Abstract
Hyperglycemia in patients with diabetes induces vascular endothelial cell apoptosis and subsequent vasculopathy. The aim of the current study was to investigate the pathological mechanism of hyperglycemia-induced endothelial cell apoptosis and vasculopathy using human umbilical vein endothelial cells. As high glucose-induced apoptosis is caused by elevated mitochondrial permeability-mediated release of mitochondrial cytochrome c, the current study examined voltage-dependent anion channel (VDAC1), the controller of mitochondrial permeability, and its regulators, hexokinase2 (HK2), Bcl-2 and Bax. The current study demonstrated that HK2 may be involved in high glucose-induced cell apoptosis, as HK2 overexpression partially reversed high glucose-induced downregulation of mitochondrial/cellular HK2 and Bcl-2 as well as upregulation of mitochondrial Bax. These results suggest that HK2 overexpression partially reversed the reduced binding of HK2 and Bcl-2 and the enhanced binding of Bax to VDAC1, which reduced the high mitochondrial permeability observed under high-glucose conditions. Furthermore, high glucose reduced HK2 transcription via down-regulation of the HK2 transcriptional factor, peroxisome proliferator activated receptor γ (PPARγ). Taken together, these results suggest that PPARγ/HK2 may be novel targets for the prevention of diabetic vasculopathy.
Keywords: hyperglycemia, hexokinase 2, voltage-dependent anion channel 1, Bcl-2, Bcl-2 associated X, mitochondria
Introduction
Diabetes mellitus is a chronic endocrine disorder, characterized by hyperglycemia and other metabolic disorders of fat, protein and electrolytes due to absolute or relative insulin deficiency (1). Epidemiological data suggest that diabetes-induced cardiovascular complications are a leading cause of diabetes-related mortality and disability (2). Diabetes-induced cardiovascular complications include macrovascular and microvascular diseases (3), of which vascular endothelial cell apoptosis is considered to be an initial event and pathological basis (4). However, the underlying mechanism of diabetes-induced endothelial cell apoptosis remains unknown.
Mitochondria, membrane-bound organelles located in the cytoplasm of almost all eukaryotic cells, are commonly referred to as cellular power plants critical to the production of energy in the form of ATP (5). In addition, mitochondria serve a central role in cell apoptosis (6). Damage to the mitochondria leads to increased mitochondrial membrane permeability and subsequent release of cytochrome c (7), an essential component of the electron transport chain (8). Upon entering the cytoplasm, cytochrome c interacts with apoptotic protease activating factor-1 that triggers the activation of caspase cascades (9). Previous studies have confirmed that hyperglycemia induces mitochondrial pathway-dependent apoptosis in vascular endothelial cells (10), however the underlying mechanisms remain unknown.
Increasing evidence suggests that voltage-dependent anion channel (VDAC), an abundant protein located in the outer mitochondrial membrane (11), serves a role in the regulation of mitochondrial membrane permeability and is essential for the release of cytochrome c during cell apoptosis (12). The VDAC protein family consists of 3 isoforms: VDAC1, VDAC2 and VDAC3, and vascular endothelial cells mainly express VDAC1 (13). VDAC provides the pathway for the movement of ATP and other small molecules out of the mitochondria (14). Mitochondrial membrane permeability is regulated by VDAC1 together with other factors including hexokinase 2 (HK2), Bcl-2 and Bax (15). HK2 and Bcl-2 decreases, while Bax increases mitochondrial permeability (16–18). Once cells are exposed to apoptogenic factors, such as oxidative stress, this leads to the VDAC1-mediated increase in mitochondrial permeability, with the release of cytochrome c and subsequent cell apoptosis (19).
The present study investigated the pro-apoptotic effect of high glucose on human umbilical vein endothelial cells (HUVECs), a commonly used cell line in diabetic vascular research (20) and the potential underlying mechanisms. The results of the current study indicated that high glucose may induce HUVEC apoptosis via downregulation of HK2 and Bcl-2 proteins, leading to decreased interactions with VDAC1. Furthermore, increased interactions between Bax and VDAC1 lead to the subsequent release of cytochrome c. Taken together, the results of the current study may provide new approaches and potential targets for the prevention of diabetes-induced cardiovascular complications.
Materials and methods
Cell culture
HUVECs were purchased from the American Type Culture Collection. HUVECs were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) containing 5.5 mM glucose with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2-humidified incubator. MG132 was utilized to prevent the potential proteasome-induced degradation of protein. HUVEC cells were pretreated with 50 µM MG132 (Selleck Chemicals) for 8 h at 37°C in a 5% CO2-humidified incubator before culture in DMEM containing 5.5, 16.5 or 33 mM glucose for a further 72 h at 37°C. To examine the effect of cyclosporine A (CyA), HUVECs were treated with 10 mM CyA (EMD Millipore) for 12 h at 37°C in a 5% CO2-humidified incubator. Following the 12-h treatment, HUVECs were cultured in DMEM containing 5.5 or 33 mM glucose for a further 72 h.
Cell infection
HUVEC cells were seeded into 6-well plates at a density of 4×105 cells/well overnight at 37°C. Virus particles (2×107)/well [HK2 adenovirus or the control virus; OBiO Technology (Shanghai) Corp., Ltd.] were subsequently added and incubated for 2 h at 37°C. Cells were cultured in fresh DMEM containing 5.5 or 33 mM glucose for a further 72 h at 37°C in a 5% CO2-humidified incubator.
