Abstract
Previous research has demonstrated that the collapsin response mediator protein (CRMP) family is involved in the formation of neural networks. A recent whole-exome sequencing study identified a de novo variant (S541Y) of collapsin response mediator protein 4 (CRMP4) in a male patient with autism spectrum disorder (ASD). In addition, Crmp4-knockout (KO) mice show some phenotypes similar to those observed in human patients with ASD. For example, compared with wild-type mice, Crmp4-KO mice exhibit impaired social interaction, abnormal sensory sensitivities, broader distribution of activated (c-Fos expressing) neurons, altered dendritic formation, and aberrant patterns of neural gene expressions, most of which have sex differences. This review summarizes current knowledge regarding the role of CRMP4 during brain development and discusses the possible contribution of CRMP4 deficiencies or abnormalities to the pathogenesis of ASD. Crmp4-KO mice represent an appropriate animal model for investigating the mechanisms underlying some ASD phenotypes, such as impaired social behavior, abnormal sensory sensitivities, and sex-based differences, and other neurodevelopmental disorders associated with sensory processing disorders.
Keywords: collapsin response mediator protein 4, autism spectrum disorder, neurodevelopmental disorder, whole-exome sequencing, animal model, sex different phenotypes
1. Introduction
The formation of neural networks is temporally and spatially regulated by numerous molecules, such as extracellular molecules regulating cell adhesion and axon guidance, and intracellular signaling molecules regulating axon elongation and the formation of dendrites, spines, and synapses. Collapsin response mediator proteins (CRMPs) are intracellular signaling molecules elicited by extracellular signals (e.g., semaphorin (Sema) 3A and reelin) during neuronal migration, differentiation, neurite network organization, and even remodeling [1,2,3]. Genome-wide studies, genetic linkage analyses, proteomic analyses, and translational approaches have revealed altered expression levels of CRMPs in neurodevelopmental disorders, such as schizophrenia, attention-deficit/hyperactivity disorder (ADHD), and autism spectrum disorder (ASD) [3,4,5,6,7,8]. Similar findings have been observed for neurological disorders such as Alzheimer’s disease [9,10,11] and hyperalgesia syndrome [12,13,14,15]. Furthermore, during the past decade, many studies using knockout (KO) mice have demonstrated the role of CRMPs in the pathogenesis of neurodevelopmental disorders, as described in Section 3 below. In our recent whole-exome sequencing study, we identified a de novo variant of CRMP4 in a male patient with ASD [8]. In this review, we discuss the functions of CRMP4 in the developing brain and the possible involvement of CRMP4 deficiencies and abnormalities in the pathogenesis of neurodevelopmental disorders.
2. Identification of CRMP4
Sema1A guides the growth cone in the proper direction during neural circuit formation in the developing brain. Sema1A was first identified as fasciclin IV in Drosophila [16] and subsequently identified as collapsin in chickens [17]. Since then, numerous members of the Sema family have been identified. Among them, Sema3A has been implicated in each step of neural circuit formation from axonal and dendritic development to synaptic assembly [18,19,20,21]. Goshima et al. [22] identified a CRMP with a relative molecular mass of 62 kDa (CRMP-62), now known as CRMP2, which is required for Sema3A-induced inward currents in the Xenopus laevis oocyte expression system. The authors further reported that introduction of anti-CRMP-62 antibodies into dorsal root ganglion neurons blocks Sema3A-induced growth cone collapse [22]. In 1995, Minturn et al. identified a 64-kDa protein in the rat embryo known as turned on after division 64 (TOAD-64), which was eventually classified as CRMP4 [23,24]. The CRMP family comprises five homologous cytosolic proteins (CRMP1 ~ 5) with high (50−70%) homology. CRMP4 is also referred to as TUC-4, unc-33-like phosphoprotein 1 (Ulip-1), dihydropyrimidase 3 (DRP3), and dihydropyrimidase-like 3 (DPYSL3) because those were found to be homologous to CRMP4 later [23,24,25,26]. These multiple names of CRMP4 have sometimes caused confusion.
