Abstract
Background:
Poor nutritional status is associated with osteoporosis. Prealbumin is a more sensitive marker than albumin to assess nutritional status. Therefore, the relationship between serum levels of prealbumin and osteoporosis in older adults with type 2 diabetes mellitus (T2DM) was investigated.
Methods:
A total of 370 older adults with T2DM were divided into two groups: older adults with osteoporosis (n = 249) and older adults without osteoporosis (n = 121). Bone mineral density (BMD) and appendicular skeletal muscle (ASM) were measured by dual-energy X-ray absorptiometry (DEXA). Serum levels of prealbumin, highly sensitive C-reactive protein (hs-CRP), interleukin-6 (IL-6), 25-hydroxyvitamin D3 [25(OH) D3] were also tested. Logistic regression analysis was performed to assess the association between prealbumin and osteoporosis.
Results:
The adults with osteoporosis had lower prealbumin levels than those without osteoporosis (235.40 ± 60.66 versus 261.34 ± 55.28 mg/l, p < 0.001). The proportion of adults with prealbumin levels below the normal range was significantly higher in individuals with osteoporosis compared with those without osteoporosis (16.53% versus 4.42%, respectively). After adjusting for age, sex, body mass index (BMI), anemia, handgrip strength and skeletal muscle index (SMI), logistic regression showed that participants with lower levels of prealbumin had a higher risk of osteoporosis [odds ratio (OR): 3.85; 95% confidence interval (CI): 1.54–6.34; p = 0.004].
Conclusion:
Our findings suggested that low levels of prealbumin were associated with an increased risk of osteoporosis in older adults with T2DM. Further longitudinal studies should be conducted to determine if there is a causative association between prealbumin and osteoporosis.
Keywords: ageing, albumin, bone mineral density, China, diabetes, nutrition, osteoporosis, prealbumin
Introduction
Osteoporosis and type 2 diabetes mellitus (T2DM) are two highly prevalent conditions of late life and quite often coexist.1 Osteoporosis and a high falls risk can often occur together as a result of advancing age, which may result severe fractures, leading to disability, poor quality of life, hospitalizations and higher mortality.2 Low bone mineral density (BMD) in older adults is known to be associated with higher incidences of osteoporotic fractures.3 While the relation of T2DM with BMD remains inconsistent across studies,4–6 the fracture risks in individuals with T2DM has been suggested to increase.7 Osteoporosis has been shown to predict fracture risks in individuals with T2DM.8 Furthermore, individuals with T2DM have even worse fracture outcomes than those with normal blood glucose.9 However, strategies to reduce fracture risks appear underutilized in older adults with T2DM, possibly due to challenges in identifying the high-risk individuals.9 Thus, it is crucial to identify those at risk of osteoporosis in this population and implement preventive strategies accordingly so as to avoid the negative consequences of osteoporosis.
The influential factors for osteoporosis include age, sex, serum vitamin D concentrations, lifestyle factors,9 chronic inflammation,10,11 and nutritional risk.12,13 Poor neuromuscular function is a risk factor for fracture risk.14 Many mechanisms have been assumed to contribute to diabetic osteoporosis, such as poor glycemic control,1,15 defects in insulin secretion or insulin action,16 low serum vitamin D concentrations,1 increased proinflammatory cytokines.10 Additionally, older adults with T2DM may have an additional risk of undernutrition, as a result of overly restrictive eating patterns to control blood sugar.17 Serum albumin and prealbumin are among widely used nutritional biomarkers.18,19 However, prealbumin has been suggested to be the earliest laboratory indicator of nutrition and is a much preferred biomarker for malnutrition compared with albumin.18 Previous studies have reported conflicting results on the association of hypoalbuminemia with osteoporosis. Few observational studies have suggested an independent association of osteoporosis with lower levels of serum albumin,19–21 while others have reported no such association.22 Nonetheless, the relationship between osteoporosis and prealbumin has been rarely investigated (to our knowledge, only two studies). A recent study has shown reduced prealbumin to be associated with BMD in postmenopausal women.23 While another study has shown such an association in patients undergoing chronic peritoneal dialysis.19 In addition, the association of osteoporosis with prealbumin in individuals with T2DM has not yet been examined.