Vectors
pGL3-HK2, pGL3-Bcl-2, pRL-TK-Renilla plasmid and pCF CREB, pCMV4 and pSV-SPORT expression vectors were gifts provided by Professor Dongping Wei (The First Affiliated Hospital of Nanjing, Nanjing, China). The mammalian expression vector pcDNA3.1 was purchased from Thermo Fisher Scientific (San Jose, CA, USA). The HK2 adenovirus and the control virus were purchased from Obio Technology Co., Ltd. (Shanghai, China). The pSV Sport PPARγ (Addgene plasmid no. 8886) and pCMV4 p65 (Addgene plasmid no. 21966) plasmids were gifted by Dr Bruce Spiegelman (Dana-Farber Cancer Institute, Harvard Medical School) and Dr Warner Greene (Howard Hughes Medical Institute, Department of Medicine, Duke University Medical Center), respectively.
Cell transfection
HUVEC cells were seeded into 6-well plates at a density of 4×105 cells/well overnight at 37°C. Cells were subsequently transfected with pCF CREB (pcDNA3.1), pCMV4-p65 (pCMV4), pSV SPORT PPARγ (pSV SPORT) and flag-tagged PPARγ plasmids (1 µg/well) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). A ratio of 1 µg plasmid to 2 µl liposome was then diluted in serum-free DMEM. After 4 h, cells were cultured in 10% FBS with DMEM for a further 48 h at 37°C in a 5% CO2-humidified incubator prior to subsequent experimentation.
Cell viability assay
Cell viability was examined by MTT assay. HUVEC cells were seeded into 96-well plates at a density of 2×104 cells/well and cultured for 24 h. Subsequently, cells were cultured in fresh DMEM containing 5.5, 16.5 or 33 mM glucose for a further 72 h or cells were infected with the HK2 adenovirus or the control virus following culture in fresh DMEM containing 5.5, 16.5 or 33 mM glucose for a further 72 h. Following incubation, MTT solution (Beyotime Institute of Biotechnology) was added to each well at a final concentration of 5 mg/ml and cells were incubated for 1 h at 37°C. The formazan precipitate was dissolved in 200 µl DMSO (Thermo Fisher Scientific, Inc.) and the absorbance was measured at 570 nm using a microplate reader (SpectraFluor; Tecan Group, Ltd.). Cell survival was expressed as a percentage of the absorbance in the control wells.
Cell apoptosis analysis
Cell apoptosis was examined by Hoechst 33258 staining. Cells were washed three times with PBS and fixed in 4% paraformaldehyde for ~12 h at 4°C. Cells were then washed a further three times with PBS and incubated with 10 µg/ml Hoechst 33258 (Beyotime Institute of Biotechnology) was added to each sample for 5 min at room temperature. Cells were washed with PBS and the coverslips were mounted with glycerol and images were captured using a fluorescence microscope (magnification, ×200) with an excitation wavelength at 346 nm and an emission wavelength at 460 nm.
Isolation of mitochondria
HUVEC cells were resuspended in mitochondria isolation reagents (Cell mitochondria separation kit; cat. no. c3601; Beyotime Institute of Biotechnology) and incubated on ice for 15 min. Following homogenization, the cell lysate was centrifuged at 600 × g for 10 min at 4°C, and the supernatant was collected and further centrifuged at 11,000 × g for 10 min at 4°C. The remaining mitochondrial pellet was collected and used for subsequent experimentation.
Flow cytometric analysis of mitochondrial membrane potential using JC-1 staining
Following trypsinization, cells were washed twice with PBS, resuspended in 1 ml JC-1 staining solution (1X; Beyotime Institute of Biotechnology) and incubated at 37°C for 20 min. Following incubation, cells were centrifuged at 600 × g for 10 min at 4°C and resuspended in 500 µl staining buffer (cat. no. c2005; Beyotime Institute of Biotechnology). Mitochondrial membrane potential was subsequently examined via flow cytometric analysis with FlowJo VX10 software (FlowJo LLC), where fluorescent emissions were detected at 530 nm (JC-1 FL1; red) and 575 nm (JC-1 FL2; green). The FL2/FL1 ratio represents mitochondrial membrane potential.
