Abstract
The glycyrrhizic acid (GA) epimers 18α- and 18β-GA exert anti-inflammatory and hepatoprotective activities, which may help to protect against alcoholic liver disease, particularly alcoholic hepatitis (AH). The aim of the present study was to investigate the optimal ratio of 18α- and 18β-GA for preventing AH in rats. Different groups of rats were administered seven different ratios of 18α- and 18β-GA (10:0, 8:2, 6:4, 5:5, 4:6, 2:8 and 0:10; 10.83 mg/kg), vehicle control, or silymarin (22.75 mg/kg) as a positive control, followed by administration of 40% alcohol (10 ml/kg) once a day for four weeks. Subsequently, livers were isolated and routinely processed for histological examination. The serum levels of 23 cytokines and chemokines associated with AH were examined with a Bio-Plex 200 Luminex assay. It was revealed that all ratios of 18α- and 18β-GA prevented alcohol-induced liver injury, as evidenced by a lesser degree of histopathological changes in the liver as compared with those in the model group. Furthermore, the levels of 15 cytokines/chemokines were significantly altered after alcohol administration, which was significantly inhibited by, pre-treatment with different proportions of 18α- and 18β-GA, particularly at a ratio of 4:6, for most cytokines/chemokines associated with AH, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-7, IL-6, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-3α, macrophage- and granulocyte macrophage colony-stimulating factor, chemokine (C-X-C motif) ligand 1(GRO/KC), vascular endothelial growth factor and C-C motif chemokine ligand 5 (RANTES). Taken together, based on these results the optimal ratio of 18α- and 18β-GA to prevent AH in model rats was considered to be 4:6.
Keywords: 18α- and 18β-glycyrrhizic acid, optimal ratio, alcoholic hepatitis, cytokines, chemokines
Introduction
Alcoholic liver disease (ALD) encompasses a spectrum of injury, including simple steatosis, alcoholic hepatitis (AH), fibrosis, cirrhosis and hepatocellular carcinoma. Among these, AH draws increasing attention due to its high incidence (1,2). The molecular mechanisms of AH are complex; however, cytokines and chemokines associated with AH are considered to be essential in the progression of AH (3–5). AH is typically characterized by inflammation, which is accompanied by a marked increase of pro-inflammatory cytokines and chemokines. Alcohol may accelerate AH by increasing the translocation of gut-derived endotoxins to the portal circulation, activating the lipopolysaccharide (LPS)/Toll-like receptor-4 (TLR-4) pathway (6), and promoting hepatocyte or Kupffer cells to release cytokines and chemokines, including interleukin (IL)-6, IL-1α, tumor necrosis factor (TNF)-α and monocyte chemotactic protein 1 (MCP-1) (7–9). Thus, modulation of cytokines and chemokines associated with AH may help to protect against AH.
Due to its health benefits and relatively low toxicity, liquorice, the root and stolon of certain Glycyrrhiza species, has been used to treat certain diseases for thousands of years (10). It has also been widely used in food products as a sweetening and flavoring component owing to its sweet taste. Glycyrrhizic acid (GA), with two different epimers, 18α- and 18β-GA, may be isolated from liquorice (11,12), and is known for its medicinal properties, including anti-inflammatory and immune regulatory actions, as well as inhibition of hepatic apoptosis and necrosis (13,14). GA was reported to attenuate the TLR-4/myeloid differentiation factor-2 complex, thus suppressing LPS-induced activation of signal cascades and production of cytokines and chemokines (15). GA also exerts anti-inflammatory effects by reducing the production of pro-inflammatory cytokines. Accumulating evidence indicates that these two epimers of GA have different pharmacological functions. 18α-GA exhibits anti-inflammatory activity, while, 18β-GA modulates bile acid metabolism (16,17). Thus, the present study hypothesized that different ratios of 18α- and 18β-GA may have different effects against AH. However, to date, the optimal ratio of 18α- and 18β-GA to synergistically prevent AH and the underlying mechanisms of these protective effects have remained elusive.
The present study was therefore designed to investigate the optimal ratio of 18α- and 18β-GA for preventing AH, and further, to explore the underlying mechanisms by detecting their effects on cytokines and chemokines associated with AH.
