Abstract
Objective
To determine the clinical course of hepatitis C virus in the first decade of infection in a group of patients who acquired their infections on a known date.
Design
Cohort study.
Setting
Clinical centres throughout the United Kingdom.
Participants
924 transfusion recipients infected with the hepatitis C virus (HCV) traced during the HCV lookback programme and 475 transfusion recipients who tested negative for antibodies to HCV (controls).
Main outcome measures
Clinical evidence of liver disease and survival after 10 years of infection.
Results
All cause mortality was not significantly different between patients and controls (Cox's hazards ratio 1.41, 95% confidence interval 0.95 to 2.08). Patients were more likely to be certified with a death related to liver disease than were controls (12.84, 1.73 to 95.44), but although the risk of death directly from liver disease was higher in patients than controls this difference was not significant (5.78, 0.72 to 46.70). Forty per cent of the patients who died directly from liver disease were known to have consumed excess alcohol. Clinical follow up of 826 patients showed that liver function was abnormal in 307 (37.2%), and 115 (13.9%) reported physical signs or symptoms of liver disease. Factors associated with developing liver disease were testing positive for HCV ribonucleic acid (odds ratio 6.44, 2.67 to 15.48), having acquired infection when older (at age ⩾ 40 years; 1.80, 1.14 to 2.85), and years since transfusion (odds ratio 1.096 per year, 1.00 to 1.20). For patients with severe disease, sex was also significant (odds ratio for women 0.38, 0.17 to 0.88). Of the 362 patients who had undergone liver biopsy, 328 (91%) had abnormal histological results and 35 (10%) of these were cirrhotic.
Conclusions
Hepatitis C virus infection did not have a great impact on all cause mortality in the first decade of infection. Infected patients were at increased risk of dying directly from liver disease, particularly if they consumed excess alcohol, but this difference was not statistically significant.
What is already known on this topic
The clinical course of HCV infection is unclear because most information has come from studies of patients with established chronic liver disease
Studies that follow patients from disease onset are rare because most HCV infections are asymptomatic
What this study adds
HCV infection does not have a great impact on all cause mortality in the first decade of infection
Infected patients have an increased risk of dying from a liver related cause, particularly if they consumed excess alcohol
Introduction
Hepatitis C virus (HCV) is a common cause of liver disease1 and a major health problem worldwide.2 Acute infection is rarely diagnosed, and information about the clinical course of HCV infection has come largely from retrospective studies of patients with established liver disease.3 Such studies exclude people with no clinical evidence of infection, and observations are often biased towards severe disease outcomes.
Opportunities for prospective studies of HCV related disease are rare, and the best known examples include cohorts of women exposed to contaminated immunoglobulin.4,5 These studies suggest that HCV related liver disease is relatively mild,4 but caution is needed because the studies included women who were young when they acquired their infections. Female sex and young age are independently associated with a favourable outcome.6 Such studies may underestimate the impact of HCV related liver disease in the wider population. Retrospective studies attempted to determine the progression of the disease from an estimated date of acquisition. The date of acquisition was based on self reported dates from patients who were injecting drugs at the time or from their first recorded exposure to blood products of high risk.6–8 The accuracy of these dates, however, has been questioned.9–11 Consequently, the rate of development of chronic liver disease and hepatocellular carcinoma is poorly understood.
In early 1995, the UK Department of Health announced that they would undertake a “lookback” at people who had received blood from donors subsequently found to be infected with the virus when transfusion took place before the introduction of testing of the blood supply for antibodies to HCV.12 Recipients were identified from hospital records, traced, and offered counselling, serological testing, and treatment for HCV infection. This process identified a large group of HCV infections with known dates of acquisition, an identifiable source, and often no observed HCV disease progression. Our study describes the HCV related disease seen after 10 years of infection and compares the mortality from liver disease in this cohort with mortality in a group of transfusion recipients who tested negative for antibodies to HCV.