Therefore, the objective of our study was to investigate whether the levels of prealbumin in older adults with T2DM with osteoporosis differed from those in older adults with T2DM without osteoporosis and whether low prealbumin was an independent risk factor for osteoporosis in this population.
Materials and methods
Study participants
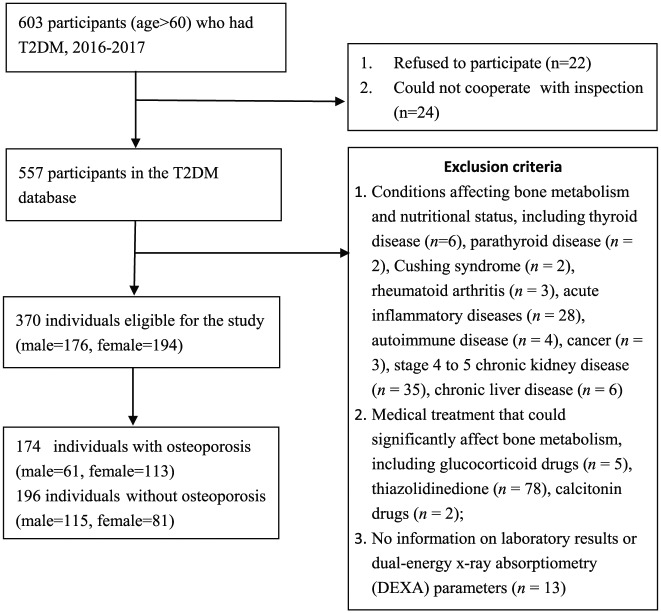
This cross-sectional study was conducted using the geriatric T2DM database of Xuanwu Hospital, Capital Medical University, China. In total, 603 older adults with T2DM aged over 60 years were recruited by the Department of Endocrinology from July 2016 to August 2017. Among which, 22 of them refused to participate in the study, and 24 could not cooperate with the protocol due to disability. A total of 557 participants aged >60 years who met the 1999 World Health Organization (WHO) diagnosis and classification criteria of T2DM were included in the database. The exclusion criteria for our current study were:
(1) Conditions affecting bone metabolism and nutritional status, including thyroid disease, parathyroid disease, Cushing’s syndrome, rheumatoid arthritis, acute inflammatory diseases, autoimmune disease, cancer, stage 4 to 5 chronic kidney disease, chronic liver disease.
(2) Medical treatment that could significantly affect bone metabolism, including glucocorticoid drugs, thiazolidinedione, calcitonin drugs.
(3) No information on laboratory results or dual-energy X-ray absorptiometry (DEXA) parameters.
Thus, a total of 370 participants were included in this study (Figure 1). The participants were divided into two groups: (1) those with osteoporosis (n = 121), and (2) those without osteoporosis (n = 249).
Figure 1.
Flow chart on the selection of participants.
The study obtained ethical approval from the Research Ethics Boards at Xuanwu Hospital of Capital Medical University, China (approval number: CTR-IPR-2019002). Written informed consent was obtained from all participants on provision of a complete study description.
Selection of risk factors
Participants were assessed for their potential risk factors for osteoporosis based on past literature such as serum vitamin D concentrations, lifestyle factors, chronic inflammation, nutritional risk and poor neuromuscular function (markers described below in detail) and included in our analyses.
Clinical and anthropometric information
Information on demographic data, lifestyle habit, disease history and medication records were obtained from all participants. The height and weight of each were measured while the participants were wearing lightweight clothing without wearing shoes. The body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2).