Materials and methods
Materials and reagents
Magnesium isoglycyrrhizinate injection (18α:18β=500:1) was provided by Chia Tai Tianqing Pharmaceutical Group Co., Ltd (Lianyungang, China) (18). Compound ammonium glycyrrhetate S for Injection (18α:18β=1:109) was purchased from Shanxi Powerdone Pharmaceutics Co., Ltd. (Datong, China) and Silibinin Capsules (purity, >99%) was supplied by Tianjin Tasly Sants Pharmaceutical Co., Ltd. (Tianjin, China) (18). Alcohol (>98%) was purchased from Tianjin Fengchuan Chemical Regent Technologies Co., Ltd. (Tianjin, China). The Bio-Plex Pro™ Rat Cytokine Group I Panel 23-Plex kit (cat. no. 12005641) was supplied by Bio-Rad Laboratories, Inc. (Hercules, CA, USA). All other reagents were of analytical grade.
Animals and animal treatments
A total of 60 Male Sprague Dawley rats (weight 200±20 g) were purchased from SPF Laboratory Animal Technology Co., Ltd. (Beijing, China). All rats were kept in a room with controlled humidity and temperature under a 12-h light/dark cycle. The animals were given free access to purified water and a standard diet.
Animal models
The rats were randomly divided into ten groups of ten rats in each group (n=6): i) normal group (0.9% saline), ii) model group [alcohol (40%; 10 ml/kg)], iii) silymarin (positive control) group [alcohol + silymarin (22.75 mg/kg)], iv-x) different 18α- and 18β-GA groups [ratios, 10:0, 8:2, 6:4, 5:5, 4:6, 2:8, 0:10; alcohol + respective 18α- to 18β-GA ratio (10.83 mg/kg)]. All drugs were diluted with 0.9% saline and intragastrically administered (per gavage) to the rats once daily for 4 weeks. The rats also received 40% alcohol (10 ml/kg) 6 h following each drug administration for 4 weeks. All animals were sacrificed 24 h after the last gavage of alcohol. Blood samples were collected from the abdominal aorta at the end of the experiment. Then blood was centrifuged at 4,000 × g for 10 min at 4°C, and serum was collected and stored at −80°C for further study.
Histological analysis
Livers were fixed in 10% formaldehyde solution, and were then embedded in paraffin. The tissues were cut into 3-µm sections, which were then de-paraffinized in xylene and rehydrated in a graded series of ethanol. The sections were assessed by morphometric evaluation of liver slides with H&E staining.
Bio-Plex Pro™ assay
Bio-Plex 200, based on the Luminex assay, has a high sensitivity and accuracy. Thus, the levels of cytokines were detected in the present study by using a Bio-Plex Pro™ Rat Cytokine Group I Panel 23-Plex (cat. no. 12005641; Bio-Rad Laboratories, Inc.), which included 23 cytokines [granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage (GM)-CSF, chemokine (C-X-C motif) ligand 1 (GRO/KC), interferon (IFN)-γ, IL-1α, IL-1β, MCP-1, IL-2, IL-4-7, IL-10, IL-12p70, IL-13, IL-17A, IL-18, M-CSF, macrophage inflammatory protein (MIP)-1α, MIP-3α, C-C motif chemokine ligand 5 (RANTES), TNF-α and vascular endothelial growth factor (VEGF)]. The levels of these cytokines or chemokines were calculated via Bio-Plex Manager v6.1 software (Bio-Rad Laboratories, Inc.). The levels of the 23 cytokines and chemokines in the serum were examined using coupled magnetic beads, which were included in the Bio-Plex Pro™ Rat Cytokine Group I Panel 23-Plex kit (Bio-Rad Laboratories, Inc., cat. no. 12005641). All experiments were performed in accordance with the manufacturer's protocols.
Statistical analysis
All statistical analyses were performed by using the Statistical Package for the Social Sciences (SPSS) version 13.0 software (SPSS Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation. Statistical significance of inter-group differences was evaluated by one-way analysis of variance followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Hepatoprotective effect of different ratios of 18α- and 18β-GA against AH
The hepatoprotective effect of different proportions of 18α- and 18β-GA was assessed by morphological observation of H&E-stained liver tissues. As presented in Fig. 1, the histological examination indicated multifocal hepatic parenchymal necrosis with inflammatory cell infiltration in the livers of alcohol-induced rats. Silymarin treatment and different ratios of 18α- and 18β-GA significantly ameliorated the degree of hepatic parenchymal necrosis, and inflammatory cell infiltration was also attenuated.