Immunoprecipitation and western blot analysis
HUVEC cells or mitochondria were lysed using radioimmunoprecipitation assay buffer (20 mM Tris, 150 mM NaCl, 1% Triton X-100 and 1 mM PMSF, pH 7.5) and incubated for 30 min on ice. Samples were then centrifuged for 10 min at 13,800 × g at 4°C. Lysates were resuspended in 3X loading buffer (2% SDS, 6% β-mercaptoethanol, 50 mM Tris-HCl, 10% glycerol and 0.05% bromophenol blue, pH 6.8). Cell lysates were incubated with primary antibody (as stated below) for 2 h at 4°C followed by incubation with protein A-sepharose beads (Sea Biotech, Shanghai, China) for 1 h at 4°C. Protein A-sepharose beads were washed three times with lysis buffer and centrifuged at 4°C for 1 min at 6,200 × g. The supernatant was discarded and 2X loading buffer (2% SDS, 6% β-mercaptoethanol, 50 mM Tris-HCl, 10% glycerol and 0.05% bromophenol blue; pH 6.8) was added to the protein A-sepharose beads. Samples were then boiled at 95°C for 5 min. Protein quantity was determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein (25 µg) was separated by 10% SDS-PAGE and transferred to PDVF membranes (EMD Millipore). Membranes were then blocked with 5% skimmed milk in TBST for 1 h at room temperature and incubated with the following primary antibodies: HK2 (1:1,000; cat. no. 2867), β-actin (1:1,000; cat. no. 3700), Bcl-2 (1:1,000; cat. no. 2872), Bax (1:1,000; cat. no. 5023), cytochrome c (1:1,000; cat. no. 4272), cleaved caspase-3 (1:1,000; cat. no. 9661), PPARγ (1:1,000; cat. no. 2435), Flag (1:1,000; cat. no. 2368), phosphorylated-CREB (1:1,000; cat. no. 9198), CREB (1:1,000; cat. no. 9197), phosphorylated-NF-κB (1:1,000; cat. no. 3033), NF-κB (1:1,000; cat. no. 8242; all Cell Signaling Technology, Inc.), COX4 (1:1,000; cat. no. 202554; Abcam) and VDAC1 (1:1,000; cat. no. sc-390996; Santa Cruz Biotechnology, Inc.) for 12 h at 4°C. Following primary antibody incubation, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, including anti-rabbit IgG (1:5,000; cat. no. 7074) and anti-mouse IgG (1:5,000; cat. no. 7076; both Cell Signaling Technology, Inc.). Protein bands were visualized using the ECL Western Blotting Substrate (EMD Millipore) and Tanon-5200 Chemiluminescence Imager (Tanon Science and Technology Co., Ltd.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HUVECs using the TRIzol® reagent (Thermo Fisher Scientific, Inc.). Reverse transcription was performed using a reverse transcription kit (Vazyme Biotech Co., Ltd.). qPCR was subsequently performed using a StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) with THUNDERBIRD® SYBR® qPCR master mix (Vazyme Biotech Co., Ltd.). The Thermocycling conditions were as follows: Initial polymerase activation step at 95°C for 30 sec; followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec. A final stage was performed at 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec. The following primer sequences were used for qPCR: HK2 forward, 5′-TGCCACCAGACTAAACTAGACG-3′ and reverse, 5′-CCCGTGCCCACAATGAGAC-3′; PPARγ forward, 5′-GGGATCAGCTCCGTGGATCT-3′ and reverse, 5′-TGCACTTTGGTACTCTTGAAGTT-3′; Bcl-2 forward, 5′-GGTGGGGTCATGTGTGTGG-3′ and reverse, 5′-CGGTTCAGGTACTCAGTCATCC-3′; and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative expression levels of target genes were quantified using the 2−ΔΔCq method (21) and normalized to the internal reference gene GAPDH.
Dual-luciferase reporter assay
HUVEC cells were seeded onto 24-well plates at a density of 1×105 cells/well and co-transfected with luciferase reporter plasmids 200 ng pGL3-HK2, pGL3-Bcl-2 or 10 ng pRL-TK-Renilla and 100 ng pSV SPORT PPARγ, pSV SPORT, pCF CREB, pcDNA3.1, pCMV4-p65 or pCMV4 expression vectors in 250 ml of serum-free DMEM using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) for 4 h at 37°C. Following incubation, HUVECs were cultured in DMEM supplemented with 10% FBS for 24 h. Following a 24-h incubation, transfected HUVECs were cultured in DMEM containing 5.5 mM glucose for a further 48 h. Luciferase activities were detected using the Dual-Luciferase Reporter assay system (Promega Corporation, Madison, WI, USA) and Firefly activity was normalized to Renilla luciferase activity. Three biological replicates were performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard error. Comparisons between groups were analyzed via one-way analysis of variance (GraphPad, Inc.). P<0.05 was considered to indicate a statistically significant result. Three biological replicates were performed in triplicate.