Biochemical measurements
Blood samples of the participants were drawn in the morning after a 10-hour overnight fast. Fasting blood glucose (FBG), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (CRE), albumin, prealbumin, fasting blood insulin (FINS), and fasting blood C peptide were detected by automatic biochemical analyzer (BioTek Instrument, Inc., Beijing, China). A low serum prealbumin level was defined as <170 mg/l.24 The homeostasis model assessment (HOMA) formula was used for calculating the insulin resistance (IR) index (HOMA-IR). HOMA-IR = fasting insulin (μIU/ml) × fasting glucose (mmol/l)/22.5. Hemoglobin A1c (HbA1c) was detected by high-performance liquid chromatography. Serum 25-hydroxyvitamin D3 [25(OH)D3] was determined by double antibody radioimmunoassay (DiaSorin, Stillwater, MN, USA and Linco Research, St. Charles, MO, USA). Serum levels of highly sensitive C-reactive protein (hs-CRP) were determined by an immunoturbidimetry assay (Kanto Chemical Co Inc, Tokyo, Japan). Serum levels of interleukin (IL)-6 were detected using an enzyme-linked immunosorbent assay kit (Beijing Biolab Science and Technology Co. Ltd., Beijing, China). The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease equation.25 The hemoglobin (Hb) level was determined using a CELL DYN 3200 hematology analyzer (Abbott Laboratories, Abbott Park, IL, USA). According to the WHO diagnostic criteria, anemia was defined as Hb levels <13 g/dl for men and <12 g/dl for women.26
Assessment of osteoporosis and appendicular muscle mass
Both body composition parameters and the BMD (g/cm2) were determined by DEXA (LUNAR iDXA, USA). A single experienced technologist performed all the scans. BMD was measured at the lumbar spine 2–4, femoral neck and total hip. The BMD T-score was calculated from the sex-matched BMD data in young adults derived in China.27 According to the criteria recommended by WHO in 1994, osteoporosis was diagnosed by a T-score <−2.5 × standard deviation (SD) at any site on the lumbar spine, femoral neck, or total hip.28 Appendicular skeletal muscle (ASM; kg) was calculated as the sum of arm and leg skeletal muscle mass. Skeletal muscle index (SMI) was calculated as ASM divided by height squared (m2) and was the indicator of relative ASM.29 Low muscle mass was defined as SMI < 7.0 kg/m2 for men and <5.4 kg/m2 for women.30
Muscle strength and gait speed measurements
Hand grip strength was measured three times on each side using the Jamar® Hydraulic Hand Dynamometer (Patterson Medical, Warrenville, IL, USA). The maximal grip strength of six measures was used in the analyses. Low grip strength was defined as <26 kg for men and <18 kg for women, as per the consensus report of the Asian Working Group for Sarcopenia.30
For the assessment of gait speed, participants were instructed to walk at their usual walking speed for a 6-meter distance.31 Trained staff recorded time for the 6-meter walk (seconds) to the nearest 0.01 s by using a stop watch. Overall, two timed trials were conducted to derive the gait speed (m/s) and the best (i.e. fastest) performance was used for the present analysis. Low gait speed was defined as a gait speed <0.8 m/s.30
Statistical analyses
All statistical analyses for this study were performed using SPSS version 17.0. Normally distributed data were reported as the mean ± SD, while variables with skewed distribution were expressed as median (interquartile range). Comparisons for categorical variables were performed via Chi-square tests, while the unpaired Student’s t test or the Mann–Whitney U test was used for continuous variables. The correlations between prealbumin level and IL-6, hs-CRP were analyzed using a Pearson’s correlation or Spearman’s rank correlation coefficient. Binary logistic regression was carried out to examine the association between prealbumin and osteoporosis. The results of the regression modeling were presented as odds ratios (ORs) and their 95% confidence intervals (CIs). The variables in the logistic regression model included age, sex, BMI, anemia, low grip strength, low SMI and low prealbumin. A two-sided p value was determined and p < 0.05 was considered to be statistically significant.
Results
A total of 176 males and 194 females with a mean age of 67.60 ± 5.78 years were included in this study (Table 1). Of the 370 participants in the study, 21.59% (38/176) of men and 42.78% (83/194) of women were classified as having osteoporosis. The mean T2DM duration of the participants was 14.44 ± 8.71 years. The mean HbA1c was 8.43 ± 1.81 %. The prealbumin levels were significantly lower in persons with osteoporosis than those without osteoporosis (235.40 ± 60.66 versus 261.34 ± 55.28, respectively), and so were the BMI and Hb levels (p < 0.001). The proportion of participants with prealbumin levels below the normal range was significantly higher in individuals with osteoporosis compared with those without osteoporosis (16.53% versus 4.42%, respectively), and so was the percentage of anemia (23.14% versus 14.46%, respectively; Table 2). The mean BMI of the participants was 25.81 ± 3.66 kg/m2. Participants with osteoporosis had a lower ASM and grip strength (p < 0.001; Table 3).