Figure 1.
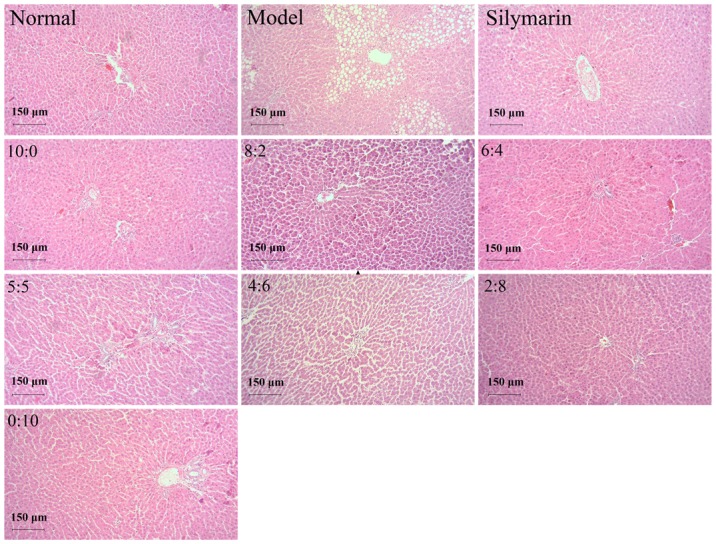
Histopathological photomicrographs of liver samples from the normal control, model and 22.75 mg/kg silymarin groups and those treated with different ratios of 18α- and 18β-glycyrrhizic acid (10:0, 8:2, 6:4, 5:5, 4:6, 2:8 and 0:10; 10.83 mg/kg) (hematoxylin and eosin staining; magnification, ×100; scale bar, 150 µm).
Effect of different ratios of 18α- and 18β-GA on pro-inflammatory cytokines and chemokines in alcohol-induced rats
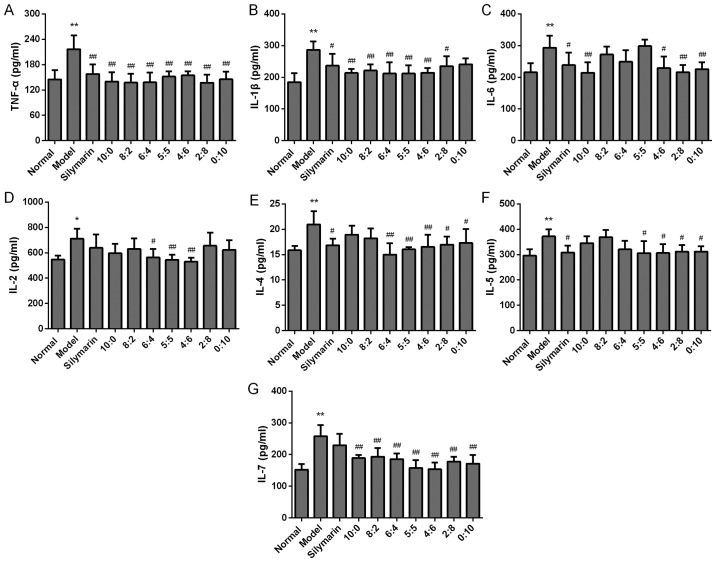
It is generally accepted that inflammatory cytokines have critical roles in AH. To identify the optimal ratio of 18α- and 18β-GA to synergistically prevent AH, the serum levels of pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, IL-6, IL-2, IL-7, IFN-γ and MCP-1 in the rats with AH were first examined. After treatment with different proportions of 18α- and 18β-GA for four weeks, the levels of pro-inflammatory cytokines and chemokines were detected. As indicated in Figs. 2–4, alcohol administration elicited a profound alteration of the levels of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-2 and IL-7, as well as pro-inflammatory chemokines, including MCP-1 (P<0.01 or <0.05), as compared with those in the normal rats. Of note, 18α- and 18β-GA at all ratios significantly decreased the levels of TNF-α, and IL-7. In addition, 18α- and 18β-GA at ratios of 10:0, 8:2, 6:4, 5:5, 4:6 and 2:8 significantly decreased the levels of IL-1β as compared with those in the model rats (P<0.01). Furthermore, compared with those in the model group, the levels of IL-6 were markedly decreased in those rats that were treated with 18α- and 18β-GA at ratios of 10:0, 4:6, 2:8 and 0:10 (P<0.05 or <0.01). In rats that were treated with 18α- and 18β-GA at ratios of 6:4, 5:5 and 4:6, the levels of IL-2 were also obviously decreased in comparison with those in the model group (P<0.01 or <0.05). In addition, 18α- and 18β-GA at proportions of 10:0, 5:5, 4:6, 2:8 and 0:10 significantly decreased the levels of MCP-1 in comparison with those in the model group (P<0.01 or <0.05). However, no significant difference was obtained in the levels of IFN-γ among all groups (Fig. 3). These results indicated that 18α- and 18β-GA at different ratios, particularly at 4:6, substantially reduced the production of most pro-inflammatory cytokines and chemokines.