Results
High glucose induces apoptosis in HUVECs
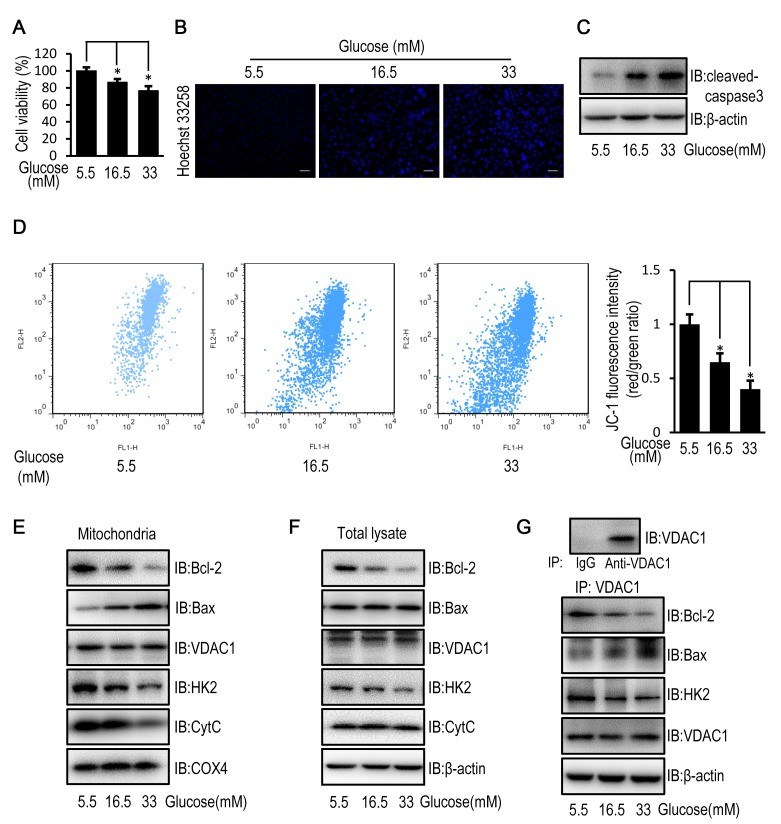
HUVECs exposed to high concentrations (16.5 and 33 mM) of glucose demonstrated a significantly decreased cell viability, increased cell apoptosis and cleaved caspase-3 levels, compared with HUVECs exposed to a low (5.5. mM) glucose concentration (Fig. 1A-C). In addition, the mitochondrial membrane potential in HUVECs exposed to high concentrations of glucose was significantly decreased compared with HUVECs exposed to a low glucose concentration (Fig. 1D). These results suggest that exposure to high glucose may induce cell apoptosis in a mitochondria-dependent manner via impairment of mitochondrial structure and function. The change in mitochondrial permeability was confirmed by a reduced mitochondrial expression level and an unchanged cellular expression level of cytochrome c (Fig. 1E and F), which is released from the mitochondria into the cytoplasm when mitochondrial function is disrupted (22). Since mitochondrial permeability is controlled by VDAC1, which is regulated by Bcl-2, Bax and HK2, Bcl-2, Bax and HK2 protein expression (19) and subcellular distribution were examined. HUVECs exposed to high glucose concentrations demonstrated decreased mitochondrial and cellular protein expression levels of HK2 and Bcl-2 compared with HUVECs exposed to a low glucose concentration (Fig. 1E and F). Although the mitochondrial protein level of VDAC1, as well as the cellular protein levels of VDAC1 and Bax remained unchanged, the mitochondrial protein level of Bax was increased in HUVECs exposed to high concentrations of glucose (Fig. 1E and F). Immunoprecipitation studies revealed that exposure to high glucose enhanced the interaction between VDAC1 and Bax, and reduced the interaction between VDAC1 and HK2 as well as Bcl-2 in HUVECs (Fig. 1G).
Figure 1.
High glucose induces apoptosis in HUVECs by disturbing the mitochondrial membrane potential. HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for 72 h. (A) MTT assay was used to examine cell viability of HUVECs. (B) Cell apoptosis was examined in HUVECs stained with the fluorescent nuclear dye Hoechst 33258. Scale bar=200 mm. (C) The protein expression level of cleaved caspase-3 was determined by western blot analysis in HUVECs. (D) Mitochondrial membrane potential was examined in HUVECs following JC-1 staining and flow cytometric analysis for fluorescence emission at 530 nm (JC-1 FL1) and 575 nm (JC-1 FL2). The FL2/FL1 ratio represents mitochondrial membrane potential. (E) The protein expression level of Bcl-2, Bax, VDAC1, HK2, CytC and COX4 were determined by western blot analysis in mitochondria isolated from HUVECs. (F) The protein expression levels of Bcl-2, Bax, VDAC1, HK2 and CytC were determined by western blot analysis in HUVECs. (G) The protein expression level of Bcl-2, Bax, HK2 and VDAC1 were determined by western blot analysis in HUVECs following immunoprecipitation with VDAC1 antibody. Data are presented as the mean ± standard error. *P<0.05 as indicated. HUVEC, human umbilical vein endothelial cell; DMEM, Dulbecco's modified Eagle's medium; Bax, Bcl-2 associated X; VDAC1, voltage-dependent anion channel 1; HK2, hexokinase 2; CytC, cytochrome c; COX4, cytochrome c oxidase subunit 4; IB, immunoblotting.
HK2 overexpression partially reverses high glucose-induced cell apoptosis
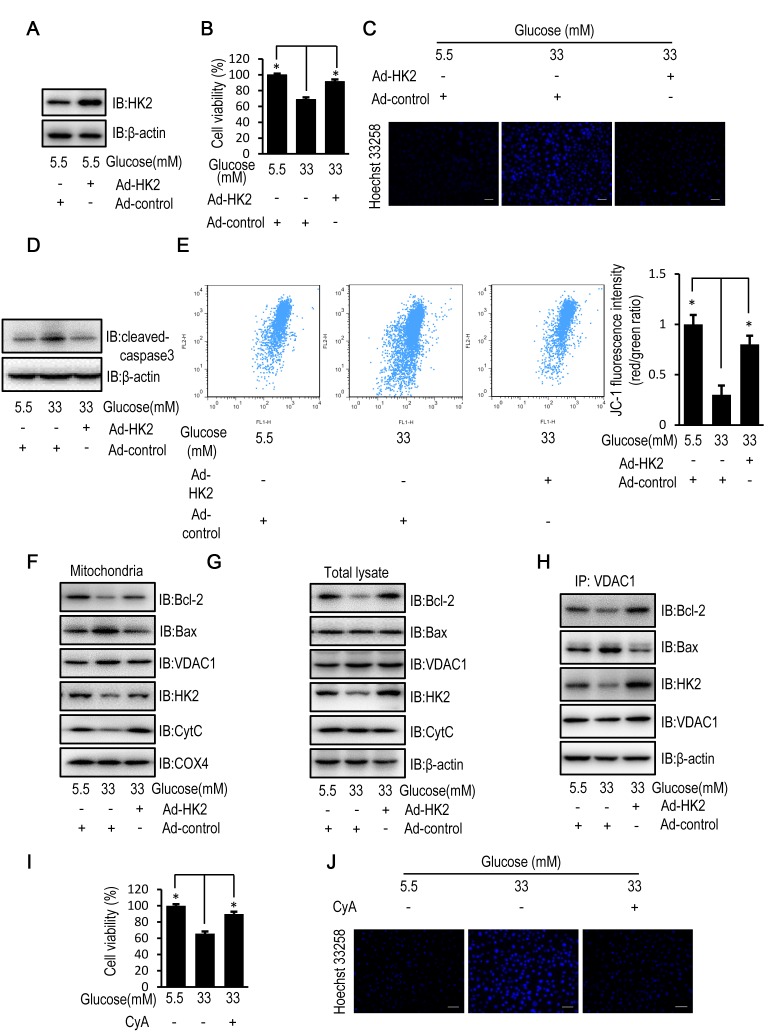