Table 1.
The demographics of the participants with and without osteoporosis.
| Total
n = 370 |
Without osteoporosis
n = 249 |
Osteoporosis
n = 121 |
p value | |
|---|---|---|---|---|
| Age (years) | 67.60 ± 5.78 | 66.59 ± 6.22 | 67.79 ± 7.73 | 0.137 |
| Sex | <0.001 | |||
| Male | 176 (47.57%) | 138 (59.42%) | 38 (31.40%) | |
| Female | 194 (52.43%) | 111 (44.58%) | 83 (68.60%) | |
| Diabetes duration (years) | 14.44 ± 8.71 | 15.18 ± 9.21 | 13.74 ± 7.51 | 0.111 |
Table 2.
The biochemical markers of the participants with and without osteoporosis.
| Total
n = 370 |
Without osteoporosis
n = 249 |
Osteoporosis
n = 121 |
p value | |
|---|---|---|---|---|
| HbA1c (%) | 8.43 ± 1.81 | 8.38 ± 1.85 | 8.50 ± 1.85 | 0.757 |
| HOMA-IR | 5.08 (2.81–9.64) | 5.47 (2.81–9.24) | 4.31 (2.74–11.06) | 0.893 |
| FBG (mmol/l) | 8.42 (6.57–11.29) | 8.58 (6.59–11.73) | 8.15 (6.40–11.20) | 0.596 |
| hs-CRP (mg/l) | 2.61 (1.73–4.62) | 2.46 (1.77–4.31) | 2.84 (1.72–4.78) | 0.445 |
| Interleukin-6 (pg/ml) | 4.12 (2.72–6.81) | 3.88 (2.76–6.81) | 4.56 (2.72–7.71) | 0.270 |
| eGFR (ml/min/1.73m2) | 91.11 ± 26.26 | 92.56 ± 24.11 | 87.41 ± 23.30 | 0.061 |
| ALT (IU/l) | 22.29 ± 12.29 | 23.45 ± 12.98 | 21.72 ± 11.29 | 0.071 |
| AST (IU/l) | 23.01 ± 8.79 | 23.59 ± 10.78 | 21.85 ± 9.29 | 0.053 |
| Albumin (g/l) | 41.50 ± 4.56 | 41.55 ± 4.23 | 40.87 ± 5.43 | 0.187 |
| Prealbumin (mg/l) | 252.22 ± 59.90 | 261.34 ± 55.28 | 235.40 ± 60.66 | <0.001 |
| Low prealbumin (n, %) | 31 (8.38%) | 11 (4.42%) | 20 (16.53%) | <0.001 |
| Hemoglobin (g/l) | 135.77 ± 14.80 | 138.06 ± 14.86 | 131.32 ± 13.87 | <0.001 |
| Anemia (n, %) | 64 (17.30%) | 36 (14.46%) | 28 (23.14%) | 0.038 |
| 25(OH)D3 (ng/ml) | 22.67 ± 8.03 | 23.28 ± 8.54 | 21.45 ± 7.36 | 0.061 |
25(OH)D3, 25-OH vitamin D3; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein.
Low prealbumin levels were defined as <170 mg/l. Data are shown as mean ± standard deviation or median (interquartile range) or percentage.
Table 3.
Anthropometry and body composition measurements of the participants with and without osteoporosis.