Methods
Patients
At the end of 1999, 996 transfusion recipients infected with HCV had been traced during the lookback.13 For most patients, transfusion was the only probable route of infection, but 18 were excluded because exposure to other possible causes meant that the date they acquired the virus was uncertain (nine had injected drugs and nine had been exposed to blood products). Seventeen recipients were excluded either because they could not be flagged within the NHS central registers (11 patients), because the recipients were transfused after the testing of the blood supply for antibodies to HCV was introduced (three patients), or because their dates of counselling were not clear (three patients). A further 37 recipients were excluded because full confirmatory testing revealed that they were not infected with the virus or because initial reactivity to antibodies to HCV was not confirmed. Of the remaining 924 eligible patients, 608 (65.8%) were known to be positive for HCV ribonucleic acid and 189 (20.5%) negative for ribonucleic acid; for 127 (13.7%) the status was unknown.
Controls
To provide a source of data on transfusion recipients who were HCV negative, all 536 recipients from the HCV lookback exercise in England who were traced and counselled and who tested negative for anti-HCV were identified.13 Four of the recipients were excluded because their records could not be flagged within the NHS central registers, and 57 because their dates of transfusion were unclear or because their transfusion took place after the introduction of anti-HCV testing of donated blood. Of the 475 controls, 443 (93%) were confirmed to be HCV ribonucleic acid negative; the ribonucleic acid status of 32 (7%) was not known.
Sources of data
Data were collected from patients and controls at the time of initial counselling during the HCV lookback and from death registration forms. Additional data on patients was obtained at entry into the HCV national register. The methods of data collection and for maximising its quality have been described elsewhere.13
To compare all cause mortality and liver related mortality in patients and controls, we reviewed the text of the death certificates; deaths in which HCV related liver disease was likely to have been a direct cause of death were also identified. For this we included certificates that mentioned hepatocellular carcinoma or end stage liver disease (varices, ascites, or hepatic encephalopathy) or where liver disease was coded as the underlying and only cause of death. Death certificates for which liver disease or hepatitis C were given as contributory factors were not included in this analysis because decisions regarding cause of death may have been influenced by knowledge of the patient's HCV status.
Data on clinical features of liver disease were available for registered patients. Features were classified as severe where there were signs or symptoms of a liver tumour or of portal hypertension; other signs or symptoms of liver disease were classified as mild.
Statistical analysis
Differences in baseline data between patients and controls at counselling were assessed by using t tests for means or χ2 tests for proportions. To test for differences in survival between eligible patients and controls, we used Cox's proportional hazards survival analysis, with survival taken from the date of counselling to death with censoring at the end of 1999. Multivariable modelling allowed adjustment for differences between patients and controls according to factors such as alcohol consumption and tests for interactions between HCV status and other factors that affect survival, such as age and sex. Risk factors for signs and symptoms of liver disease, within patients, were investigated by using logistic regression.
Results
All 924 eligible patients and 475 eligible controls were counselled between January 1995 and March 1999. At the end of 1999, the mean time since transfusion was 11.1 years (range 8.2-20.6 years) for patients and 11.8 years (8.3-25.0 years) for controls (table 1). The eligible patients did not differ significantly from the controls by age (P=0.50) or sex (P=0.72). Ethnic group and country of birth were more often unknown for controls than for patients (P<0.001). Patients were more likely to report drinking ⩾20 units of alcohol/week14 or to have unknown alcohol consumption at baseline than were controls (P<0.001). They were also more likely to have evidence of exposure to hepatitis B virus (tested positive for antibodies to hepatitis B virus core protein; P=0.001). On average the patients were counselled six weeks earlier than the controls and have therefore been followed up for slightly longer.
Table 1.
