Abstract
(1) Background: Pregnancy outcomes for both mother and child are affected by many environmental factors. The importance of pregnancy for ‘early life programming’ is well established and maternal nutrition is an important factor contributing to a favourable environment for developing offspring. We aim to assess whether following a Mediterranean Diet during pregnancy is beneficial for maternal and offspring outcomes; (2) Methods: a systematic review was performed using standardized reporting guidelines with the National Heart Lung and Blood Iinstitute quality assessment tool for selection and extraction; (3) Results: results show that being on a Mediterranean Diet during pregnancy is associated with favourable outcomes for both maternal and offspring health, particularly for gestational diabetes in mothers and congenital defects in offspring (4) Conclusions: Following a Mediterranean dietary pattern during gestation is beneficial for the health of both the mother and offspring. Pregnant women and those trying to conceive should be advised to follow a Mediterranean Diet to potentially decrease, for example, the likelihood of atopy (OR 0.55) in the offspring and Gestational Diabetes Mellitus in the mother (OR 0.73).
Keywords: maternal nutrition, Mediterranean diet, offspring health
1. Introduction
The maternal diet before conception and during pregnancy has long-term implications for maternal and offspring health, from placental development [1], risk of developing gestational diabetes [2], birth complications [3], birth weight [4] and risk of developing allergies in childhood [5,6,7,8]. Exposure to an unfavourable environment in early pregnancy is known to significantly increase the risk of diseases in adult life; this is known as ‘early life programming’ and one of the most important factors is the maternal diet [9]. Pregnancy presents an opportune time window for healthcare professionals’ intervention to improve health for both the mother and child, making the evidence surrounding maternal diet an important tool for the healthcare practice.
Maternal diet is a blueprint for the diet which children are likely to follow into adolescence. This is due to the fact that, typically, mothers are responsible for feeding their children. Without intervention, pregnant women who do not follow a healthy diet or lifestyle choices are unlikely to change their patterns of behaviour. Sometimes obstacles to changes in behaviour can present themselves even for well-known harmful habits such as smoking during pregnancy, but as a unique window of opportunity, focusing health service efforts to impact the behaviour of pregnant women is crucial.
The Mediterranean Diet (MD) and Mediterranean Diet Adherence (MDA) is characterized by a high intake of fruits, vegetables, whole grain cereals, legumes, fish and nuts; low-to-moderate consumption of dairy products and limited amounts of red meat and red wine. It is low in saturated fats and high in antioxidants, fibre and mono and polyunsaturated fatty acids mainly derived from extra virgin olive oil (evoo) (MUFAs) and oily fish (n-3 PUFAs). The MD is known to have many beneficial effects for longevity and disease prevention, demonstrated in numerous high-quality studies, reviews and meta-analyses, making it the most widely studied and evidence-based dietary approach to healthy eating and disease prevention [10,11,12,13]. The unique synergy of various health benefitting nutrients makes MD an effective approach to improving health [11].
Recording dietary patterns through food diaries and food frequency questionnaires (FFQs) is a valuable tool to analyse eating behaviours and better understand which foods and food groups are consumed. Individual foods, such as extra virgin olive oil (evoo), as well as food groups, such as pulses, provide an insight to the nutrients consumed in the recorded foods, such as monounsaturated fatty acids (MUFAs) and fibre, respectively. Asking an individual to recount specific nutrient amounts is more complex than recounting what foods were consumed as part of the daily diet, making dietary pattern analysis more attainable for patients and crucial for research.
Evidence on the impact of diet during pregnancy on health outcomes focusses on either individual nutrients, such as folic acid, or the Mediterranean Diet for their impact on specific disease outcomes such as neural tube defects [14] or leukaemia [15]. The degree of evidence available for the impact of specific nutrients in pregnancy on outcomes for offspring health is more powerful than that for dietary patterns. For instance, the necessity for appropriate levels of folic acid during gestation is well-documented in the prevention of neural tube defects, and the mechanism by which folic acid prevents neural tube defects is well understood and documented. Whereas supplements are the quicker and easier way to address certain nutritional deficiencies of individual nutrients [11], dietary patterns are a useful way of studying the effects of dietary exposure, in turn, making recommended diets a potential health improvement intervention alongside appropriate and necessary supplementation in pregnancy.
The Mediterranean Diet is well established as beneficial in the literature [16]. Previous reviews of the evidence where several dietary patterns have been evaluated highlight that maternal dietary patterns are important factors in early life programming. Maternal dietary patterns which reflect the MD showed consistent associations with a lower risk for allergic disease in children [17], appropriate infant birthweight [18], and lower risk of pre-eclampsia and preterm birth [19]. This Review investigates and presents current evidence exclusively on the Mediterranean Diet’s multifactorial impact on overall health outcomes for both the mother and offspring, as the sole dietary pattern of interest.
Our results indicate that the Mediterranean Diet has a significantly positive impact on maternal and offspring health, strengthening the evidence base to encourage its adoption as a preventive measure against diseases throughout the life course and especially in pregnancy when the outcome of two or more individuals can be positively impacted.
2. Materials and Methods
2.1. Data Sources and Search Strategy
This systematic review was performed using a predetermined, unpublished protocol and in accordance with standardized reporting guidelines [20]. Search terms identification and classification were guided by previous comprehensive relevant reviews [21]. One reviewer (FA) performed searches in the following online electronic databases (Medline, Embase, Web of Science, Scopus, Maternity & infant care and Cochrane). The search of online databases is up to date to February 2019. The search was not restricted by language or date.
The search was broken down into four main categories. To identify the relevant population, the first Boolean search was done using the term “OR” to explode (search by subject heading) and map (search by keyword) the following MeSH headings “child health” or “offspring” or “newborn” or “neonate” or “child” or “baby” or “gestation” or “pregnancy” or “pregnant woman” or “perinatal period” or “prenatal exposure” or “prenatal”. To identify relevant interventions the second Boolean search used the term “OR” to explode and map “Mediterranean diet” or “fruit” or “vegetable” or “legume” or “nut” or "olive oil” or “evoo” or “oily fish” or “seafood” or “tomato”. The third category of MeSH headings was also related to the intervention and included ((low or little or medium or moderate or less or decrease or reduce or restrict) (intake or consumption or consume or eat or amount)) AND (“dairy product” or “red meat” or “processed meat” or “red wine”). Finally, the fourth group of key terms was used to identify the study design whereby a Boolean search using the term “OR” was used to explode and map the keywords “randomized controlled trial” or “controlled clinical trial” or “placebo” or “Retrospective Studies” or “Cohort Studies”. The four search categories were then combined using the Boolean operator “AND”. The hand searching of results in the Maternity & infant care database for a Mediterranean diet highlighted that using the terminology of ‘child health’ was not capturing some relevant papers, thus, the papers which were the result of searching for a Mediterranean diet in this database were hand-searched instead. For a full list of the search terms in each database please see Appendix A.
2.2. Study Selection
Three reviewers (FA, AS and SH) independently evaluated articles for eligibility in a three-stage procedure. In stage one, all identified titles and abstracts were reviewed. In stage two, a full-text review was performed on all the articles that met the predefined inclusion criteria as well as all articles for which there was uncertainty as to eligibility. In stage three, full texts were re-evaluated for data extraction.