3. The Regulatory Mechanisms Suggested for CRMP4
CRMPs regulate intercellular signaling pathways mediated through extracellular molecules such as Sema3A, reelin, neurotrophins, and myelin-associated inhibitors (MAIs) [22,23,24,25,26,27,28]. Through transduction of these extracellular cues, CRMPs have been reported to regulate various neurodevelopmental events including neuronal apoptosis, migration, axonal elongation, dendritic elongation and branching, spine development, and synaptic plasticity [27,28,29,30,31]. CRMP functions are controlled by the dynamic spatiotemporal regulation of phosphorylation status, which is mediated by kinases such as Cdk5, Rho/ROCK, and GSK3β, which alter CRMP binding to various cytoskeletal proteins such as actin, tubulin, and tau [32,33,34,35,36]. Cytoskeletal proteins regulate neuronal polarity, axonal and dendritic outgrowth, neuronal migration, synaptic formation, and other functions of neurons like transportation of neurotransmitters-containing vesicles. Therefore, effects on cytoskeletal dynamics promote neurodevelopmental responses mediated by CRMPs.
Numerous studies have focused on the relationship between CRMP phosphorylation and the roles of CRMPs. For example, MAIs regulate neurite extension via the phosphorylation of CRMP4, which is mediated by upstream phospho-inactivation of GSK3β [28]. Loss of GSK3β phosphorylation permits L-CRMP4–RhoA binding and suppresses neurite outgrowth. Therefore, MAI−CRMP4 signaling normally contributes to myelin-dependent growth inhibition [37]. Additionally, phosphorylation of CRMP2 and CRMP4 by Cdk5 is required for the proper positioning of Rohon–Beard primary sensory neurons and neural crest cells as well as caudal primary motor neurons in the zebrafish spinal cord during neurulation [38,39].
In addition to phosphorylation, truncation of CRMP4 by calpain-mediated cleavage is found in glutamate- and N-methyl-D-aspartate (NMDA) receptor-induced excitotoxicity and oxidative stress, both of which reduce cellar viability in primary cultured cortical neurons [40,41,42]. The similar regulatory mechanism of CRMP4 is also involved in potassium deprivation-induced apoptosis in cultured cerebellar granule cells [43].
Furthermore, CRMP4 is expressed as both a short isoform (CRMP4a) and a longer isoform (CRMP4b) [44,45]. Previous studies have indicated that these two isoforms exhibit opposing functions during neurite outgrowth [44,46], though the mechanisms regulating their expressions remain unclear.
4. Potential Involvement of CRMPs Including CRMP4 in Neurodevelopmental Disorders
CRMP family genes and proteins are abundantly expressed in the developing brain, strongly suggesting that they play important roles in neuronal circuit formation [23,47,48]. Furthermore, in situ hybridization experiments have revealed that there are regional differences in Crmp4 mRNA expression during postnatal brain development [49]. In addition, while Crmp4 mRNA expression is scarcely detectable in most areas of the adult brain, it remains considerably detectable in adult neurogenic regions containing immature neurons, such as the subgranular zone of the dentate gyrus and subventricular zone–olfactory bulb (OB) migratory pathway [49]. Such findings highlight the crucial role of CRMP4 in neuronal circuit formation.
Abnormal CRMP expression in the brain has been associated with several neurodevelopmental disorders. For example, patients with schizophrenia exhibit alterations in levels of CRMP1 and CRMP2 protein (for review, see [4], [7,50,51,52]. Liu et al. [53] suggested that reduced transcription and mTOR-regulated translation of certain DPYSL2 isoforms (i.e., genes encoding CRMP2) increase the risk of schizophrenia. Lee et al. [6] further reported that two functional single-nucleotide polymorphisms of the human DRYSL2 gene are associated with susceptibility to schizophrenia. Pham et al. [7] demonstrated that allelic variants of the di-nucleotide repeat at the 5’-untranslated repeat of DPYSL2 change the interaction between CRMP2 and mTOR effector proteins. In addition, findings obtained from Crmp1- and Crmp2-KO mice suggest that impairments in CRMP1 and CRMP2 functions are involved in the pathogenesis of schizophrenia [5,54,55]. Furthermore, brain-specific Crmp2-KO mice display molecular, cellular, structural, and behavioral deficits, many of which are reminiscent of the features associated with schizophrenia [56].