Figure 2.
Effects of different ratios of 18α- and 18β-glycyrrhizic acid on the levels of the inflammatory cytokines (A) TNF-α, (B) IL-1β, (C) IL-6, (D) IL-2, (E) IL-4, (F) IL-5 and (G) IL-7 in alcohol-induced rats (n=6 per group). Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. normal group; #P<0.05, ##P<0.01 vs. model group. IL, interleukin; TNF, tumor necrosis factor.
Figure 4.
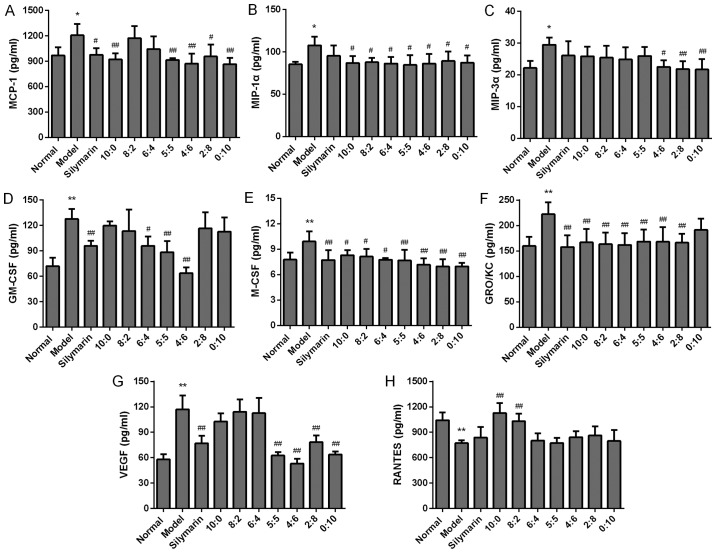
Effects of different proportions 18α- and 18β-glycyrrhizic acid on the levels of the chemokines (A) MCP-1, (B) MIP-1α, (C) MIP-3α, (D) GM-CSF, (E) M-CSF, (F) chemokine (C-X-C motif) ligand 1 (GRO/KC), (G) VEGF and (H) RANTES in alcohol-induced rats (n=6 per group). Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. normal group; #P<0.05, ##P<0.01 vs. model group. MCP-1, monocyte chemotactic protein 1; MIP, macrophage inflammatory protein; GM-CSF, granulocyte macrophage colony-stimulating factor; VEGF, vascular endothelial growth factor; RANTES, C-C motif chemokine ligand 5; GRO/KC, chemokine (C-X-C motif) ligand 1.
Figure 3.
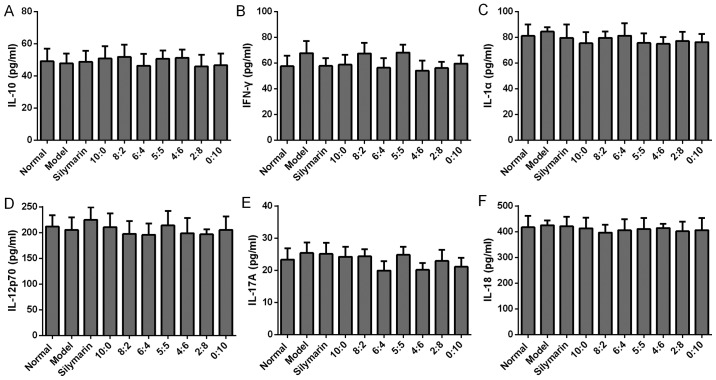
Effects of different proportions of 18α-and 18β-glycyrrhizic acid on the levels of the hepatoprotective cytokines (A) IL-10, (B) IFN-γ, (C) IL-1α, (D) IL-12p70, (E) IL-17A and (F) IL-18 in alcohol-induced rats (n=6 per group). Values are expressed as the mean ± standard deviation. IL, interleukin; IFN, interferon.