2.3. Inclusion/Exclusion Criteria
Studies, publications or reports in the English-language describing an association between the Mediterranean diet during pregnancy and infant development were eligible for inclusion. Studies, publications or reports were included for analysis if they included the following:
1. Exposure: Comprehensive dietary assessment: Food frequency questionnaire (FFQ), 24 h diet recall, food record, diet history, Use of a priori dietary score or index;
2. Outcome: Clinical neonatal outcome, disorders assessed by study staff, medical records or clinician diagnosed (for example, Foetal growth restriction (FGR) and preterm delivery (PTD)), developmental issues assessed by validated scale/questionnaire;
3. Design: Observational studies (cross-sectional, cohort, case-control), Interventional studies (clinical trials);
4. Population: Pregnant women (at any gestational age).
Articles were excluded if they investigated pregnant women with other dietary complications (non-gestational diabetes, obesity, anorexia, malnourishment) and/or if they explored a specific nutrient impact as opposed to a dietary pattern (e.g., Omega-3 supplementation as a feature of the Med Diet.)
2.4. Data Extraction and Quality Assessment
Two reviewers (FA and AS) independently extracted data from all studies that satisfied the inclusion criteria. Any disagreement in data extraction and/or study inclusion was resolved through discussion between the two reviewers and, when necessary, a third reviewer (SH).
The primary outcome was the impact on maternal and offspring health. A number of other study characteristics were also extracted including geographic location, description of the study population, primary outcomes, description of intervention and control, and results. Furthermore, data pertaining to sample size, number, and features of the intervention were also extracted.
2.5. Quality Assessment
The methodological quality of studies was assessed and scored independently by two reviewers (FA and AS) using the validated National Heart, Lung, and Blood Institute (NHLBI) study Quality Assessment Tool [22] (see Appendix B Table A1). Disagreements were resolved through discussion. The summary score for each study was calculated (minimum: 1, maximum: 10) and categorised into three categories. Studies with a score of 8 or above were considered of high-quality, studies with a score of 5–7 were considered of fair quality and studies with a score of 4 or below were considered of poor quality. These categories were used to evaluate whether outcomes significantly varied according to study quality and to determine which weight studies should be given in the synthesis of the findings.
2.6. Data Synthesis and Presentation
Since exploring heterogeneity is one of the main aims of an SLR/MA, a descriptive synthesis approach was used instead of meta-analytic procedures. Our review includes studies with diverse designs and all shared comprehensive dietary assessment tools to measure MD adherence, with neonatal and maternal clinical markers as outcomes. The results of the quality assessment are presented in Table 1. As the narrative synthesis was used to analyse and present the findings, the results of data abstraction are summarized in Table 2, which outlines the type, objective and target of intervention, and measurement of effect for each intervention. Study results are summarized in Table 3 according to categories/themes and whether the MD was found to have a Protective, Null or Negative effect on outcomes.
Table 1.
The quality assessment score values: <4 = Low, 4–7 = Medium, 8–10 = High.
| Author | Design | Score (1–10) | Quality Assessment |
|---|---|---|---|
| Assaf-Balut, C; Garcia de la Torre, N; A Duran et al. [23] | Prospective randomized interventional study | 8 | High |
| Assaf-Balut, Carla; Garcia de la Torre, et al. [24] | Prospective randomized controlled trial (The St Carlos GDM prevention study) | 9 | High |
| Botto, Lorenzo D.; Krikov, Sergey; et al. [25] | Multicentre population-based case-control study | 9 | High |
| Chatzi, L.; Rifas-Shiman, S.; [26] | Prospective Cohort study (Project Viva + Rhea Study) | 9 | High |
| Chatzi L.; Torrent, M.; et al. [27] | Prospective Cohort study | 7 | High |
| Chatzi, L.; Garcia, R.; et. al. [28] | Cohort study (INMA and Rhea Study) | 9 | High |
| E, Parlapani; et al. [29] | Cohort study | 7 | Medium |
| Fernandez-Barres, S.; et al. [30] | Birth Cohort study (INMA) | 8 | High |
| Gesteiro, E.; Rodriguez B., et al. [31] | Cohort study | 7 | Medium |
| Gesteiro, E.; Bastida S., et al. [32] | Cross-sectional study | 7 | Medium |
| Gonzalez-Nahm, S. et al. [33] | Cohort study | 8 | High |
| Haugen, M.; et al. [34] | Prospective Cohort study (MoBa) | 8 | High |
| House, J.; et al. [35] | Prospective Cohort study | 9 | High |
| Castro-Rodriguez, J.; et al. [36] | Cohort study | 7 | Medium |
| Lange, N. [37] | Longitudinal pre-birth cohort study | 9 | High |
| Mantzoros, C.; et al. [38] | Prospective cohort study (Project Viva) | 8 | High |
| Monteagudo, C.; et al. [39] | Cohort study | 8 | High |
| Peraita-Costa, I. et al. [40] | Retrospective cross-sectional population-based study | 7 | Medium |
| Saunders, L.; et al. [41] | Cohort study (TIMOUN) | 8 | High |
| Steenweg-de Graaff, J.; et al. [42] | Population-based cohort (The Generation R Study) | 9 | High |
| Vujkovic, M.; et al. [43] | Case-control study | 7 | Medium |
| Smith, L.; et al. [44] | Population-based cohort study | 7 | Medium |
GDM = gestational diabetes mellitus, INMA = INfancia y Medio Ambiente study, TIMOUN= French Caribeean Mother Child cohort study.
Table 2.
The outcomes and effect estimates for the included studies.
| Authors | Design and Cohort | Included Participants and Gestational Age | Intervention Type and Comparator | Results |
|---|---|---|---|---|
| Assaf-Balut, C; Garcia de la Torre, N; A Duran et al. [23] Spain |
Prospective randomized interventional study | 874; First Trimester |
MD nutritional therapy | As an early nutritional intervention, MD reduces the incidence of GDM. Comparison of HbA1c levels at 24–28 weeks in women with GDM and normal glucose tolerance: p = 0.001. Values became similar at 36–38 gestational weeks with intervention. |
| Assaf-Balut, Carla; Garcia de la Torre, et al. [24] Spain |
Prospective randomized controlled trial | 874; intervention group (IG), n = 434 control group (CG), n = 440; 8–12 gestational weeks (First Trimester) |
MD nutritional therapy with additional evoo and pistachios | Supplemented MD reduces the incidence of GDM as an early nutritional intervention. IG showed reduced rates of insulin-treated GDM: p =< 0.05 |
| Botto, Lorenzo D.; Krikov, Sergey; et al. [25] USA |
Multicentre population-based case-control study | Mothers of babies with major non-syndromic congenital heart defects (n = 9885); mothers with unaffected babies (n = 9468); maternal diet assessed in the year before pregnancy |
A priori defined MDS with Quartiles 1–4 (worst to best) | Better diet quality is associated with a reduced occurrence of some conotruncal and septal heart defects Overall conotruncal defects: OR 0.63, 95% CI 0.49 to 0.80 Overall tetralogy of Fallot: OR 0.76, 95% CI 0.64 to 0.91 Overall septal defects: OR 0.77, 95% CI 0.63 to 0.94 Overall atrial septal defects: OR 0.86, 95% CI 0.75 to 1.00 |
| Chatzi, L.; Rifas-Shiman, S.; [26] USA, Greece |