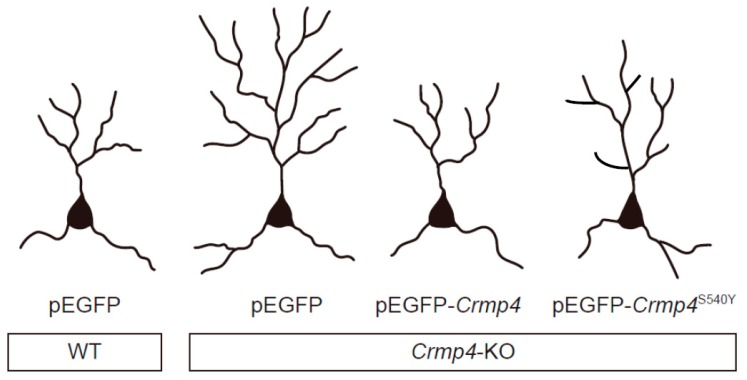
In contrast to CRMP1 and CRMP2, relatively few studies have investigated the involvement of CRMP4 in neurodevelopmental disorders [8,57,58,59]. Miller et al. [57] suggested that microRNA (miR)-132, CREB-regulated miRNA associated with NMDAR signaling, is involved in the pathogenesis of schizophrenia and revealed that expressions of several genes including CRMP4 (DPYSL3) are regulated by miR-132, though the relation between CRMP4 and miR-132 and that between CRMP4 and schizophrenia remain unknown. A missense variant and four other de novo variants of the CRMP4 gene were identified in an ASD proband from the Simons Simplex Collection [58]. A recent whole-exome sequencing study also identified another likely pathogenic missense variant in the CRMP4 gene (CRMP4S541Y) in a male patient with ASD [8]. In addition, Tsutiya et al. [8] investigated the effect of Crmp4 missense mutation, which was found in ASD patients, on dendritic extension. In their study, dendritic formation was compared among neurons from wild type (WT) mice (WT neurons) transfected with enhanced green fluorescent protein (pEGFP), and neurons from Crmp4-KO mice (Crmp4-KO neurons) transfected with either a pEGFP, pEGFP-WT Crmp4 or pEGFP-Crmp4S540Y (the site homologous to human S541) (Figure 1). Crmp4-KO neurons transfected with pEGFP had significantly longer dendrites with more branching points than WT-neurons transfected with pEGFP. Crmp4-KO neurons transfected with pEGFP-Crmp4S540Y exhibited significantly greater numbers of dendritic branching points than Crmp4-KO neurons transfected with pEGFP-WT Crmp4 (Figure 1). These results suggest that ASD-linked CRMP4 mutations alter dendritic morphology. Furthermore, accumulating evidence suggests that Crmp4-KO mice exhibit several phenotypes that resemble those observed in human patients with ASD (DSM-V [60]). In the following sections, we review the autism-like phenotypes observed in Crmp4-KO mice and other animal models of ASD.
Figure 1.
Schematic drawings showing dendritic arborization of cultured hippocampal pyramidal neurons differentially expressing CRMP4. The S540Y mutation in mouse Crmp4 is homologous to S541Y in human CRMP4, which was observed in a patient with autism spectrum disorder (ASD). Representative drawings of cultured hippocampal cells from wildtype (WT) mice transfected with control (pEGFP) vector, Crmp4-knockout (KO) mice transfected with pEGFP vector, Crmp4-KO mice transfected with pEGFP-Crmp4 vector, and Crmp4-KO mice transfected with pEGFP-Crmp4S540Y vector. CRMP: collapsin response mediator protein.
5. Behavioral and Perceptual Abnormalities Observed in Crmp4-KO Mice
5.1. Impairments in Social Behavior
Tsutiya et al. [8,61] examined behavioral deficits in young or adolescent Crmp4-KO mice. Their open-field test and elevated plus maze results suggested that Crmp4-KO mice of both sexes exhibited locomotive activity and anxiety levels similar to those observed in WT mice. Similarly, the novel object recognition test revealed no significant differences in memory acquisition/retention between WT and Crmp4-KO mice of both sexes. However, the authors also utilized the three-chamber test for investigating social behavior, which compares time spent investigating (sniffing) a stranger mouse and a novel object. Male Crmp4-KO mice spent significantly more time in the “object side chamber” than in the “stranger side chamber”, while WT mice of both sexes and female Crmp4-KO mice spent more time sniffing the stranger mouse. In addition, in the social interaction test, male Crmp4-KO mice spent significantly less time actively interacting with a stranger mouse than male WT littermates, although there were no significant differences in the amount of active interaction between WT and Crmp4-KO females. These findings indicate that male-dominant impairments in social behavior can be observed in Crmp4-KO mice [8].
