nrg3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. A., Sumanth P., Lanting L., Yuan H., Wang M., Mar D., et al. (2014). Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int. 85 362–373. 10.1038/ki.2013.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy K., Kang H. M., Hostetter T., Susztak K. (2014). Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 124 2333–2340. 10.1172/JCI72271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden D. M. (2017). Phenome-wide association studies: a new method for functional genomics in humans. J. Physiol. 595 4109–4115. 10.1113/JP273122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm N., Forsblom C., Mäkinen V. P., McKnight A. J., Osterholm A. M., He B. (2014). Genome-wide association study of urinary albumin excretion rate in patients with type 1 diabetes. Diabetologia 57 1143–1153. 10.1007/s00125-014-3202-3 [DOI] [PubMed] [Google Scholar]
- Sandholm N., Haukka J. K., Toppila I., Valo E., Harjutsalo V., Forsblom C., et al. (2018). Confirmation of GLRA3 as a susceptibility locus for albuminuria in Finnish patients with type 1 diabetes. Sci. Rep. 8:12408. 10.1038/s41598-018-29211-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm N., McKnight A. J., Salem R. M., Brennan E. P., Forsblom C., Harjutsalo V. (2013). FinnDiane Study Group and the GENIE Consortium. Chromosome 2q31.1 associates with ESRD in women with type 1 diabetes. J. Am. Soc. Nephrol. 24 1537–1543. 10.1681/ASN.2012111122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm N., Salem R. M., McKnight A. J., Brennan E. P., Forsblom C., Isakova T. (2012). New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 8:e1002921. 10.1371/journal.pgen.1002921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm N., Van Zuydam N., Ahlqvist E., Juliusdottir T., Deshmukh H. A., Rayner N. W. (2017). The genetic landscape of renal complications in type 1 diabetes. J. Am. Soc. Nephrol. 28 557–574. 10.1681/ASN.2016020231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., Lee J., Powell J., Erinle O., Yafai F., Reichert J., et al. (2011). DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 6 20–28. 10.4161/epi.6.1.13362 [DOI] [PubMed] [Google Scholar]
- Sayyed S. G., Gaikwad A. B., Lichtnekert J., Kulkarni O., Eulberg D., Klussmann S., et al. (2010). Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol. Dial. Transplant. 25 1811–1817. 10.1093/ndt/gfp730 [DOI] [PubMed] [Google Scholar]
- Seman N. A., Mohamud W. N., Östenson C. G., Brismar K., Gu H. F. (2015). Increased DNA methylation of the SLC30A8 gene promoter is associated with type 2 diabetes in a Malay population. Clin. Epigenetics 7:30. 10.1186/s13148-015-0049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q., Zheng G., Zhu A., Cao L., Lu J., Wu D., et al. (2016). Epigenetic modification of miR-10a regulates renal damage by targeting CREB1 in type 2 diabetes mellitus. Toxicol. Appl. Pharmacol. 306 134–143. 10.1016/j.taap.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Sharma I., Dutta R. K., Singh N. K., Kanwar Y. S. (2017). High glucose-induced hypomethylation promotes binding of Sp-1 to myo-inositol oxygenase: implication in the pathobiology of diabetic tubulopathy. Am. J. Pathol. 187 724–739. 10.1016/j.ajpath.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K. R., Heckler K., Stoll S. J., Hillebrands J. L., Kynast K., Herpel E., et al. (2016). ELMO1 protects renal structure and ultrafiltration in kidney development and under diabetic conditions. Sci. Rep. 6:37172. 10.1038/srep37172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman D., Pare G., Oberbauer R., Louie J. Z., Rowland C. M., Devlin J. J., et al. (2014). A gene variant in CERS2 is associated with rate of increase in albuminuria in patients with diabetes from ONTARGET and TRANSCEND. PLoS One 9:e106631. 10.1371/journal.pone.0106631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki A., Kawamura Y., Kanazawa A., Sekine A., Saito S., Tsunoda T., et al. (2005). Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes 54 1171–1178. 10.2337/diabetes.54.4.1171 [DOI] [PubMed] [Google Scholar]