Protein expression levels of HK2 were increased in HUVECs following transfection with HK2 adenovirus compared with control adenovirus (Fig. 2A). When compared with control virus infected HUVECs, HK2 overexpression partially reversed the decrease in cell viability, enhanced cell apoptosis and activation of caspase-3, and reduced mitochondrial membrane potential of HUVECs following exposure to high levels of glucose (Fig. 2B-E). In addition, HK2 overexpression partially reversed the decreased mitochondrial and cellular protein expression levels of HK2 and Bcl-2 as well as the increased mitochondrial protein expression level of Bax observed in HUVECs compared with control virus infected HUVECs following exposure to high levels of glucose. Furthermore, HK2 overexpression suppressed cytochrome c release (Fig. 2F and G). Furthermore, immunoprecipitation assays revealed that HK2 overexpression enhanced the interactions between VDAC1 and HK2 as well as Bcl-2, and attenuated the interactions between VDAC1 and Bax (Fig. 2H). CyA, a known inhibitor of mitochondrial permeability transition (23), was used to examine the effect of mitochondrial permeability on cell viability and apoptosis in HUVECs exposed to a high concentration of glucose. Treatment with CyA partially reversed the decrease in cell viability and the increase in cell apoptosis in HUVECs exposed to a high glucose concentration, similarly to the aforementioned HK2 overexpression (Fig. 2I and J).
Figure 2.
HK2 overexpression reverses high glucose-induced apoptosis in HUVECs. HUVECs were transfected with HK2 adenovirus or control virus for 24 h. Following transfection, HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for a further 72 h. (A) The protein expression level of HK2 was determined by western blot analysis in HUVECs. (B) MTT assay was used to examine cell viability of HUVECs. (C) Cells apoptosis was examined in HUVECs stained with the fluorescent nuclear dye Hoechst 33258. Scale bar=200 mm. (D) The protein expression level of cleaved caspase-3 was determined by western blot analysis in HUVECs. (E) Mitochondrial membrane potential was examined in HUVECs following JC-1 staining and flow cytometric analysis for fluorescence emission at 530 nm (JC-1 FL1) and 575 nm (JC-1 FL2). (F) The protein expression levels of Bcl-2, Bax, VDAC1, HK2, CytC and COX4 were determined by western blot analysis in mitochondria isolated from HUVECs. (G) The protein expression levels of Bcl-2, Bax, VDAC1, HK2 and CytC were determined by western blot analysis in HUVECs. (H) The protein expression levels of Bcl-2, Bax, HK2 and VDAC1 were determined by western blot analysis in HUVECs following immunoprecipitation with VDAC1 antibody. HUVECs were treated with 10 mM CyA for 12 h. Following 12-h treatment, HUVECs were cultured in DMEM containing 5.5 or 33 mM glucose for a further 72 h. (I) MTT assay was used to examine cell viability of HUVECs following treatment with CyA. (J) Cells apoptosis was examined in HUVECs stained with the fluorescent nuclear dye Hoechst 33258 following treatment with CyA. Scale bar=200 µm. Data are presented as the mean ± standard error. *P<0.05 as indicated. HK2, hexokinase 2; HUVEC, human umbilical vein endothelial cell; DMEM, Dulbecco's modified Eagle's medium; Bax, Bcl-2 associated X; VDAC1, voltage-dependent anion channel 1; CytC, cytochrome c; COX4, cytochrome c oxidase subunit 4; IB, immunoblotting.
High glucose-induced Bcl-2 downregulation is reversed by HK2 upregulation
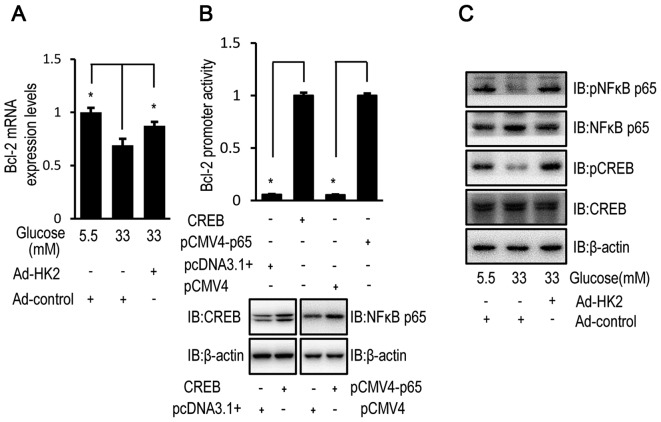
HK2 overexpression partially restored the relative mRNA expression level of Bcl-2 in HUVECs exposed to high concentrations of glucose (Fig. 3A). Luciferase assays confirmed the transcriptional regulation of Bcl-2 by CREB and NF-κB p65 in HUVECs (Fig. 3B). Furthermore, the phosphorylation level of CREB and NF-κB, as an indicator of activation, was examined by western blot analysis in HUVECs. HK2 overexpression reversed the high glucose-induced effect on the activation status of phosphorylated CREB and NF-κB in HUVECs (Fig. 3C).