5.2. Abnormalities in Sensory Perception
“Hyper-reactivity or hypo-reactivity to sensory input” is among the diagnostic criteria for ASD specified in the DSM-V. Recent studies have indicated that patients with ASD exhibit neural hyperactivity [62,63], which may account for abnormal sensory sensitivity. Neuronal hyperactivity is considered to result from membrane hyperexcitability and/or abnormal connectivity in neural circuits, such as recurrent excitation or a change in the balance between excitatory and inhibitory synaptic input. Altered neural activity has also been observed in animal models of ASD. For example, mice with null mutations in the Fmr1 gene exhibit social deficits [64] and impaired sensory adaptation [65], which may be due to cortical hyper-excitability [66]. In addition, mice with null mutations of Shank2 exhibit social deficits [67,68] and have been reported to exhibit hypo-excitability to mechanical and noxious heat stimuli as well as to inflammatory and neuropathic pain [69].
Several studies have examined sensory perception in Crmp4-KO mice. Tsutiya et al. [8,61] reported that Crmp4-KO pups exhibit alterations in temperature and olfactory perceptions when compared to WT mice. Infant mice produce ultrasonic vocalizations (UVs) as a normal response to sensory stimulation [70], and the number of UVs is usually used to evaluate the sensory perception ability.
The numbers of UVs emitted by WT and Crmp4-KO pups of both sexes are similar at room temperature (RT, 23 °C). However, when WT pups of both sexes and Crmp4-KO females were subjected to a 19 °C environment, they produced significantly more UVs. When moved from RT to 9 °C, both groups emitted significantly fewer UVs. Surprisingly, when Crmp4-KO males were moved from RT to a 19 °C environment, the authors observed no increases in the number of UVs emitted. However, the number of UVs significantly increased when Crmp4-KO males were moved from RT to 9 °C. These findings indicate that temperature perception markedly differs between Crmp4-KO males and WT mice of both sexes/Crmp4-KO females [8].
In addition, Crmp4-KO mice of both sexes demonstrate impaired olfactory sensitivity when compared to WTs [8,61]. In these previous studies, WT pups of both sexes produced more UVs during exposure to unfamiliar bedding than during exposure to familiar bedding. In contrast, there was no significant difference in the number of UVs emitted by Crmp4-KO pups of both sexes when exposed to the different smells.
Many people diagnosed with ASD have difficulty processing sensory information, which often manifests as hyper- or hypo-sensitivity to sensory stimuli. To examine whether Crmp4 KO is associated with hyper- or hypo-sensitivity to olfactory stimuli, a previous study utilized immunohistochemical experiments to examine the expression of the neuronal activity marker c-Fos following exposure to the odorant ethyl acetate (EA) [61]. Cells positive for c-Fos were counted in each layer of the OB (glomerular layer (GL), external plexiform layer (EPL), mitral cell layer (MCL), granule cell layer (GCL)) and compared among male WT pups and Crmp4-KOs with or without EA exposure. In WT and Crmp4-KO males without EA stimulation, only a few c-Fos-positive cells were observed in sections of the OB. In accordance with a study by Van der Gucht et al. [71], who reported that c-Fos is expressed by neurons after sensory induction, many c-Fos-positive cells were detected after EA exposure. Research has indicated that specific odorants induce neuronal activity in a spatially restricted area known as the odorant map [72,73]. The study has reported that c-Fos-positive cells can be observed in restricted areas of the EPL, MCL, and GCL of WT pups after EA exposure, and that the distribution of these cells is similar for the previous work performed in adult WT mice exposed to EA [74]. In contrast, Crmp4-KO pups exhibited broad, dramatic increases in the number of c-Fos-positive cells in all OB layers following EA exposure. Therefore, the number of active neurons with c-Fos expression is much greater in Crmp4-KO pups than in WT pups after exposure to a single odorant (EA), suggesting that the altered olfactory perception observed in Crmp4-KO pups stems from neuronal hyperactivity in the OB and may be other brain areas related to perception.