Cohort study; Project Viva | Mother-child pairs from USA: 997 Greece: 569 MDA measured during pregnancy with follow-up at median 4.2 and 7.7 years |
MDA with a priori defined MDS through FFQ | Greater adherence to MD during pregnancy may protect against excess offspring cardiometabolic risk. For each 3-point increase in MDS, offspring BMI decreased by 0.14 units (95% confidence interval, −0.15 to −0.13) |
| Chatzi L.; Torrent, M.; et al. [27] Spain |
Cohort study | 507 mothers during the gestational period; 460 children at 6.5 years post-gestational follow-up |
Impact of MDA during pregnancy on asthma and atopy in childhood using a priori defined MDS | Adherence to Med Diet during pregnancy support protective effect against asthma-like symptoms and atopy in childhood Persistent wheeze: OR 0.22; 95% CI 0.08 to 0.90 Atopic wheeze: OR 0.30; 95% CI 0.10 to 0.90 Atopy: (OR 0.55; 95% CI 0.31 to 0.97 |
| Chatzi, L.; Garcia, R.; et al. [28] Spain, Greece |
Cohort study; INMA (Spain) RHEA (Greece) |
During pregnancy with follow-up within 1 year post-gestational; 1771 mother-newborn pairs (Spain); 745 pairs (Greece) |
MDA calculated through completed FFQ | High meat intake during pregnancy may increase the risk of a wheeze in the first year of life; high dairy intake may decrease it RR 0.83, 95% CI 0.72, 0.96 |
| E, Parlapani; et al. [29] Greece |
Cohort study | 82 women delivering preterm singletons ≤34 weeks | FFQ and MDA | High adherence to MD, may favourably affect intrauterine growth (IUGR), premature birth and maternal hypertension (HTN); Low-MDA neonates group had a higher rate of IUGR: OR 3.3 Low-MDA mothers had a higher rate of prematurity: OR 1.6 Low-MDA mothers had a higher gestational HTN: OR 3.8 |
| Fernandez-Barres, S.; et al. [30] Spain |
Cohort study; INMA |
1827 mother-child pairs, assessed during pregnancy | FFQ and MDA | Adherence to MD during pregnancy not associated with a risk of childhood obesity, but is linked to a lower waist circumference; p-value for trend = 0.009 |
| Gesteiro, E.; Rodriguez B., et al. [31] Spain |
Cohort study | 35 women with ‘adequate’ or ‘inadequate’ diets according to HEI (healthy eating index) and MDA score; 1st trimester |
13 point MDA score via FFQ | Maternal diets during the 1st trimester with low HEIs or adherence to MD have a negative effect on insulin markers at birth; Low MDA-score diets had low-fasting glycaemia: p = 0.025 and delivered infants with high insulinaemia: p = 0.049 |
| Gesteiro, E.; Sanchez-Muiz FJ, et al. [32] Spain |
Cross-sectional study | 53 mother-neonate pairs; GDM screening at 24–28 gestational weeks |
Maternal MDA and offspring lipoprotein profile | Neonates of mothers who consumed low adherence of MD during pregnancy presented impaired lipoprotein and higher homocysteine levels; Mothers’ diet in the nAA + AT x mTT group (neonates carrying FTO rs9939609 T allele x Mothers homozygous for FTO rs9939609 T allele) had a significantly lower MDA score: p = 0.05 |
| Gonzalez-Nahm, S. et al. [33] USA |
Cohort study | 390 women whose infants had DNA methylation cord blood data available; FFQ at preconception or 1st trimester |
MDA via FFQ | Suggests that maternal diet can have a sex-specific impact on infant DNA methylation at specific imprinted DMRs; OR = 7.40, 95% CI = 1.88–20.09 |
| Haugen, M.; et al. [34] Norway |
Cohort study; MoBa |
MD criteria met: 569 women; 1–4 criteria met: 25,397 women; 0 MD criteria met: 159 women; 18–24 gestational weeks |
MDA via FFQ | Women who adhered to the MD criteria did not have a reduced risk of preterm birth compared to women who met none of the criteria; OR: 0.73, 95% CI: 0.32, 1.68 Intake of fish twice a week or more associated with lower preterm birth; OR: 0.84; 95% CI: 0.74, 0.95 |
| House, J.; et al. [35] USA |
Cohort study; NEST |
325 mother-infant pairs; 1st trimester; follow-up at 2 years post-gestation |
MDA via FFQ | Offspring of women with high MDA less likely to exhibit neurobehavioural effects: Depression; OR = 0.28 Anxiety; OR = 0.42 Social relatedness; OR = 2.38 |
| Castro-Rodriguez, J.; et al. [36] Spain, Chile |
Cohort study | Gestational period; follow-up in 1000 preschoolers (at 1.5 yrs and 4 yrs) | MDA via FFQ | Low fruit and high meat consumption by the child had a negative effect on allergic responses (wheezing, rhinitis, or dermatitis); as did the high consumption of pasta and potatoes by the mother |
| Lange, N. [37] USA |
Longitudinal prebirth cohort study; Project Viva |
1376 mother-infant pairs; 1st and 2nd trimesters with follow-up at 3 years post-gestation |
MDA via FFQ | Dietary pattern during pregnancy not associated with recurrent wheeze; OR per 1-point increase in MD: 0.98, 95% CI, 0.98–1.08 |
| Mantzoros, C.; et al. [38] USA |
Prospective cohort study; Project Viva |
780 women; 1st and 2nd trimesters; post-gestational cord blood |
MDA | Adherence to MD during pregnancy not associated with cord blood leptin or adiponectin; p-value = 0.38 |
| Monteagudo, C.; et al. [39] Spain |
Cohort study | 320 umbilical cord serum samples | MDS-p (med diet score adapted to pregnancy) | Adherence to the MD and folic acid supplementation during pregnancy may indicate being overweight in newborns; OR = 3.33 (p = 0.019) |
| Peraita-Costa, I. et al. [40] Spain |
Retrospective cross-sectional population-based study | 492 mother-child pairs; immediately post-delivery and for 6 months thereafter |
MDA with two groups identified: low and high adherence | Low adherence to an MD was not associated with a higher risk of a low birthweight newborn; aOR = 1.68; 95% CI 1.02–5.46 |
| Saunders, L.; et al. [41] French West Indies |
Cohort study; TIMOUN |
728 pregnant women who delivered liveborn singletons with no malformations | Semi-quantitative FFQ analysed for MDA | Results suggest that adherence to a Caribbean diet may include benefits of MD, contributing to a reduction in preterm delivery in overweight women; A OR: 0.7, 95% CI 0.6, 0.9 |
| Steenweg-de Graaff, J.; et al. [42] The Netherlands |
Population-based cohort; Generation R Study |
During pregnancy at median 13.5 weeks; Post-gestation in 3104 children at 1.5, 3, and 6 years of age | MDA via FFQ | High adherence to traditional Dutch diet and low adherence to MD are linked to an increased risk of child externalizing problems; OR per SD in MDS: 0.90, 95% CI: 0.83–0.97 OR per SD in Traditionally Dutch Score: 1.11, 95% CI: 1.03–1.21 |
| Vujkovic, M.; et al. [43] The Netherlands |
Case-control study | 50 mothers of children with Spinal Bifida; 81 control mothers post-gestation |
Dietary assessment via FFQ | MD seems to show an association with reducing the risk of offspring being affected by SB; Weak MDA; OR: 2.7 (95% CI 1.2–6.1) High MDA; OR: 3.5 (95% CI 1.5–7.9) |
| Smith, L.; et al. [44] United Kingdom |
Population-based cohort study | 922 LMPT; 965 term births; 32–36 weeks gestation (3rd Trimester) |
Maternal interview for dietary factors: MDA, low fruit and vegetable intake, use of folic acid supplements | Women with 0 adherence to MD were nearly twice as likely to deliver LMPT; RR 1.81 (1.04 to 3.14) Smokers and low consumption of fruit and vegetables had a particularly high risk; RR 1.81 (1.29 to 2.55) |
MD = Mediterranean Diet, MDS = Mediterranean Diet Score, MDA = Mediterranean Diet Adherance, FFQ = Food Frequency Questionnaire, HEI = Healthy Eating Index.
Table 3.