Effect of different ratios of 18α- and 18β-GA on the levels of hepatoprotective cytokines in alcohol-induced rats
Next, the effect of different proportions of 18α- and 18β-GA on anti-inflammatory cytokines, including IL-4 and IL-5, was detected. As presented in Fig. 2, IL-4 and IL-5 increased significantly in the model group compared with that in the normal rats (P<0.01). Of note, 18α- and 18β-GA at ratios of 5:5, 4:6, 2:8 and 0:10 significantly inhibited the increase of IL-4 and IL-5 induced by alcohol administration (P<0.01 or <0.05). In addition, no significant difference in the levels of IL-10, IL-1α, IL-12p70, IL-17A and IL-18 was observed among all groups (Fig. 3). The level of IL-13 was below the detection limit (data not shown). Overall, the results indicated that 18α- and 18β-GA at ratios of 5:5, 4:6, 2:8 and 0:10 modulated the levels of anti-inflammatory cytokines, including IL-4 and IL-5; however, 18α- and 18β-GA at all ratios had no significant effect on the production of IL-10, IL-1α, IL-12p70, IL-17A and IL-18 (Fig. 3), which have also been considered to be associated with the progression of AH (19).
Effect of different ratios of 18α- and 18β-GA on the production of chemokines in alcohol-induced rats
Chemokines are small molecular proteins that regulate the migration and activation of hepatocytes and Kupffer cells and contribute to the pathogenesis of AH. Thus, the effects of different ratios of 18α- and 18β-GA on chemokines in alcohol-induced AH rats were then assessed. As presented in Fig. 4, alcohol administration significantly increased levels of MCP-1, MIP-1α, MIP-3α, GM-CSF, M-CSF, GRO/KC and VEGF, while markedly reducing the production of RANTES compared with that in the normal rats (P<0.01 or P<0.05). 18α- and 18β-GA at ratios of 10:0 and 8:2 significantly increased the levels of RANTES as compared with those in the model group (P<0.01). The production of VEGF was markedly suppressed by administration of 18α- and 18β-GA at proportions of 5:5, 4:6, 2:8 and 0:10, and the production of MCP-1 was significantly suppressed by the administration of 18α- and 18β-GA at proportions of 10:0, 5:5, 4:6, 2:8 and 0:10 (P<0.01). In addition, 18α- and 18β-GA at proportions of 4:6, 2:8 and 10:0 significantly decreased levels of MIP-3α in comparison with those in the model group (P<0.05). When rats were treated with 18α- and 18β-GA at ratios of 6:4, 5:5 or 4:6, the levels of GM-CSF decreased significantly as compared with those in the model group (P<0.05). 18α- and 18β-GA at all proportions markedly reduced levels of MIP-1α and M-CSF compared with those in the model group (P<0.05). Furthermore, 18α- and 18β-GA at all proportions except 0:10 significantly decreased the levels of GRO/KC when compared with those in the model group (P<0.01). G-CSF expression was below the detection limit (data not shown). Taken together, the results indicated that 18α- and 18β-GA at different proportions, particularly at 4:6, substantially reduced the production of most chemokines associated with AH.
Discussion
Alcohol intake is able to alter the expression and release of multiple cytokines and chemokines, which have been reported to participate in local inflammatory response of AH in animal models (20). Inflammation, the early response of the liver to alcohol abuse, is characterized by infiltration of inflammatory cells and hepatocellular injury. The histologic characteristics of alcoholic inflammation range from centrilobular ballooning of hepatocytes to fibrosis (21,22). Infiltration of inflammatory cells may have two opposing functions: Beneficial effects, including clearing out damaged and dying cells, as well as an uncontrolled inflammatory response, which may further exacerbate hepatocellular damage. In the present study, the effect of different proportions of 18α-, 18β-GA on ameliorating AH was investigated, and the effect on the production of cytokines and chemokines associated with inflammation was further explored. The present study was the first, to the best of our knowledge, to have determined that the optimal compatibility proportion of 18α- and 18β-GA against alcohol-induced AH was 4:6, which was associated with the modulation of the levels of most cytokines and chemokines associated with AH.