6. Altered Dendritic Arborization in Crmp4-KO Mice and Crmp4-Knockdown (KD) Neurons
As shown in Section 3, transfection of cultured hippocampal Crmp4 −/− neurons with mutated mouse CRMP4S540Y (homologous to human CRMP4S541Y found in a patient with ASD) increases dendritic branching when compared to transfection of WT Crmp4. Several studies have also reported altered dendritic involvement in other mouse models of ASD [75,76,77,78]. Indeed, animal models of ASD exhibit hippocampal and cortical pyramidal neurons with significantly longer apical and basal dendrites, as well as significantly greater branching, than WT neurons.
Recent studies have reported that dendritic morphology and axon elongation are altered in Crmp4-KO mice and Crmp4-knockdown (KD) cells [46,59,79,80,81,82]. Niisato et al. [79,80] revealed that deficiency of CRMP4 increases the bifurcation of pyramidal neuron apical dendrites in the mouse hippocampus and in primary cultures. Cha et al. [81] further reported that overexpression of the C-terminal actin-interacting site of CRMP4 facilitates dendritic growth in cultured hippocampal neurons. Using the DiI tracing method, Tsutiya et al. [8] reported that the extension of apical dendrites from OB mitral cells in vivo is enhanced in Crmp4-KO neonates, to those in WT animals [59]. Exaggerated elongation of neurites has also been observed in hippocampal neuronal cell line (HT22) cells transfected with Crmp4 siRNA (Crmp4-KD HT22 cells) [59]. In addition, dendritic length and branching are greater in cultured hippocampal pyramidal neurons derived from Crmp4-KO neonates than in those derived from WT mice [8]. In contrast, overexpression of Crmp4 suppresses dendritic elongation and branching in these cells [8]. Collectively, these results suggest that deficiencies in CRMP4 can increase dendritic elongation and branching in various types of neurons.
7. Altered Expressions of Genes Related to Excitatory and Inhibitory Synaptic Transmission in the Brain of Crmp4-KO Mice
ASD has been reported to be associated with alterations in the expression of several genes related to receptors, transporters, and synthesis enzymes for neurotransmitters such as glutamate, γ-aminobutyric acid (GABA), dopamine, serotonin, acetylcholine, and histamine (for review, see [83]). In addition, Crmp4-KO mice exhibit alterations in the expression of genes mainly related to the glutamatergic and GABAergic systems [8,61]. Since abnormalities in glutamate and GABA have been hypothesized to underlie ASD symptoms, recent translational proton magnetic resonance spectroscopy (MRS) studies have investigated levels of glutamate and GABA in adult humans with ASD as well as rodent ASD models. Such studies have reported that glutamate concentrations in the striatum are decreased in human patients with ASD and some animal models, although no such alterations in GABA levels were observed [84]. Glutamatergic abnormalities are well known to occur in models of ASD (for review, see [85]): For example, proline-rich synapse-associated protein 1 (ProSAP1/Shank2)-KO mice exhibit early, region-specific upregulation of ionotropic glutamate receptors at the synapse [67]. In addition, telomerase reverse transcriptase-overexpressing (TERT transgenic, TERT-tg) mice exhibit male-specific autism-like behaviors, as well as increases in the expression of the NMDA receptor NR2A and NR2B subunits and AMPA receptor GluR1 and GluR2 subunits. TERT-tg mice also exhibit increases in vesicular glutamate transporter (vGluT) 1 levels in the prefrontal cortex [86]. Furthermore, research has indicated that glutamatergic modulators may aid in the treatment of ASD in humans [83,87] and animal models [85,88,89], supporting the notion that the glutamatergic system plays a role in ASD and ASD-like phenotypes.
Male Crmp4-KO pups exhibit significantly greater mRNA and protein expressions of AMPA receptor subunits GluR1 and GluR2 than their WT counterparts [61]. Adult Crmp4-KO mice also exhibit sex- and region-dependent differences in levels of GluR1, GluR2, vGluT1, vGluT2, GABAAα1, GABAAγ2, GABAB receptor 1, and vesicular GABA transporter expressions. However, no significant differences in the expression of other genes (e.g., serotonin transporter mRNA in the raphe nucleus and dopamine D2 receptors (D2Rs) in the cortex) are observed between Crmp4-KO and WT mice of either sex [8]. These data support the notion that Crmp4 deficiency induces alterations in glutamatergic- and some GABAergic-associated genes, which may be associated with the pathogenesis of certain autism-like features in Crmp4-KO mice.