A summary of findings from our review of the evidence.
| Health Outcomes | Impact of Maternal MD | ||
|---|---|---|---|
| Outcome Variable | Protective | Negative | Inconclusive |
| Allergic Disorders | 3 (27,28,36) | 1 (37) | |
| Premature birth, birth weight, childhood obesity | 5 (29,30,39,41,44) | 2 (30,36) | |
| Cardiometabolic and congenital defects | 3 (25,26,43) | ||
| Gestational diabetes & pre-eclampsia | 2 (23,24,29) | ||
| DNA Methylation | 2 (33,35) | ||
| Biomarkers | 2 (31,32) | 1(38) | |
| Behavioural development | 1 (42) | ||
3. Results
3.1. Literature Selection
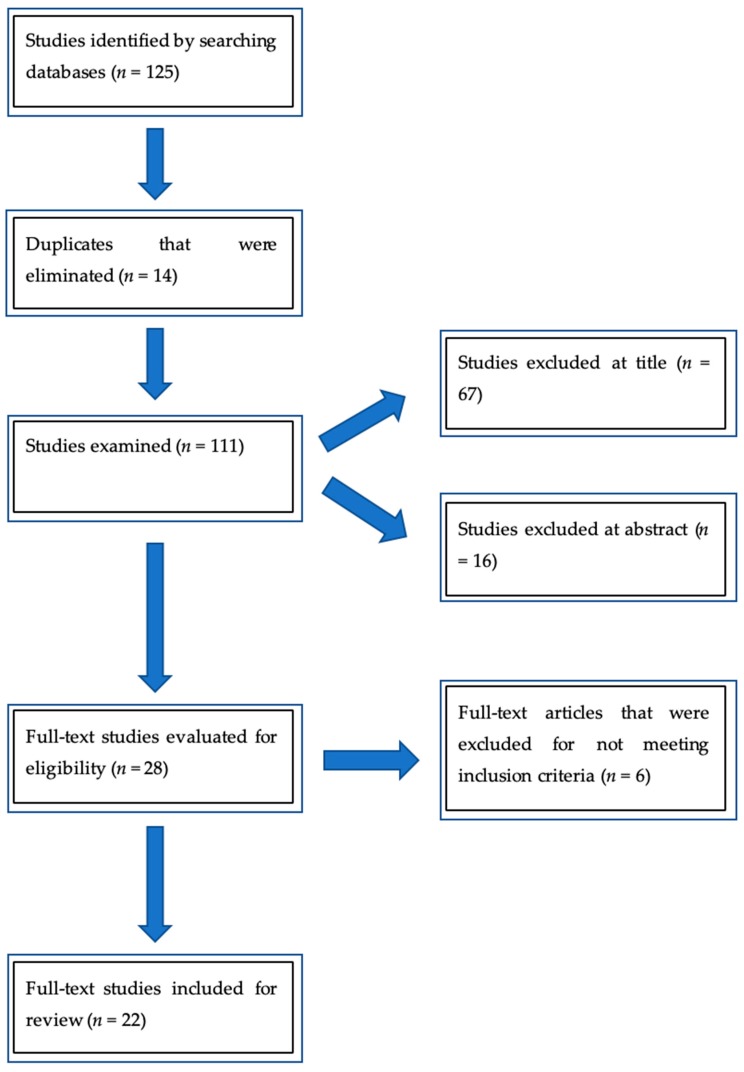
Our initial database search is outlined in Figure 1, showing that a total of 125 articles were initially identified based on our search criteria, 14 duplicates were removed, leaving 111 articles for title and abstract screening, of which 67 were excluded for the title (25 were the wrong type of article for this review, 19 targetted the non-objective population, 8 were studies that included dietary complications or risk factors, and 15 focused on a specific nutrient impact); 16 at abstract following the inclusion/exclusion criteria (9 were the wrong type of article for this review, 3 targetted a non-objective population, 3 for studies that included dietary complications or risk factors, and 2 focused on specific nutrient impact); and 6 after full text screening, included in the Appendix C (2 were the wrong type of article for this review, 3 targetted a non-objective population, and 1 focused on specific nutrient impact), for a total of 22 articles reviewed and included in this paper.
Figure 1.
The flow diagram of the process for study selection.
The studies included all used a comprehensive dietary assessment tool to measure MD adherence, with neonatal and maternal clinical markers as outcomes. The selected studies included observational, interventional studies, randomized trials, and all studies included a population of pregnant women at any gestational age with neonatal or child follow-ups. Table 1 shows our quality assessment results and Appendix C Figure A1 shows papers excluded after full text review.
3.2. Study Characteristics
The studies included (n = 22) were published between 2008 and 2018 and were conducted in 8 countries, which included Spain, USA, Greece, Holland, Norway, Chile, French West Indies, and the United Kingdom. The common dietary measurements used were the adherence to the Mediterranean Diet Score (MDS) and an adapted MDS for pregnancy (MDS-p).
The MDS is an a priori defined score developed to measure compliance to a high intake of Mediterranean Diet foods with a score ranging from 0 to 9, where 9 indicates greater adherence to the diet [13]. The MDS-p is a scoring system that was designed by Montegaudo et al. [45] for pregnant women to better quantify the micronutrients in their diets such as calcium, folic acid, and iron, which are especially relevant in pregnancy [39].
Of the included studies, 16 were cohort studies, 1 was a randomized interventional study, 1 was a randomized controlled trial, 2 were case-control studies, and 2 were cross-sectional studies. All studies were conducted during the gestational period with follow-ups after birth. Table 2 shows the summarized characteristics of the studies including design, measures used, and results reported. The total number of participants for all of the studies is 63,336.
3.3. MAIN RESULTS
3.3.1. Allergic Disorders
Four studies focused on the relationship between maternal adherence to a Mediterranean Diet during pregnancy and neonatal or childhood outcomes of asthma and atopy. A cohort study by Chatzi, L; Torrent, M.; et al. [27] found that adherence to the MD during pregnancy supports a protective effect against asthma-like symptoms and atopy in childhood at 6.5 years old. Chatzi, L.; Garcia, R.; et al. [28] found that while adherence to the MD was not associated with the risk of wheeze and eczema in any cohort, high meat intake during pregnancy might increase the risk of wheeze during the first year of life. Through questionnaires of epidemiological factors, maternal diet during pregnancy, and childhood diet, Castro-Rodriguez, J.; M. Ramirez-Hernandez et al. [36], found that wheezing, rhinitis or dermatitis were negatively affected by high potato and pasta consumption by the mother and subsequent low fruit and high meat consumption by the child. Lange, N. et al. [37] however, examined 1376 mother-infant pairs and found that the dietary pattern is not associated with recurrent wheeze.
3.3.2. Premature Birth, Birth Weight, Childhood Obesity
Six studies examined the implications of the diet on premature birth, gestational diabetes (please see Table 3), birth weight, and/or childhood obesity. Parlapani, E. et al. [29] found that neonates of mothers with low adherence to the MD had significantly higher intrauterine growth restriction and lower birth weights. Haugen, M. et al. [34] investigated the association between women who met the MD adherence criteria and those who did not. The study showed that those who met the MD criteria of fish ≥2 times a week, fruit and vegetables ≥5 times a day, use of olive/canola oil, red meat intake ≤2 times a week, and ≤2 cups of coffee a day, did not have a lower risk of preterm birth compared to women who met none of the criteria. A population-based cohort study involving 922 late and moderate preterm births (LMPT) by Smith, L. et al. [44], identified that women who did not include any aspects of the MD during pregnancy were nearly twice as likely to have LMPT in comparison to higher adherence mothers. Fernandez-Barres, et al. [30] evaluated associates between adherence to the MD during pregnancy, but found no risk to childhood obesity, however, it was associated with a lower waist circumference, which is related to abdominal obesity and is an important health marker. Peraita-Costa et al. [40] administered the “Kidmed” questionnaire to collect dietary information from mothers on adherence to the MD. Of the 492 women, 40.2% showed low adherence to the MD, but this study did not directly correlate the results to low birth weight due to potentially confounding factors including smoking, low education levels, and low dairy intake. Saunders, L. et al. [41] found that low adherence to the MD could implicate a positive correlation between maternal diet and small birth weight.