The mechanisms of AH are complex, involving innate immunity and associated cytokines and chemokines. It is generally considered that Kupffer cells, LPS/TLR4 signaling and the complement system participate in the regulation of cytokines and chemokines associated with AH (4). Alcohol consumption damages the function of the intestinal barrier and increases the flux of LPS (derived from the bacterial cell wall) to the portal vein, thus leading to the activation of Kupffer cells (19). Kupffer cells, one of the major cell types in the liver, are able to produce pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, and anti-inflammatory cytokines, e.g., IL-10. TNF-α has a critical role in the development of AH by inducing the expression of associated cytokines or chemokines, including IL-6, IL-8 and IL-18 (6). In addition, IL-4 activates signal transducer and activator of transcription (STAT)6, which regulates eotaxin expression and induces IL-5 expression, leading to hepatitis (23). IL-6 is another cytokine linked to inflammation and the severity of AH (24). The present study indicated that the levels of TNF-α, IL-1β, IL-2, IL-4, IL-5 and IL-6 in the model group were significantly higher than those in the normal group, which was consistent with the results of previous studies (6,25,26). Of note, 18α- and 18β-GA at ratios of 5:5 and 4:6 significantly decreased the levels of TNF-α, IL-1β, IL-4, IL-7 and IL-2 as compared with those in the model group.
IL-10, known as an anti-inflammatory cytokine, has been previously reported to activate STAT3 in hepatocytes and macrophages/Kupffer cells (4,27); however, in the present study, no significant difference in the serum IL-10 levels was identified between the model and the drug administration groups. Alcohol consumption inhibits the anti-fibrotic activity of IFN-γ, thus leading to acceleration of liver fibrosis (28). In the present study, no significant difference in IFN-γ levels between the alcohol model group and the drug administration groups was identified.
It was observed that the levels of chemokines in the model group, including those of MCP-1, MIP-1α and MIP-3α, were higher than those in the normal group. 18α- and 18β-GA at the ratio of 4:6 markedly reduced the expression of VEGF, MCP-1, GM-CSF and GRO/KC when compared with that in the model group. A previous study by Mandrekar et al (26) also reported that MCP-1 increased in the liver and hepatocytes of mice after oral alcohol administration. They also identified a decreased expression of RANTES in the model group compared with that in the normal group, which was consistent with the results of the present study. This further indicated that alcohol may induce AH by decreasing the level of RANTES and that the hepatoprotective effect of 18α- and 18β-GA may be associated with the upregulation of RANTES. The present study indicated that 18α- and 18β-GA, particularly at the ratio of 4:6, is promising for the treatment of AH by modulating chemokines associated with AH.
In conclusion, the present study was the first, to the best of our knowledge, to determine that the optimal ratio of 18α-GA and 18β-GA for protecting against AH was 4:6. The hepatoprotective effect of GA in alcohol-treated rats was associated with the modulation of cytokines or chemokines associated with AH. These results may help to understand the roles of cytokines and chemokines in AH and provide novel results supporting the clinical use of 18α- and 18β-GA. However, other mechanisms, including the improvement of bile acid and cholesterol metabolism and transport, may be responsible for the hepatoprotective effect of GA16, and thus, further studies, including immunohistochemical analysis of liver tissue and western blot analysis, still require to be performed to demonstrate the hepatoprotective effect of GA and the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
This study was supported by the Fund of education department of Hebei province (grant no. QN2018153), the Special Foundation Construction Project of Medical Discipline of Hebei University (grant no. 2014A1003) and Hebei university innovation funding project (grant no. hbu2018ss74).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YZ and XH designed the current study. XS, JQ and XM performed the experiments. SY analyzed the data and ZC wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Ethical approval and consent to participate