| Total
n = 370 |
Without osteoporosis
n = 249 |
Osteoporosis
n = 121 |
p value | |
|---|---|---|---|---|
| BMI (kg/m2) | 25.81 ± 3.66 | 26.36 ± 3.26 | 24.70 ± 3.74 | <0.001 |
| ASM (kg) | 19.30 ± 4.13 | 20.13 ± 3.94 | 17.43 ± 3.68 | <0.001 |
| SMI (kg/m2) | 7.03 ± 1.03 | 7.21 ± 0.99 | 6.59 ± 0.96 | <0.001 |
| Low SMI (n, %) | 51 (13.78%) | 22 (8.83%) | 29 (23.96%) | <0.001 |
| Grip strength (kg) | 29.86 ± 10.04 | 31.88 ± 9.87 | 26.68 ± 9.38 | <0.001 |
| Low grip strength (n, %) | 49 (13.24%) | 21 (8.43%) | 28 (23.14%) | <0.001 |
| Low gait speed (n, %) | 47 (12.70%) | 29 (11.65%) | 18 (14.87%) | 0.382 |
| BMD (g/cm2) | ||||
| Lumbar spine 2–4 | 1.126 ± 0.252 | 1.200 ± 0.180 | 0.959 ± 0.134 | <0.001 |
| Right femoral neck | 0.883 ± 0.156 | 0.940 ± 0.131 | 0.767 ± 0.138 | <0.001 |
| Right total hip | 0.952 ± 0.158 | 1.004 ± 0.134 | 0.814 ± 0.125 | <0.001 |
ASM, appendicular skeletal muscle mass; BMD, bone mineral density; BMI, body mass index; SMI, skeletal muscle mass index.
Low grip strength was defined as <26 kg for men and <18 kg for women. Low muscle mass was defined as SMI <7.0 kg/m2 for men and <5.4 kg/m2 for women.
Results of the logistic regression model for the association of prealbumin and risk factors associated with osteoporosis are shown in Table 4. Anemia (yes or no), low grip strength (yes or no), and low SMI (yes or no), as well as age, sex, BMI, and low prealbumin level (yes or no), were included in the multivariate logistic regression model. We found that low prealbumin (OR: 3.85, 95% CI: 1.54–6.34) was significantly associated with an increased risk of osteoporosis. Female, low BMI and low grip strength were the associated risk factors for osteoporosis in older adults with T2DM.
Table 4.
Logistic regression models for risk factors associated with osteoporosis.
| Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | P value | Adjusted OR | 95% CI | p value | |
| Low prealbumin | 4.36 | 1.88–7.10 | 0.001 | 3.85 | 1.54–6.34 | 0.004 |
| Age | / | / | 1.01 | 0.97–1.05 | 0.639 | |
| Female | / | / | 2.98 | 1.75–5.08 | 0.000 | |
| BMI | / | / | 0.88 | 0.81–0.95 | 0.001 | |
| Low grip strength | / | / | 1.77 | 1.17–3.58 | 0.045 | |
| Anemia | / | / | 1.22 | 0.66–2.66 | 0.525 | |
| Low SMI | / | / | 1.19 | 0.60–2.37 | 0.620 | |
BMI, body mass index; CI, confidence interval; OR, odds ratio; SMI, skeletal muscle mass index.
Low grip strength was defined as <26 kg for men and <18 kg for women; Low prealbumin levels were defined as <170 mg/l. Low muscle mass was defined as SMI < 7.0 kg/m2 for men and <5.4 kg/m2 for women.
Model 1: unadjusted.
Model 2: adjusted by age, sex, BMI, anemia, low grip strength, low SMI.
Discussion
In this study, we found that low levels of prealbumin were associated with an increased risk of osteoporosis in older adults with T2DM. The association remained significantly independent of several confounders such as age, sex, BMI, metabolic parameters, muscle mass and grip strength.