3.3.3. Cardiometabolic and Congenital Defects
Three studies examined the link between better maternal diet quality with congenital heart defects or metabolic factors. Chatzi, L.; Rifas-Shiman, S.; et al. [26] studied 997 mother-child pairs, finding that improved adherence to the MD during pregnancy showed lower systolic and diastolic blood pressures and may protect offspring against cardiometabolic risk. Botto, L. [25] et al. found, in a high-quality study, that diet quality was a factor of reduced conotruncal and atrial septal heart defects. Vujkovic, M. et al. [43] found that because of the higher levels of serum and RBC folate, Vitamin B12 and lower plasma homocysteine contained in the MD, offspring have a reduced risk of spina bifida.
3.3.4. Gestational Diabetes and Pre-Eclampsia
Two studies found that gestational nutrition based on the MD reduces the incidence of Gestational Diabetes (GDM). In a prospective randomized interventional study by A Duran, [24] 177 women out of 874 were diagnosed with GDM and found that with early intervention using the MD, gestational outcomes were improved. Likewise, the results of a randomized controlled trial conducted by Assaf-Balut, C. et al. [24] show that the incidence of GDM was reduced by early dietary intervention with a diet rich in EVOO and pistachios. Parlapani et al. [29] found that MDA was an independent predictor of gestational hypertension and pre-eclampsia.
3.3.5. DNA Methylation
Two studies examined the association between maternal adherence to the MD and DNA methylation in infants. One study conducted by Gonzalez-Nahm et al. [33] showed evidence that maternal diet of low MD adherence had an increased risk of female sex-linked hypo-methylation at the MEG3-IG differentially methylated region. House, J. et al. [35] also found an association between maternal adherence to the MD and female sex-linked methylation at MEG3, IGF2, and SGCE/PEG10 DMRs, and identified an association between MD and favourable neurobehavioral outcomes in early childhood.
3.3.6. Biomarkers
Four studies were associated with adherence to the MD and subsequent repercussions on biomarkers and implications of the pregnancy and on the newborn. Gesteiro, E. et al. [31] aimed to determine the relationship between diet within the first trimester and biomarkers of insulin resistance at birth. They found that women consuming low MD adherence diets had low-fasting glycaemia and delivered infants with high insulinaemia. A follow up cross-sectional study by Gasteiro, E., Bastida, S. et al. [32] aiming to identify the relationship between diet quality during pregnancy and serum lipid, arylesterase and homocysteine values at birth identified that neonates whose mothers had low adherence to the MD presented impaired levels of the referenced biomarkers. In a cohort study by Mantzoros et al. [38], multivariable linear regression was used to analyse the correlation between maternal diet during the 1st and 2nd trimesters and cord blood levels of leptin and adiponectin. High adherence to the MD was not found to be associated with these levels. Conversely, a study by Monteagudo et al. [39] on the exposure to organochlorine pesticides and maternal diet indicated that higher folic acid supplementation and greater exposure to the endocrine-disrupting residues were related to higher newborn weight.
3.3.7. Behavioural Development
One cohort study by Steenweg-de Graaff et al. [42], examining the pregnancies and follow-up of the 3104 children born, found that the MD was not associated with internalizing problems such as anxiety or depression, while low adherence to the MD was positively associated with increased child externalizing problems, such as aggression or inattention.
Of the 22 studies included, 18 found that adherence to the Mediterranean Diet during pregnancy had protective factors on the health of the newborn and 4 of the studies were inconclusive or showed no correlation. None of the studies showed a negative association between the MD and the outcomes.
4. Discussion
The mechanisms by which the MD exerts its effect on fetal development and maternal health are complex and need further research. By reviewing the available literature focusing specifically on MD interventions/exposure, as opposed to all dietary patterns as in previous reviews, we have a good overview of how the MD pattern effects mothers and their offspring on a variety of outcomes. Particularly interesting insights can be gleaned from the numerical values associated with some of the risk factors. The studies in this review are of good quality and represent a combined study population >63,000.
The observed heterogeneity of interventions, study populations and outcomes measured, allows for a big picture view of how the MD diet exerts its effects at all gestational ages and in the early years of life. For instance, when looking at behavioural outcomes, this review concluded that adherence to the MD in pregnancy has a statistically significant impact on decreasing the likelihood of offspring exhibiting depressive behaviours (OR 0.28) [35] in an ethnically diverse cohort, and another study added to this with a reduced OR of 0.90 [42] for developing externalising behaviours (such as aggression) for offspring of mothers with a high MDA.
Though some of the outcome categories we have identified only have 2 or 3 studies, they give a good starting point for future research to build on the current knowledge. Berti et al.’s comprehensive literature review of early life nutritional exposures on life-long health splits its findings into ‘sections’ [46] which, again, give a big picture overview on the vast impact that maternal, early life and even pre-conceptional nutrition can have. Unlike some more focused reviews, investigating the impact of maternal diet on allergic diseases [47], for instance, we hope that our review’s heterogeneity contributes to a wider understanding of maternal dietary impact.
This review brings together some ideas of how the MD may be contributing to in-utero development through specific mechanistic pathways. The methylation of specific sites in two of the studies (see Table 3) present results on how the MD, as opposed to specific nutrients, contribute to this very time-sensitive window of change of DNA methylation during fetal development.
The results on atopy, asthma and eczema highlight that whilst the MD is not always protective, it is associated with a reduced likelihood of allergic disorders as a result of maternal MD diet adherence and compounded further by offspring MD adherence. This type of follow up warrants attention; further investigation into the impacts which MD adherence for offspring of mothers who had high adherence to the MD during pregnancy and lactation, compared to non-MD adherent mother-child pairs would allow for dietary recommendations to be evidence-based through the course of pregnancy and early life as a one-time course. Intuitively, this would be a good way to approach dietary recommendations for improving health, as most children adopt their mother’s dietary choices as the main provider of food in early life.
With regards to premature birth, low birth weight and childhood obesity, the evidence is more mixed. The studies that found no association between the MD and decreased likelihood of premature birth were of high quality. Some of the uncertainty is due to the limitations of the studies, some not controlling for confounders of specific outcomes like intrauterine growth, such as smoking. Despite this, the studies that were inconclusive on the impact of the MD on their primary chosen outcome, for instance the INMA birth cohort study for childhood obesity [30] and Parlapani et al.’s work [29] on birth complications and prematurity, still found statistically significant positive associations between MD adherence and other health outcomes in their results, such as waist circumference and likelihood of pre-eclampsia and necrotizing enterocolitis for each study, respectively.
A strong case for the positive impact of the MD can be seen in the results of the studies focusing on cardiometabolic and congenital defects, GDM and DNA methylation. Though these studies investigated hugely different markers and outcomes, it is clear that the MD has a protective effect on them all. The evidence presented here regarding DNA methylation and biomarkers reflects a novel way to measure the impact of dietary changes and contributes to the understanding of the mechanisms behind the long-term impacts which ‘early-life programming’ has. The nature of DNA methylation in the fetal development time-course makes it possible to observe the impact which specific intra-uterine exposures have on offspring DNA methylations 20, 30 or 40 years later. For example, studies on the impact of intra-uterine exposure to famine showed that lower methylation of 5 CpG dinucleotides within the insulin-like growth factor-II differentially methylated region (DMR) [48] was detected in affected offspring decades later. Thus, the potential for future research on the impact of the MD on methylation sites of interest is vast, as dietary exposure has such a large impact.