Figure 3.
High glucose downregulates Bcl-2 expression via HK2 expression. HUVECs were transfected with HK2 adenovirus or control virus for 24 h. Following transfection, HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for 72 h. (A) The relative mRNA expression level of Bcl-2 was determined by RT-qPCR in HUVECs. (B) HUVECs were co-transfected with pCF CREB (or pcDNA3.1) and pGL3-Bcl-2 (or pRL-TK-Renilla) or pCMV4-p65 (NF-κB) (or pCMV4) and pGL3-Bcl-2 (or pRL-TK-Renilla) for 48 h and luciferase activity was detected using the Dual-Luciferase reporter assay. The protein expression of CREB or NF-κB were increased in HUVECs following transfection with pCF CREB or pCMV4-p65 compared with pcDNA3.1 or pCMV4, respectively. (C) The protein expression levels of p-NFκB, NFκB, p-CREB and CREB were determined by western blot analysis in HUVECs. Data are presented as the mean ± standard error. *P<0.05 as indicated. HK2, hexokinase 2; HUVEC, human umbilical vein endothelial cell; DMEM, Dulbecco's modified Eagle's medium; NFκB, nuclear factor-κB; CREB, cyclic AMP response element binding protein; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; IB, immunoblotting.
High glucose reduces HK2 transcription via downregulation of PPARγ in HUVECs
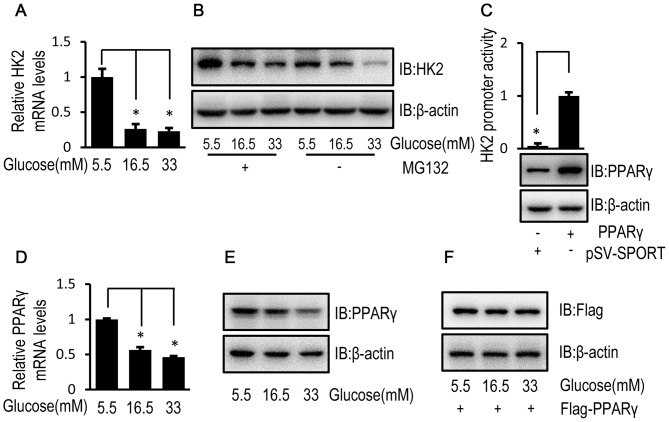
To investigate the mechanisms underlying the inhibitory effect of high glucose on HK2 expression, the relative mRNA and protein expression levels of HK2 were determined by RT-qPCR and western blotting, respectively, in HUVECs exposed to high and low glucose concentrations. The relative mRNA and protein expression levels of HK2 were decreased in HUVECs exposed to high glucose concentrations compared with HUVECs exposed to a low glucose concentration (Fig. 4A and B). In addition, when MG132, a cell-permeable proteasome inhibitor, was used to block HK2 protein degradation, a high glucose-induced decrease in HK2 protein expression level was observed (Fig. 4B). These results suggest that high glucose may regulate HK2 expression at a transcriptional level. Furthermore, PPARγ is reported to be a transcription factor for HK2 (24). In the current study, luciferase activity confirmed the transcriptional regulation of HK2 by PPARγ (Fig. 4C). Although HUVECs exposed to high glucose concentrations demonstrated decreased mRNA and protein expression levels of endogenous PPARγ (Fig. 4D and E), high glucose had no obvious effect on the protein expression level of flag-tagged PPARγ level in HUVECs (Fig. 4F).
Figure 4.
High glucose inhibits HK2 transcription of HK2 via PPARγ downregulation in HUVECs. (A) HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for 72 h and the relative mRNA expression level of HK2 was determined by RT-qPCR. (B) HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for 72 h followed by treatment with MG132 (50 µM) for 8 h. Following 8-h treatment, the protein expression level of HK2 was determined by western blot analysis. (C) HUVECs were co-transfected with pSV SPORT PPARγ (or pSV-SPORT) and pGL3-HK2 (or pRL-TK-Renilla) into cells for 48 h and luciferase activity was detected using the Dual-Luciferase assay. HUVECs were cultured in DMEM containing 5.5, 16.5 or 33 mM glucose for 72 h. (D) The relative mRNA expression level of PPARγ was determined by RT-qPCR in HUVECs. (E) The protein expression level of PPARγ was determined by western blot analysis in HUVECs. (F) HUVECs were transfected with the Flag tagged PPARγ for 24 h. Following transfection, HUVECs were cultured in medium containing 5.5, 16.5 or 33 mM glucose for 72 h. The protein expression level of Flag was determined by western blot analysis in HUVECs. Data are presented as the mean ± standard error. *P<0.05 as indicated. HK2, hexokinase 2; PPARγ, peroxisome proliferator-activated receptor γ; HUVEC, human umbilical vein endothelial cell; DMEM, Dulbecco's modified Eagle's medium; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; MG132, cell-permeable proteasome inhibitor; IB, immunoblotting.