It is undeniable that nutrition plays an extremely important role in bone health. Albumin and prealbumin are the widely used biomarkers of nutrition. A number of studies have concluded that hypoalbuminemia is associated with osteoporosis,20,21 but these findings remain contradictory.22,32 In the NHANES database, Afshinnia20 reported an independent association of osteoporosis with hypoalbuminemia at the femoral neck, total femur, and lumbar spine in the general population of the United States. However, in the Rancho Bernardo study, Lunde and colleagues22 failed to show such an association. The discrepancy in the results of these studies may be due to a different selected population and methodological limitations including low sample size, lack of enough confounders and narrow range of variables. To our knowledge, studies investigating the association of prealbumin and osteoporosis are sparse. A recent study has found reduced prealbumin to be associated with BMD in women with osteoporosis.23 Another study reported that albumin and prealbumin to be related predictors of BMD in patients undergoing chronic peritoneal dialysis.19 However, in our study, low levels of prealbumin but not albumin were found to be significantly associated with osteoporosis in older adults with T2DM. The possible explanation for such findings could be as suggested before, that prealbumin is a more sensitive nutritional marker than albumin for assessing nutritional status because of its short half-life. Prealbumin is known to be a good marker of visceral protein status and affected earlier by acute variations in protein balance.33 The association between prealbumin and osteoporosis might be explained by the nutrition risk in older people with osteoporosis. Proper osseous metabolism is significantly affected by calcium, vitamin D, proteins, magnesium and other vitamins.34 Nutrition risk induces osteoporosis and fragility fracture.12
The mechanism underlying the association between hypoalbuminemia with osteoporosis is unclear. Albumin and prealbumin are not only markers of nutritional status but also belong to the negative acute-phase reactants, the concentrations of which fall during inflammation.35 Inflammation plays a potential role in both the normal bone remodeling process and the pathogenesis of osteoporosis. Numerous proinflammatory cytokines have been implicated in the regulation of osteoblasts and osteoclasts.10 For example, IL-6 promotes osteoclast differentiation and activation. Hs-CRP is regarded as a sensitive marker of systemic inflammation and its production in the liver is regulated by IL-1, IL-6 and tumor necrosis factor (TNF)-α.36 The association between hs-CRP levels and BMD has been observed in several studies.11 Hypoalbuminemia is the result of inflammation which reduces the effective use of dietary protein and augments catabolism of the key somatic protein.37 Consistent with previous studies, our present study demonstrated that prealbumin was inversely correlated with hs-CRP and IL-6 (p < 0.001). However, although there was an increased trend for serum levels of hs-CRP and IL-6 in individuals with osteoporosis, the result did not reach statistical significance. The results are different from previous studies.10 The discrepancies could be mainly associated with selection bias of the study populations. Hypoalbuminemia may directly suppress osteogenesis and activate osteoclasts via nuclear factor (NF)-κB, which is considered a potent mediator of inflammatory osteolysis; or it may be indirectly linked with NF-κB-mediated osteoclastogenesis via cytokines such as the receptor activator of NF-κB ligand, TNF-α, IL-1, and oxygen radicals.21,38 Indeed, further studies are required to help better understand the pathophysiological link between osteoporosis and albumin metabolism.
Anemia, BMI and muscle mass are commonly used indicators of nutritional status. Thus, we also performed Hb, BMI and muscle mass measurements to assess nutritional status. Previous studies showed that Hb and anemia status were associated with BMD and risk of fracture in the general population.39 In addition, previous studies suggested that the impairment of muscle status (i.e. muscle mass and muscle strength) is associated with a deterioration in bone mass.13 In our study significant differences were found according to BMI, Hb, and DEXA parameters (ASM, SMI) between participants with and without osteoporosis. However, low grip strength but not low SMI was found to be associated with osteoporosis. The possible explanation could be that muscle strength does not depend solely on muscle mass and may decline more with age than muscle mass.40,41
Many mechanisms have been assumed to contribute to diabetic osteoporosis. One of them is that metabolic alterations in T2DM can trigger disorders of calcium homeostasis, skeletal metabolism, and bone mass.42 Some studies have reported BMD to decrease more severely in T2DM individuals with poor glycemic control.15 The explanation for this may be that a higher concentration of advanced glycation end-products as a result of higher glucose levels in collagen may reduce bone strength and stimulate apoptosis of osteoblasts, thereby contributing to deficient bone formation.43,44 Another mechanism is that a defect in the insulin secretion or insulin action in individuals with diabetes can lead to diabetic osteopenia due to a deficiency in the anabolic activation of insulin.16 For instance, one previous study indicated that there was a positive correlation between the levels of insulin secretion and BMD in older Japanese adults with T2DM.6 Our study showed there was no significant difference according to HbA1c, FINS, and HOMA-IR between the individuals with T2DM with and without osteoporosis. One of the reasons for such findings could be that our study was performed in older adults with T2DM under fairly controlled and stable conditions.