Baseline characteristics of patients infected with hepatitis C virus and of controls who tested negative for antibodies to hepatitis C virus. Results are numbers (percentages) unless otherwise stated
| Characteristic | Patients (n=924) | Controls (n=475) |
|---|---|---|
| Mean (range) years since exposure by end of 1999* | 11.1 (8.2-20.6) | 11.8 (8.3-25.0) |
| Mean (range) age at exposure in years | 43.6 (0.0-87.2) | 41.5 (0.0-84.5) |
| Sex: | ||
| Male | 445 (48.2) | 224 (47.2) |
| Female | 479 (51.8) | 251 (52.8) |
| Ethnic group: | ||
| White | 783 (84.7) | 325 (68.4) |
| Non-white | 54 (5.8) | 37 (7.8) |
| Not known | 87 (9.4) | 113 (23.8) |
| Country of birth: | ||
| United Kingdom | 740 (80.1) | 333 (70.1) |
| Not United Kingdom | 68 (7.4) | 35 (7.4) |
| Not known | 116 (12.6) | 107 (22.5) |
| Alcohol consumption reported at counselling or first diagnosis: | ||
| Nil | 274 (29.7) | 249 (52.4) |
| Less than 20 units/week | 421 (45.6) | 181 (38.1) |
| At least 20 units/week or alcoholism reported | 125 (13.5) | 29 (6.1) |
| Not known | 104 (11.3) | 16 (3.4) |
| Hepatitis B status at counselling or first diagnosis: | ||
| Chronic infection† | 2 (0.2) | 0 (0.0) |
| Resolved infection‡ | 20 (2.2) | 0 (0.0) |
| No evidence of current infection§ | 706 (76.4) | 390 (82.1) |
| Evidence of past infection, but current status unknown¶ | 24 (2.6) | 8 (1.7) |
| Not known | 172 (18.6) | 77 (16.2) |
Excluding those who had died before the end of 1999.
Positive for hepatitis B surface antigen (HBsAg).
Negative for hepatitis B surface antigen but positive for hepatitis B core protein (HBc) antibody.
Negative for HBsAg or negative for HBc antibody.
HBsAg unknown, but positive for HBc antibody.
Deaths
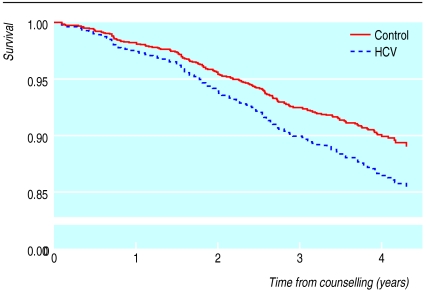
By the end of 1999, 117 of 924 eligible patients (12.7%) had died, including 112/826 (13.6%) of the patients registered with the national HCV register. Of the 117 patients who died, 29 (25%) had one or more liver related conditions mentioned on their death certificate: hepatocellular carcinoma (n=3), liver encephalopathy (n=1), ascites (n=1), hepatic failure (n=5), cirrhosis (n=11), chronic hepatitis or hepatitis C (n=20). Of these 29, only 10 were considered to have died directly from liver disease and they had been infected for a mean of 11 years (SE 0.77, range 8.42-15.88). Nine were known to be negative for hepatitis B surface antigen (including one who tested positive for antibodies to hepatitis B core protein); hepatitis B markers were unknown for one. Two of the 10 had alcohol mentioned on their death certificate and a further two had reported excessive alcohol consumption at counselling. Of the controls, 43/475 (9%) had died by the end of 1999 (figure), and one (2%) had a liver related condition mentioned on the death certificate. This person died from a hepatocellular carcinoma, was known to be negative for HCV ribonucleic acid and antibodies to hepatitis B virus core protein, and was reported at counselling to consume no alcohol.
The survival analysis with all cause mortality showed that the difference between patients and controls was significant only at the 10% level, with a hazards ratio of 1.41 (95% confidence interval 0.95 to 2.08, P=0.08). Factors that significantly worsened survival were older age, being male, and the level of alcohol consumption (P=0.003). Compared with drinkers of 1-20 units, survival was worse for patients with unknown consumption and zero consumption of alcohol (2.71, 1.58 to 4.64 and 1.76, 1.18 to 2.62, respectively) and for patients consuming ⩾20 units/week (1.28, 0.75 to 2.18). The relation between survival and age, sex, or alcohol use did not differ between patients and controls. A significant difference in survival to death certified as liver related (30 deaths) was observed between patients and controls (12.84, 1.73 to 95.44, P=0.013). Comparison of survival to a death directly from liver disease (11 deaths) showed a large excess in patients but this excess was not significant (5.78, 0.72 to 46.70, P=0.10).