Discussion
Hyperglycemia-induced apoptosis of vascular endothelial cells serves a role in the pathogenesis of both macrovascular and microvascular complications of diabetes (25). In the present study, an in vitro model of chronic hyperglycemia was established in HUVECs following exposure to high glucose, in order to investigate the potential mechanisms and therapeutic targets for diabetes-induced vascular complications.
In the present study, high glucose-induced apoptosis in HUVECs was associated with the release of mitochondrial cytochrome c, as a result of the increase in mitochondrial membrane permeability demonstrated by the reduced mitochondrial membrane potential. VDAC1 plays a role in regulating mitochondrial membrane permeability (26), following exposure to high glucose, and its function is regulated by HK2, Bcl-2 and Bax (27). While HK2 and Bcl-2 reduce mitochondrial permeability, Bax increases mitochondrial permeability (16–18). In the current study, high glucose-induced downregulation of mitochondrial and cellular HK2 and Bcl-2 expression, and therefore decreased interactions with VDAC1. In addition, high glucose induced upregulation of mitochondrial Bax by enhancing interactions with VDAC1 without affecting total lysate Bax expression levels. As HK2 and Bax competitively bind to VDAC1 (28), the decreased protein expression level of HK2 is likely to be affect the interactions between Bax and VDAC1. It was therefore hypothesized that downregulation of HK2 was involved in high glucose-induced cell apoptosis. To examine whether HK2 was involved in high glucose-induced cell apoptosis, HK2 was overexpressed in HUVECs exposed to high glucose. HK2 overexpression partially suppressed high glucose-induced cell apoptosis, by reducing mitochondrial Bax and its interaction with VDAC1. The antiapoptotic effect of HK2 may be achieved by directly competing with Bax for binding to VDAC1, as there was no upregulation of intracellular Bax observed. Furthermore, HK2 overexpression increased the expression, mitochondrial abundance and interaction of Bcl-2 with VDAC1. Bcl-2 is an apoptotic regulator which plays an antiapoptotic role by binding to the N-terminal domain of VDAC1 (29). Therefore, upregulation of Bcl-2 may also contribute to the antiapoptotic effect of HK2.
Consistent with the findings of the current study, previous studies demonstrated that HK2 upregulated Bcl-2 expression in several types of cancer cell lines (30), through the activation of CREB, a Bcl-2 transcription factor, via phosphorylation (31). Despite the lack of studies regarding the effect of long-term exposure to high glucose on CREB phosphorylation, the current study indicated that high glucose effectively attenuated CREB phosphorylation in HUVECs. In addition, the current study demonstrated that high glucose attenuated phosphorylation of NF-κB, another Bcl-2 transcriptional factor (32). However, HK2 overexpression partially reversed the high glucose-induced inhibition of NF-κB and CREB phosphorylation. Therefore, the inhibition of CREB and NF-κB phosphorylation by high glucose may reduce Bcl-2 expression, however this was partially reversed by HK2 overexpression. The underlying mechanism of CREB and NF-κB regulation by high glucose and HK2 remains unknown.
To investigate the underlying mechanism by which high glucose reduces HK2 expression in HUVECs, the mRNA and protein levels of HK2 were examined. The current study demonstrated that high glucose reduced the transcription of HK2 without affecting its protein degradation rate. As high glucose may increase the proteasome-mediated degradation of HK2, MG132 was used to prevent this protein degradation, which resulted in a reduced difference of protein levels between high and low glucose treatment. A previous study revealed that PPARγ is an important transcription factor for HK2 (25), which was confirmed in the current study using a luciferase assay. As was the case with HK2, high glucose reduced PPARγ expression by decreasing its mRNA level. Therefore, high glucose-induced downregulation of PPARγ led to suppressed HK2 transcription. However, the mechanism underlying high glucose-mediated regulation of PPARγ expression in HUVECs requires further investigation.
In conclusion, high glucose reduced HK2 expression by suppressing the expression of the HK2 transcription factor (PPARγ), which subsequently reduced Bcl-2 expression in HUVECs. These changes weakened the interaction of mitochondrial VDAC1 with HK2 and Bcl-2 leading to the enhanced binding of Bax to VDAC1, which increased mitochondrial membrane permeability and induced cell apoptosis. It is hypothesized that upregulating HK2 or PPARγ may reduce vascular endothelial cell apoptosis and prevent diabetes-induced vascular complications.
Acknowledgements
The authors would like to thank Dongping Wei (The First Affiliated Hospital of Nanjing, Nanjing, China) for the luciferase reporter plasmids pGL3-HK2 and pGL3-Bcl-2, and the pCF CREB, pCMV4 and pSV-SPORT expression vectors. The authors would also like to acknowledge Dr Bruce Spiegelman (Dana-Farber Cancer Institute, Harvard Medical School) and Dr Warner Greene (Howard Hughes Medical Institute, Department of Medicine, Duke University Medical Center) pSV SPORT PPARγ and pCMV4 p65 expression vectors, respectively.