The pattern of MD that may influence the reduction of adverse effects most appears to be one where there is purposefully added evoo and nuts. As seen in the present literature, increased vegetables and oily fish and decreased processed foods delivered positive results, and the effects are seen both in metabolically healthy and compromised gestation, indicating that the MD could be a diet with beneficial outcomes for GDM and metabolically healthy pregnancies.
The results of this review add to the growing body of evidence that the Mediterranean Diet is a beneficial dietary recommendation throughout the life course. By collating the evidence on outcomes of the MD for the mother and child, we highlight the broad range of effects this dietary intervention has. The limitations of this work are the small number of studies per outcome group, with the biggest group here being ‘premature birth and birth weight’ at only 7 studies total. Regular updates of this review are important as research around the topic is growing, and with an expanding evidence base, the possibility of meta-analysis for each category is likely. The biggest strength of this review is that, even with small numbers of studies, it highlights the impact which MDA in pregnancy and early childhood can have on several different health outcomes. The implications for clinical practice are great, as prescribing an MD pattern to women of reproductive age is a simple intervention, with important clinical potential for both the mother and offspring.
5. Conclusions
The Mediterranean Diet in pregnancy and early infancy is safe and beneficial for a wide range of maternal and offspring outcomes. Further research to ascertain the relationship and mechanisms maternal MD has with health outcomes of interest in different populations is needed to position it as a public health intervention for all populations.
Acknowledgments
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under the Collaborations for Leadership in Applied Health Research and Care (CLAHRC) programme for North West London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Appendix A. Search Strategy
Database: MEDLINE <1946 to February 04, 2019>:
exp Fruit/ or Fruit.mp. (131831)
edible grain/ or exp vegetables/ (42391)
Leguminosae.mp. or exp Fabaceae/ (69062)
nut.mp. or Nuts/ (6941)
exp Olive Oil/ or evoo.mp. (4609)
extra virgin olive oil.mp. (931)
monounsaturated fatty acids.mp. or exp Fatty Acids, Monounsaturated/ (45287)
Fishes/ or oily fish.mp. or Fatty Acids, Omega-3/ or Seafood/ (75289)
Lycopersicon esculentum/ or tomato*.mp. (23549)
((high or more or increase* or elevat* or much or rais*) adj6 (intake or consumption or consume or eat* or amount*)).tw. (179734)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (348823)
10 and 11 (14459)
Milk/ or exp Dairy Products/ or dairy.mp. (120218)
red meat.mp. or exp Red Meat/ (4259)
Meat Products/ or processed meat.mp. (7285)
Wine/ or red wine.mp. (11501)
13 or 14 or 15 or 16 (141175)
((low or little or medium or moderate or less or decrease* or reduc* or restrict*) adj6 (intake or consumption or consume or eat* or amount*)).tw. (177151)
17 and 18 (6736)
Mediterranean Diet.mp. or exp Diet, Mediterranean/ (4636)
exp Pregnancy/ or pregnan*.mp. (966432)
maternal diet.mp. (1885)
prenatal.mp. or Prenatal Nutritional Physiological Phenomena/ or Prenatal Exposure Delayed Effects/ (161569)
21 or 22 or 23 (994947)
child health.mp. or Child/ or Child Health/ (1617258)
newborn.mp. or exp Infant, Newborn/ (717150)
25 or 26 (2178391)
exp animals/ not humans.sh. (4543138)
randomized controlled trial.mp. or Randomized Controlled Trial/ (498499)
controlled clinical trial.mp. or Controlled Clinical Trial/ (105353)
placebo.de. (0)
trial.mp. (1103671)
Retrospective Studies/ or Cohort Studies/ or cohort stud*.mp. (994814)
29 or 30 or 31 or 32 or 33 (2049754)
34 not 28 (1998518)
((12 and 19) or 20) and 24 and 27 and 35 (22)
Database: Embase Classic+Embase <1947 to 2019 February 04>:
exp Fruit/ (127584)
fruit.mp. (110586)
vegetable*.mp. or leafy vegetable/ or vegetable/ (128062)
exp legume/ or legume.mp. (79855)
exp nut/ or nut*.mp. (670576)
extra virgin olive oil/ or olive oil/ or evoo.mp. (12921)
monounsaturated fatty acids.mp. or exp monounsaturated fatty acid/ (8992)
exp fish/ or oily fish.mp. or omega 3 fatty acid/ (240566)
sea food/ (9239)
tomato*.mp. or tomato/ (24952)
((high or more or increase* or elevat* or much or rais*) adj6 (intake or consumption or consume or eat* or amount*)).tw. (242744)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (1171930)
11 and 12 (49587)
exp dairy product/ (111325)
red meat/ or red meat.mp. (5486)
processed meat.mp. or exp processed meat/ (2238)
red wine.mp. or exp red wine/ (5583)
14 or 15 or 16 or 17 (122438)
((low or little or medium or moderate or less or decrease* or reduc* or restrict*) adj6 (intake or consumption or consume or eat* or amount*)).tw. (234104)
18 and 19 (7553)
Mediterranean Diet.mp. or exp Mediterranean diet/ (7990)
gestation*.mp. (314747)
maternal diet.mp. or maternal nutrition/ (11842)
pregnancy/ or pregnant woman/ or pregnant.mp. or pregnancy.mp. (1002075)
perinatal.mp. or perinatal period/ (133359)
prenatal period/ or prenatal exposure/ or prenatal.mp. (242919)
22 or 23 or 24 or 25 or 26 (1257012)
child health.mp. or child health/ (76176)
offspring.mp. or progeny/ (100561)
newborn.mp. or newborn/ (696375)
neonat*.mp. (351653)
child/ (1815590)
baby.mp. or exp baby/ (71511)
28 or 29 or 30 or 31 or 32 or 33 (2643576)
(exp animal/ or nonhuman/) not exp human/ (6800562)
clinical trial.de. (974978)
randomized controlled trial.de. (537435)
randomization.de. (81390)
single blind procedure.de. (33905)
double blind procedure.de. (160469)
crossover procedure.de. (58496)
placebo.de. (340801)
prospective study.de. (502412)
(randomi?ed controlled adj1 trial*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (744347)
rct.mp. (32969)
(random* adj1 allocat*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (37316)
(single adj1 blind*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (45468)
(double adj1 blind*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (245874)
((treble or triple) adj1 (blind* or placebo*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (1080)
36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 (2093989)
(exp animal/ or nonhuman/) not exp human/ (6800562)
((13 and 20) or 21) and 27 and 34 and 50 (26)
52 not 51 (26)
Database: Web of Science to February 04, 2019
(fruit*) (263,892)
(vegetable*) (112,459)
(legume*) (36,972)
(nut or nuts) (23,002)
(“olive oil*”) (20,491)
(evoo) (626)
(oily fish) (417)
(seafood or “sea food”) (11,311)
(tomato*) (71,792)
9 or 8 or 7 or 6 or 5 or 4 or 3 or 2 or 1 (461,691)
((high*or more or increase* or elevat* or raise*) Near/2 (intake* or consumption or cosume* or eat* or amount)) (263,946)
11 and 10 (21,325)
(dairy) (120,668)
(“red meat*”) (3894)
(“processed meat*”) (2493)
(“red wine*”) (12,350)
16 or 15 or 14 or 13 (138,033)
((low* or little or moderate or less or decrease* or reduc* or restric*) Near/2 (intake of consumption of consume or eat* or amount*)) (278,089)
18 and 17
19 and 12 (960)
(Mediterranean Near/2 diet*) (7.892)
21 or 20 (8715)
(pregnancy or pregnant) (448,186)
(gestation) (97,107)
(maternal near/2 nutrition*) (3839)
(maternal near/2 diet*) (4936)
(perinatal) (65,900)
27 or 26 or 25 or 24 or 23 (529,985)
(child* near/2 health*) (73,055)
(offspring) (84,383)
(baby or babies) (62,022)
(newborn*) (139,175)
(f?etus*) (6686)
33 or 32 or 31 or 30 or 29 (347,022)
34 and 28 (76,415)
35 and 22 (76)
Cochrane: <xx to February 04, 2019>:
TITLE-ABS-KEY (“Mediterranean Diet” AND (pregnan* OR prenatal OR perinatal OR pregnan* OR maternal) AND (offspring OR baby OR child AND health OR newborn))
Appendix B
Table A1.