Liver function and disease
Data on the clinical features of and laboratory investigation for liver disease were available for 826 (89.4%) of the eligible patients registered between February 1998 and the end of 1999. Patients who were not registered did not differ from patients who were, with respect to age at and time since transfusion, sex, alcohol consumption, and markers of exposure to hepatitis B.
Liver function, as assessed by the serum concentrations of liver transaminases (alanine aminotransferase or aspartate aminotransferase), was known to be abnormal in 37.2% (307/826) of registered cases (table 2). Liver biopsy had been performed on 362 patients and 328 (90.6%) patients had abnormal results (table 2).
Table 2.
Clinical characteristics at the time of registration of 826 patients with hepatitis C virus
| Characteristic | No (%) of patients |
|---|---|
| Concentration of serum liver transaminases: | |
| Substantially raised* | 120 (14.5) |
| Mildly raised† | 182 (22.0) |
| Raised‡ | 5 (0.6) |
| Within normal range | 451 (54.6) |
| Not known | 68 (8.2) |
| Physical signs or symptoms of liver disease: | |
| Severe liver disease | 34 (4.1) |
| Liver tumour | 1 (0.1) |
| Varices | 9 (1.1) |
| Ascites | 7 (0.8) |
| Splenomegaly | 17 (2.1) |
| Mild liver disease | 81 (9.8) |
| No physical signs or symptoms | 640 (77.5) |
| Not known | 71 (8.6) |
| Results of liver biopsy§: | |
| Biopsy taken | 362 (43.8) |
| Cirrhosis | 35 (9.7) |
| Chronic hepatitis | 270 (74.6) |
| Minimal change | 23 (6.4) |
| Normal | 26 (7.2) |
| Not known | 8 (2.2) |
| Biopsy not performed | 408 (49.4) |
| Not known if biopsied | 56 (6.8) |
Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >twice the upper limit of normal.
ALT or AST one to two times the upper limit of normal.
ALT or AST reported as abnormal, but degree unknown.
Percentages are of patients who had a biopsy.
Some physical signs or symptoms of liver disease were reported for 115 (13.9%) of the registered patients (table 2). Twenty one (18%) patients with features of liver disease had other reported factors that may have contributed to the severity of the disease (11 alcohol, 4 iron overload, 2 cryptogenic cirrhosis, 2 congenital hepatic fibrosis, 1 β-thalassaemia major, and 1 drug induced liver abscess). Single variable logistic regression was used to see which factors may have been involved in the disease (mild or severe). Factors that showed some evidence of an association (P<0.2) were then included in a multivariable model (table 3). The factors dropped (P>0.2) were ethnicity, country of birth, hepatitis B status, and the presence of another chronic viral infection. In the multivariable logistic regression model, testing positive for HCV ribonucleic acid, age at transfusion, and a longer time since transfusion were associated with liver disease. When age at transfusion was categorised as <40 and ⩾40 years, the odds ratio for transfusion when older was 1.80 (1.14 to 2.85). Sex, smoking, and alcohol were not significantly associated with disease, but the direction of the effect was as in other studies.6 The analysis was repeated comparing severe disease with no disease or mild disease. Testing positive for HCV ribonucleic acid and being male were significant (table 3). Age at transfusion and time since transfusion showed similar effects as in the analysis of any disease, but differences were not significant.
Table 3.