Many studies have implicated altered excitatory (glutamatergic)/inhibitory (GABAergic) balance in the pathogenesis of ASD [90,91,92,93]. However, altered gene expressions in Crmp4-KO mice do not necessarily mean the excitatory/inhibitory balance. Future physiological investigations of Crmp4-KO mice may help to reveal the functional meaning of alterations in glutamatergic and GABAergic gene expressions, and whether such alterations are associated with autism-like phenotypes.
8. Sex-Specific Phenotypes Observed in Crmp4-KO Mice and Other Animal Models of ASD
ASD is more prevalent among boys than girls, and there are substantial sex-based differences in ASD phenotypes [94,95,96,97]. For example, male-biased differences have been observed in patients with ASD exhibiting mutations in genes that encode the synaptic cell adhesion protein neuroligin (NLGN), including NLGN 3, and NLGN4X [98,99]. Sex-based differences in autistic-like phenotypes have also been reported in animal models of ASD generated by exposure to chemicals or genetic manipulation [8,67,86,100,101,102,103]. For example, Schneider et al. [102] demonstrated that prenatal exposure to valproic acid (VPA), which is well known to induce autism-like phenotypes in rats or mice [104], induces some male-specific alterations in behavior and immunological function. Kim et al. [86,101] further revealed that rats exposed to VPA in utero exhibit male-specific alterations in social interactions, hyperactive behavior, and impaired postsynaptic development. Konopko et al. [103] reported sex-based differences in the induction of some exons of the brain-derived neurotrophic factor (Bdnf) gene in the brains of fetal mice exposed to VPA during the prenatal period, indicating that female sex may confer neuroprotection against ASD-like phenotypes.
Some animal models of ASD developed by deleting genes found to be mutated or deficient in human patients with ASD exhibit sex-based differences in autism-like phenotypes. For example, UVs in response to brief separation from the mother are more prominent in female Nlgn4-KO pups than in their male counterparts [105]. In addition, adult Shank2 −/− mice exhibit limited male-biased differences in the call rate and duration of UVs [100]. Tsutiya et al. [8] identified a rare Crmp4 mutation in male patients with ASD. Furthermore, Crmp4-KO mice exhibit male-biased alterations in social behavior, sensory perception, and gene expression, as described in the preceding sections (Section 3, Section 4, Section 5 and Section 6). Iwakura et al. [106] further revealed that CRMP4 is among the candidate proteins involved in the sexual differentiation of the anteroventral periventricular nucleus (AVPV) in the preoptic area of the hypothalamus. The AVPV, which is known to regulate ovulatory cycles, is larger in females than in males. This difference is due to the effects of testosterone (T) secreted from the testes of perinatal males [107]. In our previous study, which involved proteomics analysis followed by real-time PCR analysis, we observed that CRMP4 and Crmp4 mRNA expression in the AVPV is sex-dependent during the critical period of sexual differentiation. In addition, prenatal testosterone propionate (TP) treatment increased the expression of Crmp4 mRNA during the critical period in females [106]. Moreover, the number of dopaminergic neurons in the nucleus was influenced by Crmp4 deletion in females, suggesting that CRMP4 plays a sex-dependent role in the regulation of dopaminergic neuronal death or survival in this region [106]. However, the relationship between decreases in the number of dopaminergic neurons in the AVPV and ASD-like phenotypes in Crmp4-KO females remains uncertain.
Ferri et al. [108] argued that the male predominance of ASD may be associated with interactions between risk genes, which may not be sex-specific themselves, and sex-specific hormonal or immune-related pathways. Although several candidate hormones have been proposed, it has been suggested that androgens are associated with such differences because it is well known that natural secretion of androgens from the testes during the prenatal period contributes to the increased risk of ASD in males (i.e., prenatal sex steroid theory) [109,110,111,112,113,114]. Accumulating evidence has demonstrated the important role of prenatal androgens in many aspects of neural network formation through their effects on developing neurons and glia–neuron interactions [115,116]. In these studies, it is reported that prostaglandin and gonadal steroids including T and estradiol converted from T by aromatase influence synaptogenesis via their effects on developing glial cells, astrocytes and microglia. In an intensive post mortem study, Werling et al. [117] observed increased expression of astrocyte and microglia marker genes in the brains of male patients with ASD, suggesting that interactions between glial cells and neurons may be involved in sex-based differences in ASD phenotypes. Although the mechanisms underlying the pathogenesis of ASD and the target cells of androgens and/or estrogens converted from androgens remain unclear, perinatal androgen exposure may contribute to the sexually dimorphic pathophysiology of ASD. According to the prenatal sex steroid theory of autism, loss of the suppressive role of CRMP4 in Crmp4-KO mice in neuronal development may exaggerate the promotive effect of prenatal sex steroids, androgens and/or estrogens converted from androgens secreted from the testes, on neuronal network development, thereby resulting in male-biased ASD-like phenotypes.