The based on National Institutes of Health (NIH) study quality assessment tools for controlled trials 1.
| Criteria | Yes | No | Other |
|---|---|---|---|
| Cohort/cross-sectional studies | (CD, NR, NA) * | ||
| 1. Was the research question or objective in this paper clearly stated? | |||
| 2. Was the study population clearly specified and defined? | |||
| 3. Was the participation rate of eligible persons at least 50%? | |||
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | |||
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | |||
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | |||
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | |||
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | |||
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | |||
| 10. Was the exposure(s) assessed more than once over time? | |||
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | |||
| 12. Were the outcome assessors blinded to the exposure status of participants? | |||
| 13. Was loss to follow-up after baseline 20% or less? | |||
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | |||
| Quality Rating (Good, Fair, or Poor) | |||
| Rater #1 FA: | |||
| Rater #2 AS: | |||
| Additional Comments (If POOR, please state why): | |||
| Randomized Control Trials | |||
| 1. Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT? | |||
| 2. Was the method of randomization adequate (i.e., use of randomly generated assignment)? | |||
| 3. Was the treatment allocation concealed (so that assignments could not be predicted)? | |||
| 4. Were study participants and providers blinded to the treatment group assignment? | |||
| 5. Were the people assessing the outcomes blinded to the participants’ group assignments? | |||
| 6. Were the groups similar at baseline on important characteristics that could affect outcomes (e.g., demographics, risk factors, co-morbid conditions)? | |||
| 7. Was the overall drop-out rate from the study at endpoint 20% or lower of the number allocated to treatment? | |||
| 8. Was the differential drop-out rate (between treatment groups) at the endpoint 15 percentage points or lower? | |||
| 9. Was there high adherence to the intervention protocols for each treatment group? | |||
| 10. Were other interventions avoided or similar in the groups (e.g., similar background treatments)? | |||
| 11. Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants? | |||
| 12. Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power? | |||
| 13. Were outcomes reported or subgroups analyzed prespecified (i.e., identified before analyses were conducted)? | |||
| 14. Were all randomized participants analyzed in the group to which they were originally assigned, i.e., did they use an intention-to-treat analysis? | |||
| Quality Rating (Good, Fair, or Poor) | |||
| Rater #1 initials: | |||
| Rater #2 initials: | |||
| Additional Comments (If POOR, please state why): |
* CD, cannot determine; NA, not applicable; NR, not reported; 1 National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. NHLBI. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Appendix C
The studies excluded after full text screening:
Figure A1.
The papers excluded after full-text screening.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Reijnders I.F., Mulders A.G.M.G.J., van der Windt M., Steegers E.A.P., Steegers-Theunissen R.P.M. The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: A systematic review. Hum. Reprod. Update. 2019;25:72–94. doi: 10.1093/humupd/dmy037. [DOI] [PubMed] [Google Scholar]
- 2.Kampmann U., Madsen L.R., Skajaa G.O., Iversen D.S., Moeller N., Ovesen P. Gestational diabetes: A clinical update. World J. Diabetes. 2015;6:1065–1072. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kind K.L., Moore V.M., Davies M.J. Diet around conception and during pregnancy – effects on fetal and neonatal outcomes. Reprod. Biomed. Online. 2006;12:532–541. doi: 10.1016/S1472-6483(10)61178-9. [DOI] [PubMed] [Google Scholar]
- 4.Zerfu T.A., Pinto E., Baye K. Consumption of dairy, fruits and dark green leafy vegetables is associated with lower risk of adverse pregnancy outcomes (APO): A prospective cohort study in rural Ethiopia. Nutr. Diabetes. 2018;8:52. doi: 10.1038/s41387-018-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sewell D.A., Hammersley V.S., Devereux G., Robertson A., Stoddart A., Weir C., Worth A., Sheikh A. Investigating the effectiveness of the Mediterranean diet in pregnant women for the primary prevention of asthma and allergy in high-risk infants: Protocol for a pilot randomised controlled trial. Trials Electron. Resour. 2013;14:173. doi: 10.1186/1745-6215-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyedrezazadeh E., Moghaddam M.P., Ansarin K., Vafa M.R., Sharma S., Kolahdooz F. Fruit and vegetable intake and risk of wheezing and asthma: A systematic review and meta-analysis. Nutr. Rev. 2014;72:411–428. doi: 10.1111/nure.12121. [DOI] [PubMed] [Google Scholar]
- 7.Nurmatov U., Devereux G., Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011;127:724–733. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Venter C., Brown K.R., Maslin K., Palmer D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017;28:135–143. doi: 10.1111/pai.12682. [DOI] [PubMed] [Google Scholar]
- 9.Moody L., Chen H., Pan Y.-X. Early-Life Nutritional Programming of Cognition—The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process12. Adv. Nutr. 2017;8:337–350. doi: 10.3945/an.116.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estruch R., Martínez-González M.A., Corella D., Salas-Salvadó J., Ruiz-Gutiérrez V., Covas M.I., Fiol M., Gómez-Gracia E., López-Sabater M.C., Vinyoles E., et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Int. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs D.R., Gross M.D., Tapsell L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009;89:1543S–1548S. doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet | Diabetes Care. [(accessed on 6 March 2019)]; Available online: http://care.diabetesjournals.org/content/34/1/14.short.