Multivariable logistic regression analyses for signs and symptoms of liver disease in patients infected with hepatitis C virus, n=755. Values are odds ratio (95% confidence intervals)
| Factor/levels | Mild or severe infection v no infection
|
Severe infection v no or mild infection
|
|||
|---|---|---|---|---|---|
| Odds ratio | P value | Odds ratio | P value | ||
| Sex: | |||||
| Male | 1 | 0.11 | 1 | 0.019 | |
| Female | 0.69 (0.44 to 1.10) | 0.38 (0.17 to 0.88) | |||
| Alcohol consumed (units/week): | |||||
| <20 | 1 | 0.13 | 1 | 0.14 | |
| None | 1.20 (0.70 to 2.06) | 1.97 (0.77 to 5.07) | |||
| ⩾20 | 1.97 (1.10 to 3.51) | 2.84 (1.09 to 7.41) | |||
| Unknown | 0.93 (0.37 to 2.36) | 1.19 (0.23 to 6.16) | |||
| Age at transfusion (years): | |||||
| 0-19 | 1 | 0.028 | 1 | 0.24 | |
| 20-39 | 1.15 (0.53 to 2.51) | 2.19 (0.50 to 9.63) | |||
| 40-49 | 1.96 (0.86 to 4.53) | 4.48 (1.00 to 20.08) | |||
| 50-59 | 2.76 (1.27 to 6.03) | 3.52 (0.79 to 15.42) | |||
| ⩾60 | 1.38 (0.65 to 2.95) | 3.22 (0.79 to 13.06) | |||
| Time since transfusion | |||||
| Years | 1.096 (1.00 to 1.20) | 0.045 | 1.098 (0.95 to 1.26) | 0.22 | |
| HCV ribonucleic acid status: | |||||
| No | 1 | <0.001 | 1 | 0.008 | |
| Yes | 6.44 (2.67 to 15.48) | 4.18 (0.94 to 18.44) | |||
| Unknown | 2.06 (0.68 to 6.18) | 0.94 (0.12 to 6.95) | |||
| Smoker: | |||||
| No | 1 | 0.35 | 1 | 0.43 | |
| Past | 1.31 (0.72 to 2.39) | 0.52 (0.17 to 1.58) | |||
| Current | 1.78 (0.92 to 3.44) | 0.69 (0.20 to 2.34) | |||
| Unknown | 1.33 (0.75 to 2.35) | 1.28 (0.53 to 3.10) | |||
Discussion
Survival
During the first decade of infection, all cause mortality among transfusion recipients with positive or indeterminate results on antibody tests for hepatitis C virus was 1.4 times greater than that observed in a similarly traced group of transfusion recipients negative for HCV. Although the excess mortality failed to reach statistical significance, the survival curves for the two groups were diverging, suggesting that differential mortality may increase in the future. The risk of dying directly from liver disease was almost six times higher for people infected with HCV than for negative controls, but this difference was not significant. Excessive alcohol consumption was implicated in 40% of the deaths from liver disease among patients.
Liver function and disease
The majority of infected patients had no signs or symptoms of liver disease, but nearly 40% had abnormal liver function and 91% of patients biopsied had abnormal liver histology. Patients who had developed physical signs or symptoms of liver disease were more likely to have been infected for longer, to be positive for HCV ribonucleic acid, and to have acquired their infections at an older age. Patients with clinical features of severe liver disease were also more likely to be male.
Study limitations
Our study has provided data on a group of patients infected with HCV for which the time since infection is accurately known. The median time from acquisition of the virus to cirrhosis has been estimated to be 30 years,6 and so the morbidity described here occurred after a relatively short observation period. Loss to follow up has been minimised by flagging all patients and controls in the NHS central registers; mortality data is complete. The use of death certification to establish cause of death is a potential information bias.7,15 Knowledge of HCV status is likely to influence content of the death certificate and this may partly explain the excess risk of liver related deaths among patients. By examining the text of the death certificates, however, we restricted one of our analyses to conditions likely to be clinically apparent at the time of death.
The cohort was limited to transfusion recipients who were traceable and had survived long enough to be tested in the national lookback.14 To reduce confounding, deaths have been compared with those in negative recipients also recruited by this mechanism.13 Analysis of the lookback shows that the HCV ribonucleic acid status of the source was the biggest influence on infection status and that recipient factors, like age or sex, did not differ between recipients who tested negative and recipients who tested positive for the virus.14 Information is also available on important confounding factors among the controls, including alcohol consumption and hepatitis B markers.