Our study showed that low levels of prealbumin were associated with a higher risk of osteoporosis which has tremendous clinical implications, given the rapid ageing of populations worldwide. Comprehensive clinical evaluation of prealbumin in older adults with T2DM might be helpful for the early identification of those at high risk of osteoporosis. Early recognition of osteoporosis and initiation of therapy for osteoporosis with bone-active agents might prevent bone loss and thus improve patient outcomes. Such findings could play an extremely important role in reducing the increasing burden of osteoporosis in older people with T2DM; however, our study had some limitations. First, this study is cross-sectional; thus, a causal pathway from low levels of prealbumin to osteoporosis cannot be definitively proven. Therefore, follow-up studies should be conducted in the future. Second, our study consisted of individuals selected only from Xuanwu Hospital. Hence, the present study population may not be representative of the general T2DM population and the results of our study are not generalizable to other populations. Third, dietary habits and medications that potentially affect osteoporosis and albumin/prealbumin levels were not included in the models.
In conclusion, our study demonstrated that low levels of prealbumin were associated with an increased risk of osteoporosis in older adults with T2DM. Further longitudinal studies are warranted to clarify the association of prealbumin levels and the development of osteoporosis.
Acknowledgments
The authors thank all of the doctors, and participants who were involved in the study.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Shuangling Xiu  https://orcid.org/0000-0002-8549-4374
https://orcid.org/0000-0002-8549-4374
Contributor Information
Shuangling Xiu, Department of Endocrinology, Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, No.45 ChangChun Street, XiCheng District, Beijing, 100053, China.
Jagadish K Chhetri, Department of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China; National Clinical Research Center for Geriatric Disorders, Beijing, China.
Lina Sun, Department of Endocrinology, Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China.
Zhijing Mu, Department of Endocrinology, Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China.
Li Wang, Department of Endocrinology, Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China.
References
- 1. Paschou SA, Dede AD, Anagnostis PG, et al. Type 2 diabetes and osteoporosis: a guide to optimal management. J Clin Endocrinol Metab 2017; 102: 3621–3634. [DOI] [PubMed] [Google Scholar]
- 2. Watts NB, Manson JE. Osteoporosis and fracture risk evaluation and management: shared decision making in clinical practice. JAMA 2017; 317: 253–254. [DOI] [PubMed] [Google Scholar]
- 3. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int 2014; 25: 2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 2012; 27: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaturu S, Humphrey S, Landry C, et al. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit 2009; 15: 5–9. [PubMed] [Google Scholar]
- 6. Majima T, Komatsu Y, Yamada T, et al. Decreased bone mineral density at the distal radius, but not at the lumbar spine or the femoral neck, in Japanese type 2 diabetic patients. Osteoporos Int 2005; 16: 907–913. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011; 305: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maíra Viégas, Costa C, Lopes A, et al. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications 2011; 25: 216–221. [DOI] [PubMed] [Google Scholar]
- 9. Sellmeyer DE, Civitelli R, Hofbauer LC, et al. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes 2016; 65: 1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000; 21: 115–137. [DOI] [PubMed] [Google Scholar]
- 11. Wu ZJ, He JL, Wei RQ, et al. C-reactive protein and risk of fracture: a systematic review and dose-response meta-analysis of prospective cohort studies. Osteoporos Int 2015; 26: 49–57. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Hui M, Chang X, et al. BMI reduction and vitamin D insufficiency mediated osteoporosis and fragility fractures in patients at nutritional risk: a cross-sectional study. Eur J Clin Nutr 2018; 72: 455–459. [DOI] [PubMed] [Google Scholar]
- 13. Offord EA, Karagounis LG, Vidal K, et al. Nutrition and the biology of human ageing: bone health and osteoporosis / sarcopenia / immune deficiency. J Nutr Health Aging 2013; 17: 712–716. [DOI] [PubMed] [Google Scholar]