- Shimazaki A., Tanaka Y., Shinosaki T., Ikeda M., Watada H., Hirose T., et al. (2006). ELMO1 increases expression of extracellular matrix proteins and inhibits cell adhesion to ECMs. Kidney Int. 70 1769–1776. 10.1038/sj.ki.5001939 [DOI] [PubMed] [Google Scholar]
- Smyth L. J., Maxwell A. P., Benson K. A., Kilner J., McKay G. J., McKnight A. J. (2018). Validation of differentially methylated microRNAs identified from an epigenome-wide association study; Sanger and next generation sequencing approaches. BMC Res. Notes 11:767. 10.1186/s13104-018-3872-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Reddy M. A., Yuan H., Lanting L., Kato M., Natarajan R. (2010). Epigenetic histone methylation modulates fibrotic gene expression. J. Am. Soc. Nephrol. 21 2069–2080. 10.1681/ASN.2010060633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. Y., Qin H. J., Zhang Z., Xu Y., Yang X. C., Zhao D. M., et al. (2016). Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 13 661–668. 10.3892/mmr.2015.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan E. J., Maxwell A. P., McKnight A. J. (2015). Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. 32 1110–1115. 10.1111/dme.12775 [DOI] [PubMed] [Google Scholar]
- Tanaka N., Babazono T., Saito S., Sekine A., Tsunoda T., Haneda M., et al. (2003). Association of solute carrier family 12 (sodium/chloride) member 3 with diabetic nephropathy, identified by genome-wide analyses of single nucleotide polymorphisms. Diabetes 52 2848–2853. 10.2337/diabetes.52.11.2848 [DOI] [PubMed] [Google Scholar]
- Teumer A., Tin A., Sorice R., Gorski M., Yeo N. C., Chu A. Y. (2016). Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes 65 803–817. 10.2337/db15-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thameem F., Igo R. P., Jr., Freedman B. I., Langefeld C., Hanson R. L., Schelling J. R. (2013). Family investigation of nephropathy and diabetes research group. A genome-wide search for linkage of estimated glomerular filtration rate (eGFR) in the family investigation of nephropathy and diabetes (FIND). PLoS One 8:e81888. 10.1371/journal.pone.0081888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. C. (2016). Epigenetic mechanisms in diabetic kidney disease. Curr. Diab. Rep. 16:31. 10.1007/s11892-016-0723-9 [DOI] [PubMed] [Google Scholar]
- Thomas M. C., Brownlee M., Susztak K., Sharma K., Jandeleit-Dahm K. A., Zoungas S., et al. (2015). Diabetic kidney disease. Nat. Rev. Dis. Primers 1:15018. 10.1038/nrdp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. C., Groop P. H., Tryggvason K. (2012). Towards understanding the inherited susceptibility for nephropathy in diabetes. Curr. Opin. Nephrol. Hypertens. 21 195–202. 10.1097/MNH.0b013e328350313e [DOI] [PubMed] [Google Scholar]
- Tikoo K., Meena R. L., Kabra D. G., Gaikwad A. B. (2008). Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br. J. Pharmacol. 153 1225–1231. 10.1038/sj.bjp.0707666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen T. J., Salzberg S. L. (2011). Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat. Rev. Genet. 13 36–46. 10.1038/nrg3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E. L., Jaszczyszyn Y., Naquin D., Thermes C. (2018). The third revolution in sequencing technology. Trends Genet. 34 666–681. 10.1016/j.tig.2018.05.008 [DOI] [PubMed] [Google Scholar]