Comparison with other studies
Many of the reported differences in the clinical course of the virus reflect the stage at which patients were recruited and the length of time under observation. Studies that recruit patients with acute infections after transfusion16 include patients whose infections resolve spontaneously and patients who die from other causes before developing HCV related liver disease. These same patients are usually excluded from studies that recruit patients from tertiary referral centres.3 Overall, patients who have been infected for longer tend to be more sick than patients who have been infected more recently. Some patients, however, progress rapidly to end stage liver disease, whereas others remain unaffected. This is likely to be due to individual effects (like genetic differences), as well as other risk factors such as sex, age, and alcohol intake. Male sex is independently associated with an increased risk of progressive disease,6 and this might explain the relatively low rate of disease seen in female cohorts.4,5 Acquisition of disease over 40 years of age is also associated with increased progression of fibrosis in paired liver biopsies,6 and mathematical models estimate annual progression rates to be 300 times greater for men aged 61-70 than for men aged 21-40.17 Although fibrosis in men infected at younger ages initially progresses less rapidly than in older men, it may progress more rapidly as they age.18 The baseline prevalence of risk factors, such as excess alcohol use, may also explain the differential rates of progression observed in different cohorts. An excess of deaths from liver disease was seen in only two of the five cohorts studied by Seeff et al,16 and these were the only two cohorts not to have excluded patients with alcoholic liver disease.
Transfusion is a recognised risk factor for HCV transmission, but transfusion recipients make up a small proportion of people with known HCV infections in the United Kingdom.19 As the reason for the transfusion may be associated with reduced life expectancy, our study may have diluted the impact of the virus on morbidity and mortality.13 Most people with the virus in the United Kingdom have acquired infection by injecting drugs,19 and they may also have a shortened life expectancy due to factors other than HCV infection.20 The clinical course of the virus may depend on the route of infection, being less severe in patients infected by injecting drugs than by transfusion.21,22 A cross sectional study in the United Kingdom, however, showed no evidence of a difference in the extent of liver fibrosis between these two groups.23 Patients with a history of injecting may be infected with different HCV genotypes23 and are likely to differ in other important ways (such as age at infection and alcohol use).
Conclusions
Our study supports the view that HCV infection does not have a great impact on all cause mortality in the first decade of infection.4,16,24,25 Like other studies,6,26,27 our results show that the influence of alcohol is independent of other factors and is exerted only at high levels of intake. If patients can keep their alcohol consumption to a minimum, their prognosis in the first decade of infection is likely to be improved. Continued observation of this cohort will determine the outcome of HCV infection in the longer term and enable us to evaluate the impact of antiviral treatment.
Figure.
Survival (Cox's proportional hazards model) among HCV infected patients and controls
Acknowledgments
The Hepatitis C Virus National Register Steering Group: Graeme Alexander (consultant hepatologist, Addenbrooke's Hospital, Cambridge), Brian Gunson (lay representative, Patients' Association, Harrow), Helen Harris (research associate, PHLS CDSC, London), Julia Heptonstall (consultant virologist, London), Virge James (consultant haematologist, National Blood Service, Sheffield), Giorgina Mieli-Vergani (consultant paediatric hepatologist, King's College Hospital, London), Hugh Nicholas (senior medical officer, UK department of health, London), Bernard Portmann (consultant histopathologist, King's College Hospital, London), Mary Ramsay (consultant epidemiologist, PHLS CDSC, London), and Angela Robinson (medical director, National Blood Authority, Watford).
We thank all of the blood centres throughout the United Kingdom for their help in collating the initial HCV lookback data. We are grateful to all the clinicians and research nurses who have supported this national project by enrolling their patients. We thank Kate Soldan for her comments on the manuscript.
Footnotes
Funding: This research was funded by the Department of Health; all views and any errors are the responsibility of the authors alone.
Competing interests: None declared.