- 13.Widmer R.J., Flammer A.J., Lerman L.O., Lerman A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015;128:229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer M., Stronati M., Lanari M. Mediterranean diet, folic acid, and neural tube defects. J. Pediatr. 2017;43:74. doi: 10.1186/s13052-017-0391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dessypris N., Karalexi M.A., Ntouvelis E., Diamantaras A.-A., Papadakis V., Baka M., Polychronopoulou S., Sidi V., Stiakaki E., Petridou E.T. Association of maternal and index child’s diet with subsequent leukemia risk: A systematic review and meta analysis. Cancer Epidemiol. 2017;47:64–75. doi: 10.1016/j.canep.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Sofi F., Abbate R., Gensini G.F., Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 17.Netting M.J., Middleton P.F., Makrides M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition. 2014;30:1225–1241. doi: 10.1016/j.nut.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Grieger J.A., Clifton V.L. A Review of the Impact of Dietary Intakes in Human Pregnancy on Infant Birthweight. Nutrients. 2014;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Zhao D., Mao X., Xia Y., Baker P.N., Zhang H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients. 2016;8:351. doi: 10.3390/nu8060351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees K., Hartley L., Flowers N., Clarke A., Hooper L., Thorogood M., Stranges S. “Mediterranean” dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013;12:CD009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute (NHLBI) Study Quality Assessment Tools. [(accessed on 23 March 2019)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 23.Assaf-Balut C., Garcia de la Torre N., Durán A., Fuentes M., Bordiú E., del Valle L., Valerio J., Familiar C., Jiménez I., Herraiz M.A., et al. Medical nutrition therapy for gestational diabetes mellitus based on Mediterranean Diet principles: A subanalysis of the St Carlos GDM Prevention Study. BMJ Open Diabetes Res. Care. 2018;6:e000550. doi: 10.1136/bmjdrc-2018-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assaf-Balut C., Garcia de la Torre N., Duran A., Fuentes M., Bordiu E., Del Valle L., Familiar C., Ortolá A., Jiménez I., Herraiz M.A., et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE. 2017;12:e0185873. doi: 10.1371/journal.pone.0185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botto L.D., Krikov S., Carmichael S.L., Munger R.G., Shaw G.M., Feldkamp M.L. Lower rate of selected congenital heart defects with better maternal diet quality: A population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2016;101:43–49. doi: 10.1136/archdischild-2014-308013. [DOI] [PubMed] [Google Scholar]
- 26.Chatzi L., Rifas-Shiman S.L., Georgiou V., Joung K.E., Koinaki S., Chalkiadaki G., Margioris A., Sarri K., Vassilaki M., Vafeiadi M., et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017;12:47–56. doi: 10.1111/ijpo.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzi L., Torrent M., Romieu I., Garcia-Esteban R., Ferrer C., Vioque J., Kogevinas M., Sunyer J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507–513. doi: 10.1136/thx.2007.081745. [DOI] [PubMed] [Google Scholar]
- 28.Chatzi L., Kogevinas M. Prenatal and childhood Mediterranean diet and the development of asthma and allergies in children. Public Health Nutr. 2009;12:1629–1634. doi: 10.1017/S1368980009990474. [DOI] [PubMed] [Google Scholar]
- 29.Parlapani E., Agakidis C., Karagiozoglou-Lampoudi T., Sarafidis K., Agakidou E., Athanasiadis A., Diamanti E. The Mediterranean diet adherence by pregnant women delivering prematurely: Association with size at birth and complications of prematurity. J. Mater. Fetal Neonatal Med. 2017;13:1–8. doi: 10.1080/14767058.2017.1399120. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Barres S., Romaguera D., Valvi D., Martinez D., Vioque J., Navarrete-Munoz E.M., Amiano P., Gonzalez-Palacios S., Guxens M., Pereda E., et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: The INMA birth cohort study. Pediatr. Obes. 2016;11:491–499. doi: 10.1111/ijpo.12092. [DOI] [PubMed] [Google Scholar]
- 31.Gesteiro E., Rodriguez Bernal B., Bastida S., Sanchez-Muniz F.J. Maternal diets with low healthy eating index or Mediterranean diet adherence scores are associated with high cord-blood insulin levels and insulin resistance markers at birth. J. Clin. Nutr. 2012;66:1008–1015. doi: 10.1038/ejcn.2012.92. [DOI] [PubMed] [Google Scholar]
- 32.Gesteiro E., Sanchez-Muniz F.J., Ortega-Azorin C., Guillen M., Corella D., Bastida S. Maternal and neonatal FTO rs9939609 polymorphism affect insulin sensitivity markers and lipoprotein profile at birth in appropriate-for-gestational-age term neonates. J. Physiol. 2016;72:169–181. doi: 10.1007/s13105-016-0467-7. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Nahm S., Mendez M., Robinson W., Murphy S.K., Hoyo C., Hogan V., Diane R. Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ. Epigenetics. 2017;3 doi: 10.1093/eep/dvx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haugen M., Meltzer H.M., Brantsaeter A.L., Mikkelsen T., Osterdal M.L., Alexander J., Olsen S.F., Bakketeig L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): A prospective cohort study. Acta Obstet. Gynecol. Scand. 2008;87:319–324. doi: 10.1080/00016340801899123. [DOI] [PubMed] [Google Scholar]
- 35.House J.S., Mendez M., Maguire R.L., Gonzalez-Nahm S., Huang Z., Daniels J., Susan KMurphy B., Fuemmeler F.A., Wright C.H. Periconceptional Maternal Mediterranean Diet Is Associated with Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front. Cell Dev. Biol. 2018;6 doi: 10.3389/fcell.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro-Rodriguez J.A., Ramirez-Hernandez M., Padilla O., Pacheco-Gonzalez R.M., Pérez-Fernández V., Garcia-Marcos L. Effect of foods and Mediterranean diet during pregnancy and first years of life on wheezing, rhinitis and dermatitis in preschoolers. Allergol. Immunopathol. (Madr) 2016;44:400–409. doi: 10.1016/j.aller.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Lange N.E., Rifas-Shiman S.L., Camargo C.A., Gold D.R., Gillman M.W., Litonjua A.A. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J. Allergy Clin. Immunol. 2010;126:250–255. doi: 10.1016/j.jaci.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantzoros C.S., Sweeney L., Williams C.J., Oken E., Kelesidis T., Rifas-Shiman S.L., Gillman M.W. Maternal diet and cord blood leptin and adiponectin concentrations at birth. Clin. Nutr. 2010;29:622–626. doi: 10.1016/j.clnu.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monteagudo C., Mariscal-Arcas M., Heras-Gonzalez L., Ibanez-Peinado D., Rivas A., Olea-Serrano F. Effects of maternal diet and environmental exposure to organochlorine pesticides on newborn weight in Southern Spain. Chemosphere. 2016;1:135–142. doi: 10.1016/j.chemosphere.2016.04.103. [DOI] [PubMed] [Google Scholar]
- 40.Peraita-Costa I., Llopis-Gonzalez A., Perales-Marin A., Sanz F., Llopis-Morales A., Morales-Suarez-Varela M. A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn. J. Environ. Res. 2018;15:1530. doi: 10.3390/ijerph15071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders L., Guldner L., Costet N., Kadhel P., Rouget F., Monfort C., Thomé J.P., Multigner L. Cordier S.Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: Results from a French Caribbean Mother-Child Cohort Study (TIMOUN) Paediatr. Perinat. Epidemiol. 2014;28:235–244. doi: 10.1111/ppe.12113. [DOI] [PubMed] [Google Scholar]
- 42.Steenweg-de Graaff J., Tiemeier H., Steegers-Theunissen R.P.M., Hofman A., Jaddoe V.W.V., Verhulst F.C., Roza S.J. Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clin. Nutr. 2014;33:115–121. doi: 10.1016/j.clnu.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Vujkovic M., Steegers E.A., Looman C.W., Ocké M.C., Spek P., van der Steegers-Theunissen R.P. The maternal Mediterranean dietary pattern is associated with a reduced risk of spina bifida in the offspring. BJOG Int. J. Obstet. Gynaecol. 2009;116:408–415. doi: 10.1111/j.1471-0528.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 44.Smith L.K., Draper E.S., Evans T.A., Field D.J., Johnson S.J., Manktelow B.N., Seaton S.E., Marlow N., Petrou S., Boyle E.M. Associations between late and moderately preterm birth and smoking, alcohol, drug use and diet: A population-based case-cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2015;100:F486–F491. doi: 10.1136/archdischild-2014-307265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariscal-Arcas M., Rivas A., Monteagudo C., Granada A., Cerrillo I., Olea-Serrano F. Proposal of a Mediterranean diet index for pregnant women. J. Nutr. 2009;102:744–749. doi: 10.1017/S0007114509274769. [DOI] [PubMed] [Google Scholar]
- 46.Berti C., Agostoni C., Davanzo R., Hyppönen E., Isolauri E., Meltzer H.M., Steegers-Theunissen R.P., Cetin I. Early-life nutritional exposures and lifelong health: Immediate and long-lasting impacts of probiotics, vitamin D, and breastfeeding. Nutr. Rev. 2017;75:83–97. doi: 10.1093/nutrit/nuw056. [DOI] [PubMed] [Google Scholar]
- 47.Beckhaus A.A., Garcia-Marcos L., Forno E., Pacheco-Gonzalez R.M., Celedón J.C., Castro-Rodriguez J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy. 2015;70:1588–1604. doi: 10.1111/all.12729. [DOI] [PubMed] [Google Scholar]
- 48.Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., Slagboom P.E., Lumey L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]