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (USA) and the experimental procedures were approved by the Animal Care and Ethics Committee at Hebei University (no. IACUC-2016031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Anderson P. Global use of alcohol, drugs and tobacco. Drug Alcohol Rev. 2006;25:489–502. doi: 10.1080/09595230600944438. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Amet T, Xing Y, Yang D, Liangpunsakul S, Puri P, Kamath PS, Sanyal AJ, Shah VH, Katz BP, et al. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: A prospective observational study. Hepatology. 2017;66:575–590. doi: 10.1002/hep.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: Innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015;37:185–197. [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Yeh W, Ohashi P. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Bird G, Sheron N, Goka A, Alexander G, Williams RS. Increased plasma tumor necrosis factor in severe alcohol hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 8.Degré D, Lemmers A, Gustot T, Ouziel R, Trépo E, Demetter P, Verset L, Quertinmont E, Vercruysse V, Le Moine O, et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin Exp Immunol. 2012;169:302–310. doi: 10.1111/j.1365-2249.2012.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachakonda V, Gabbert C, Raina A, Li H, Malik S, DeLany JP, Behari J. Stratification of risk of death in severe acute alcoholic hepatitis using a panel of adipokines and cytokines. Alcohol Clin Exp Res. 2014;38:2712–2721. doi: 10.1111/acer.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Mcintosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B: A systematic review. Liver. 2001;21:280–286. doi: 10.1034/j.1600-0676.2001.021004280.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Sudo H. Economic importance of licorice. Plant Biotechnol. 2009;26:101–104. doi: 10.5511/plantbiotechnology.26.101. [DOI] [Google Scholar]
- 12.Ming J, Yin A. Therapeutic effects of glycyrrhizic acid. Nat Prod Commun. 2013;8:415–418. [PubMed] [Google Scholar]
- 13.Guo X, Liang B, Wang X, Fan FG, Jin J, Lan R, Yang JH, Wang XC, Jin L, Cao Q. Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J Gastroenterol. 2013;19:3781–3791. doi: 10.3748/wjg.v19.i24.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JY, Cao HY, Liu P, Cheng GH, Sun MY. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed Res Int. 2014;2014:872139. doi: 10.1155/2014/872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honda H, Nagai Y, Matsunaga T, Saitoh S, Akashi-Takamura S, Hayashi H, Fujii I, Miyake K, Muraguchi A, Takatsu K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukoc Biol. 2012;91:967–976. doi: 10.1189/jlb.0112038. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Fang ZZ, Meng R, Cao YF, Tanaka N, Krausz KW, Gonzalez FJ. Glycyrrhizin and glycyrrhetinic acid inhibits alpha-naphthyl isothiocyanate-induced liver injury and bile acid cycle disruption. Toxicology. 2017;386:133–142. doi: 10.1016/j.tox.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu R, Xiao Q, Cao Y, Yang J. Comparison of the exposure of glycyrrhizin and its metabolites and the pseudoaldosteronism after intravenous administration of alpha- and beta-glycyrrhizin in rat. Drug Res (Stuttg) 2013;63:620–624. doi: 10.1055/s-0033-1349837. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Shi M, Liu L, Han Y, Li YQ, Li Y. Analysis of content differences and variation trends of the principal component isomers and related substances in the four generations of glycyrrhizin preparations. Chin J Pharma Anal. 2014;34:247–254. [Google Scholar]
- 19.Xu MJ, Zhou Z, Parker R, Gao B. Targeting inflammation for the treatment of alcoholic liver disease. Pharmacol Ther. 2017;180:77–89. doi: 10.1016/j.pharmthera.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung P, Pyrsopoulos N. Emerging concepts in alcoholic hepatitis. World J Hepatol. 2017;9:567–585. doi: 10.4254/wjh.v9.i12.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucey M, Mathurin P, Morgan T. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea R, Dasarathy S, McCullough A, Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 23.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: Up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 24.Hill D, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J Lab Clin Med. 1992;119:547–552. [PubMed] [Google Scholar]
- 25.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. doi: 10.1016/0270-9139(95)90643-6. [DOI] [PubMed] [Google Scholar]
- 26.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for MCP-1 in alcoholic liver injury: Regulation of pro-inflammatory cytokines and hepatic steatosis. Hepatology. 2011;54:2185–219. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Kasmi KC, Smith AM, Williams L, Neale G, Panopoulos AD, Watowich SS, Häcker H, Foxwell BM, Murray PJ. Cutting edge: A transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179:7215–7219. doi: 10.4049/jimmunol.179.11.7215. [DOI] [PubMed] [Google Scholar]
- 28.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: Immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/JLB.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.