- 14. Locquet M, Beaudart C, Bruyère O, et al. Bone health assessment in older people with or without muscle health impairment. Osteoporos Int 2018; 29: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krakauer JC, McKenna MJ, Buderer NF, et al. Bone loss and bone turnover in diabetes. Diabetes 1995; 44: 775–782. [DOI] [PubMed] [Google Scholar]
- 16. Kocián J, Brunová J. Diabetic osteopathy. 4. Laboratory findings. Vnitr Lek 1990; 36: 460–466. [PubMed] [Google Scholar]
- 17. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ingenbleek Y, Van Den Schrieck HG, De Nayer P, et al. Albumin, transferrin and the thyroxine-binding prealbumin/retinol-binding protein (TBPA-RBP) complex in assessment of malnutrition. Clin Chem Acta 1975; 63: 61–67. [DOI] [PubMed] [Google Scholar]
- 19. Jeong JU, Lee HK, Kim YJ, et al. Nutritional markers, not markers of bone turnover are related predictors of bone mineral density in chronic peritoneal dialysis patients. Clin Nephrol 2010; 74: 336–342. [DOI] [PubMed] [Google Scholar]
- 20. Afshinnia F, Pennathur S. Association of Hypoalbuminemia with Osteoporosis: Analysis of the National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2016; 101: 2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Afshinnia F, Wong KK, Sundaram B, et al. Hypoalbuminemia and osteoporosis: reappraisal of a controversy. J Clin Endocrinol Metab 2016; 101: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lunde AV, Barrett-Connor E, Morton DJ. Serum albumin and bone mineral density in healthy older men and women: the Rancho Bernardo Study. Osteoporos Int 1998; 8: 547–551. [DOI] [PubMed] [Google Scholar]
- 23. Li XS, Zhang JR, Zhao YL, et al. Reduced prealbumin is associated with bone mineral density in women with osteoporosis. Nutrition 2017; 33: 338–342. [DOI] [PubMed] [Google Scholar]
- 24. Zhang SQ, Peng B, Stary CM, et al. Serum prealbumin as an effective prognostic indicator for determining clinical status and prognosis in patients with hemorrhagic stroke. Neural Regen Res 2017; 12: 1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis-a position statement from KDOQI and KDIGO. Am J Kidney Dis 2009; 53: 915–920. [DOI] [PubMed] [Google Scholar]
- 26. WHO Scientific Group. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405: 5–37. [PubMed] [Google Scholar]
- 27. Cheng X, Dong S, Wang L, et al. Prevalence of osteoporosis in China: a multicenter, large-scale survey of a health checkup population. Chin J Health Manage 2019; 13: 51–58. [Google Scholar]
- 28. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368–381. [DOI] [PubMed] [Google Scholar]
- 29. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 30. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 31. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13: 881–889. [DOI] [PubMed] [Google Scholar]
- 32. D’Erasmo E, Pisani D, Ragno A, et al. Relationship between serum albumin and bone mineral density in postmenopausal women and in patients with hypoalbuminemia. Horm Metab Res 1999; 31: 385–388. [DOI] [PubMed] [Google Scholar]
- 33. Unal D, Orhan O, Eroglu C, et al. Prealbumin is a more sensitive marker than albumin to assess the nutritional status in patients undergoing radiotherapy for head and neck cancer. Contemp Oncol (Pozn) 2013; 17: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woźniak-Holecka J, Sobczyk K. Nutritional education in the primary prevention of osteoporosis in perimenopausal and postmenopausal women. Prz Menopauzalny 2014; 13: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448–454. [DOI] [PubMed] [Google Scholar]
- 36. Koh JM, Khang YH, Jung CH, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre-and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporosis Int 2005; 16: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 37. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 38. Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int 2013; 24: 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee EA, Shin DW, Yoo JH, et al. Anemia and Risk of Fractures in Older Korean Adults: A Nationwide Population-based Study. J Bone Miner Res. Epub ahead of print 28 January 2019. 2019: e3675 DOI: 10.1002/jbmr.3675. [DOI] [PubMed] [Google Scholar]
- 40. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009; 90: 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adil C, Aydın T, Taşpınar Ö, et al. Bone mineral density evaluation of patients with type 2 diabetes mellitus. J Phys Ther Sci 2015; 27: 179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamagishi S, Nakamura K, Inoue H. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med Hypotheses 2005; 65: 1013–1015. [DOI] [PubMed] [Google Scholar]
- 44. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 2007; 40: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]