References
- 1.Seeff LB. The natural history of hepatitis C—a quandary. Hepatology. 1998;28:1710–1712. doi: 10.1002/hep.510280636. [DOI] [PubMed] [Google Scholar]
- 2.Hepatitis C—global prevalence (update) Wkly Epidemiol Rec. 1999;74:425–427. [PubMed] [Google Scholar]
- 3.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 4.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 5.Dittmann S, Roggendorf M, Durkop J, Wiese M, Lorbeer B, Deinhardt F. Long-term persistence of hepatitis C virus antibodies in a single source outbreak. J Hepatol. 1991;13:323–327. doi: 10.1016/0168-8278(91)90076-n. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 7.Darby SC, Ewart DW, Giangrande PL, Spooner RJ, Rizza CR, Dusheiko GM, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors' Organisation. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 9.Behrman AJ. Long-term prognosis of hepatitis C virus infection. JAMA. 2000;284:2592. doi: 10.1001/jama.284.20.2592. [DOI] [PubMed] [Google Scholar]
- 10.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–263. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 11.Hope VD, Judd A, Hickman M, Lamagni T, Hunter G, Stimson GV, et al. Prevalence of hepatitis C virus in current injecting drug users in England and Wales: is harm reduction working? Am J Public Health. 2001;91:38–42. doi: 10.2105/ajph.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health. Hepatitis C and blood transfusion lookback. London: HMSO; 1995. . (PL CMO(95)1.) [Google Scholar]
- 13.Harris HE, Ramsay ME, Heptonstall J, Soldan K, Eldridge KP. The HCV national register: towards informing the natural history of hepatitis C infection in the UK. J Viral Hepat. 2000;7:420–427. doi: 10.1046/j.1365-2893.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 14.English national blood service HCV lookback collation collaborators. Transfusion transmission of HCV infection prior to anti-HCV testing of blood donations in England: results of the national HCV lookback programme. Transfusion (in press). [DOI] [PubMed]
- 15.Percy C, Ries LG, Van Holten VD. The accuracy of liver cancer as the underlying cause of death on death certificates. Public Health Rep. 1990;105:361–367. [PMC free article] [PubMed] [Google Scholar]
- 16.Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung and Blood Institute Study Group. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 17.Deuffic S, Buffat L, Poynard T, Valleron AJ. Modeling the hepatitis C virus epidemic in France. Hepatology. 1999;29:1596–1601. doi: 10.1002/hep.510290528. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay ME, Balogun MA, Collins M, Balraj V. Laboratory surveillance of hepatitis C virus infection in England and Wales: 1992 to 1996. Commun Dis Public Health. 1998;1:89–94. [PubMed] [Google Scholar]
- 20.Ghodse H, Oyefeso A, Kilpatrick B. Mortality of drug addicts in the United Kingdom 1967-1993. Int J Epidemiol. 1998;27:473–478. doi: 10.1093/ije/27.3.473. [DOI] [PubMed] [Google Scholar]
- 21.Gordon SC, Elloway RS, Long JC, Dmuchowski CF. The pathology of hepatitis C as a function of mode of transmission: blood transfusion vs. injecting drug use. Hepatology. 1993;18:1338–1343. [PubMed] [Google Scholar]
- 22.Lopez-Morante A, Saez-Royuela F, Echevarria C, Llanos C, Martin-Lorente JL, Yuguero L, et al. Influence of the transmission route and disease duration in the histopathology of chronic hepatitis C: a study of 101 patients. Eur J Gastroenterol Hepatol. 1998;10:15–19. doi: 10.1097/00042737-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Mohsen AH Trent HCV Study Group. The epidemiology of hepatitis C in a UK health regional population of 5.12 million. Gut. 2001;48:707–713. doi: 10.1136/gut.48.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeff LB, Miller RN, Rabkin CS, Buskell-Bales Z, Straley-Eason KD, Smoak BL, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 25.Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31:1014–1018. doi: 10.1053/he.2000.5762. [DOI] [PubMed] [Google Scholar]
- 26.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 27.Wiley TE, McCarthy M, Breidi L, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]