- van Zuydam N. R., Ahlqvist E., Sandholm N., Deshmukh H., Rayner N. W., Abdalla M. S. (2018). A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes 67 1414–1427. 10.2337/db17-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderJagt T. A., Neugebauer M. H., Morgan M., Bowden D. W., Shah V. O. (2015). Epigenetic profiles of pre-diabetes transitioning to type 2 diabetes and nephropathy. World J. Diabetes 6 1113–1121. 10.4239/wjd.v6.i9.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve L. M., Natarajan R. (2010). The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Renal Physiol. 299 F14–F25. 10.1152/ajprenal.00200.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhang Y., Wang N., Liu Q., Wang Z., Liu B., et al. (2018). Synergistical action of the β2 adrenoceptor and fatty acid binding protein 2 polymorphisms on the loss of glomerular filtration rate in Chinese patients with type 2 diabetic nephropathy. Int. Urol. Nephrol. 50 715–723. 10.1007/s11255-018-1812-2 [DOI] [PubMed] [Google Scholar]
- Wanic K., Placha G., Dunn J., Smiles A., Warram J. H., Krolewski A. S. (2008). Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case-control and follow-up studies. Diabetes 57 2547–2551. 10.2337/db07-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Marumo T., Kawarazaki W., Nishimoto M., Ayuzawa N., Ueda K., et al. (2018). Aberrant DNA methylation of pregnane X receptor underlies metabolic gene alterations in the diabetic kidney. Am. J. Physiol. Renal Physiol. 314 F551–F560. 10.1152/ajprenal.00390.2017 [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Wang Y., Chen M., Zhang X., Wang D., Pan Y., et al. (2013). Association of ELMO1 gene polymorphisms with diabetic nephropathy in Chinese population. J. Endocrinol. Invest. 36 298–302. 10.3275/8525 [DOI] [PubMed] [Google Scholar]
- Yang L., Zhang Q., Wu Q., Wei Y., Yu J., Mu J., et al. (2018). Effect of TET2 on the pathogenesis of diabetic nephropathy through activation of transforming growth factor β1 expression via DNA demethylation. Life Sci. 207 127–137. 10.1016/j.lfs.2018.04.044 [DOI] [PubMed] [Google Scholar]
- Yang X. H., Cao R. F., Yu Y., Sui M., Zhang T., Xu J. Y., et al. (2016). A study on the correlation between MTHFR promoter methylation and diabetic nephropathy. Am. J. Transl. Res. 8 4960–4967. [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Reddy M. A., Deshpande S., Jia Y., Park J. T., Lanting L. L., et al. (2016). Epigenetic histone modifications involved in profibrotic gene regulation by 12/15-lipoxygenase and its oxidized lipid products in diabetic nephropathy. Antioxid. Redox Signal. 24 361–375. 10.1089/ars.2015.6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Cai X., Yi B., Huang J., Wang J., Sun J. (2014). Correlation of CTGF gene promoter methylation with CTGF expression in type 2 diabetes mellitus with or without nephropathy. Mol. Med. Rep. 9 2138–2144. 10.3892/mmr.2014.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Q., Liu S., Chen Y., Li R., Lin T., et al. (2017). DNA methyltransferase 1 may be a therapy target for attenuating diabetic nephropathy and podocyte injury. Kidney Int. 92 140–153. 10.1016/j.kint.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhuang L., Li M., Zhang J., Zhao W., Ge X., et al. (2018). Arg913Gln of SLC12A3 gene promotes development and progression of end-stage renal disease in Chinese type 2 diabetes mellitus. Mol. Cell. Biochem. 437 203–210. 10.1007/s11010-017-3120-z [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wu X., Cai T., Gao W., Zhou X., Zhao J., et al. (2015). Matrix metalloproteinase 9 gene promoter (rs3918242) mutation reduces the risk of diabetic microvascular complications. Int. J. Environ. Res. Public Health 12 8023–8033. 10.3390/ijerph120708023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T. B., Drummen G. P., Jiang Z. P., Li H. Y. (2015). Methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism and diabetic nephropathy susceptibility in patients with type 2 diabetes mellitus. Ren. Fail. 37 1247–1259. 10.3109/0886022X.2015.1064743 [DOI] [PubMed] [Google Scholar]