Glossary
Abbreviations
- HK2
hexokinase 2
- VDAC1
voltage-dependent anion channel 1
- CyA
cyclosporine A
- CREB
cyclic AMP response element binding protein
- NF-κB
nuclear factor-κB
- PPARγ
peroxisome proliferator-activated receptor γ
Funding
The present study was funded by grants from the Natural Science Foundation of Jiangsu Province of China (grant no. BK20151015) and the Jiangsu Health International Exchange Program.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JZ performed experiments and prepared the manuscript. YG performed the cell apoptosis analysis. WG and XZ performed the cell viability assay. MP designed and directed the experiments, and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang N, Cheng J, Han B, Li Q, Chen Y, Xia F, Jiang B, Jensen MD, Lu Y. Exposure to severe famine in the prenatal or postnatal period and the development of diabetes in adulthood: An observational study. Diabetologia. 2017;60:262–269. doi: 10.1007/s00125-016-4148-4. [DOI] [PubMed] [Google Scholar]
- 2.Ervasti J, Virtanen M, Lallukka T, Pentti J, Kjeldgård L, Mittendorfer-Rutz E, Tinghög P, Alexanderson K. Contribution of comorbid conditions to the association between diabetes and disability pensions: A population-based nationwide cohort study. Scand J Work Environ Health. 2016;42:209–216. doi: 10.5271/sjweh.3556. [DOI] [PubMed] [Google Scholar]
- 3.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diab Vasc Dis Res. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, Zou MH. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes. 2017;66:193–205. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturm A, Mollard V, Cozijnsen A, Goodman CD, McFadden GI. Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proc Natl Acad Sci U S A. 2015;112:10216–10223. doi: 10.1073/pnas.1423959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2011;16:233–238. doi: 10.1177/1074248411409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balk J, Leaver CJ, Mccabe PF. Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett. 1999;463:151–154. doi: 10.1016/S0014-5793(99)01611-7. [DOI] [PubMed] [Google Scholar]
- 8.Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalaeva DN, Dibrova DV, Galperin MY, Mulkidjanian AY. Modeling of interaction between cytochrome c and the WD domains of Apaf-1: Bifurcated salt bridges underlying apoptosome assembly. Biol Direct. 2015;10:29. doi: 10.1186/s13062-015-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safi SZ, Batumalaie K, Mansor M, Chinna K, Mohan S, Karimian H, Qvist R, Ashraf MA, Yan GO. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics (Sao Paulo) 2015;70:569–576. doi: 10.6061/clinics/2015(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A. VDAC1 functions in Ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium. 2017;69:81–100. doi: 10.1016/j.ceca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Hail D, Begas-Shvartz R, Shalev M, Shteinfer-Kuzmine A, Gruzman A, Reina S, De Pinto V, Shoshan-Barmatz V. Novel compounds targeting the mitochondrial protein VDAC1 inhibit apoptosis and protect against mitochondria dysfunction. J Biol Chem. 2016;291:24986–25003. doi: 10.1074/jbc.M116.744284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoshan-Barmatz V, Mizrachi D. VDAC1 (voltage-dependent anion channel 1) Atlas Genet Cytogenet Oncol Haematol. 2012 doi: 10.4267/2042/47493. [DOI] [Google Scholar]
- 14.Shoshanbarmatz V, Mizrachi D. VDAC1: From structure to cancer therapy. Front Oncol. 2012;2:164. doi: 10.3389/fonc.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Chen J, Weng C, Chen R, Zheng Y, Chen Q, Tang H. Identification of the protein–protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem Biophys Res Commun. 2003;305:989–996. doi: 10.1016/S0006-291X(03)00871-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63:e12413. doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 20.Zitman-Gal T, Green J, Pasmanik-Chor M, Golan E, Bernheim J, Benchetrit S. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Cardiovasc Diabetol. 2014;13:8. doi: 10.1186/1475-2840-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta) C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Varela AT, Gomes AP, Simões AM, Teodoro JS, Duarte FV, Rolo AP, Palmeira CM. Indirubin-3′-oxime impairs mitochondrial oxidative phosphorylation and prevents mitochondrial permeability transition induction. Toxicol Appl Pharmacol. 2008;233:179–185. doi: 10.1016/j.taap.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, et al. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amore A, Cirina P, Conti G, Cerutti F, Bagheri N, Emancipator SN, Coppo R. Amadori-configurated albumin induces nitric oxide-dependent apoptosis of endothelial cells: A possible mechanism of diabetic vasculopathy. Nephrol Dial Transplant. 2004;19:53–60. doi: 10.1093/ndt/gfg428. [DOI] [PubMed] [Google Scholar]
- 26.Zamarin D, García-Sastre A, Xiao X, Wang R, Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. Plos Pathog. 2005;1:e4. doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L, Han J, Ben-Hail D, He L, Li B, Chen Z, Wang Y, Yang Y, Liu L, Zhu Y, et al. A new fungal diterpene induces VDAC1-dependent apoptosis in bax/bak-deficient cells. J Biol Chem. 2015;290:23563–23578. doi: 10.1074/jbc.M115.648774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasello MF, Guarino F, Reina S, Messina A, De Pinto V. The voltage-dependent anion selective channel 1 (VDAC1) topography in the mitochondrial outer membrane as detected in intact cell. Plos One. 2013;8:e81522. doi: 10.1371/journal.pone.0081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, Bultynck G. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem. 2015;290:9150–9161. doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rho M, Kim J, Jee CD, Lee YM, Lee HE, Kim MA, Lee HS, Kim WH. Expression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissues and cell lines. Anticancer Res. 2007;27:251–258. [PubMed] [Google Scholar]
- 31.Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–346. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- 32.Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, Liu SH. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.