9. Conclusions
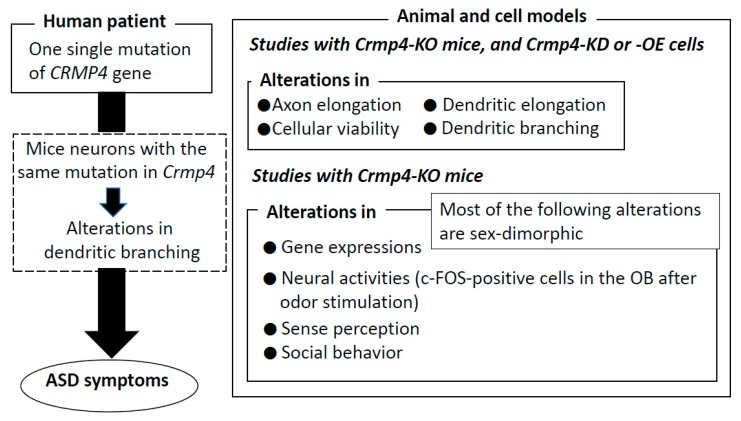
CRMPs are known to regulate various aspect of neural development, playing key roles in neurodevelopmental disorders. As summarized in Figure 2, a previous whole-exosome sequencing study identified a single mutation of the Crmp4 gene in a patient with ASD. Neurons from Crmp4-KO mice or neurons transfected with the mutation observed in the patient with ASD exhibit alterations in dendritic branching and/or extension (Figure 1). In addition, axonal elongation and cell viability are affected in Crmp4-KO mice, and in Crmp4-KD or -OE cells. Crmp4-KO mice also exhibit alterations in the expression of multiple genes contributing to glutamatergic and GABAergic neurotransmission, and most of these differences are sex- and region-specific. Single odorant stimulation induces hyperactivity (i.e., an increase in the number of c-Fos-positive cells) in the OB of Crmp4-KO pups. Furthermore, male Crmp4-KO mice exhibit more severe social and sensory deficits than females. Since most of their ASD-like phenotypes are sexually dimorphic, Crmp4-KO mice may represent a powerful model for investigating the pathogenesis of ASD and the prenatal sex steroid theory of autism in addition to Crmp4-KO mice possibly providing an animal model for investigating some other developmental disorders including ADHD and learning disabilities associated with sensory processing issues.
Figure 2.
Summary of features associated with deficiency, overexpression, and mutation of Crmp4. Crmp4: collapsing response mediator protein; Crmp4-KO: Crmp4-knock out; Crmp4-KD: Crmp4-knockdown, Crmp4-OE: Crmp4-overexpression; OB: olfactory bulb; ASD: autism spectrum disorder.
Acknowledgments
The author expresses sincere thanks to Y. Goshima, M. Nishihara, N. Yamashita, T. Iwakura, A. Tsutiya, Y. Nakano, A. Kitsu, K. Sato, M. Sakou, H. Watanabe, A. Kawahara, T. Kawachi, and S. Noma for their cooperation.
Abbreviations
| ADHD | attention-deficit/hyperactivity disorder |
| AMPA | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ASD | autism spectrum disorder |
| AVPV | anteroventral periventricular nucleus |
| CRMP | collapsin response mediator protein |
| DRP | dihydropyrimidase; DPYSL3, dihydropyrimidase-like 3 |
| EA | ethyl acetate |
| EPL | external plexiform layer |
| FMR1 | fragile X mental retardation 1 gene |
| GCL | granule cell layer |
| GL | glomerular layer |
| GluR1 | glutamate receptor 1 |
| GluT1 | glutamate transporter 1 |
| KO | knockout |
| MAI | myelin-associated inhibitor |
| MCL | mitral cell layer |
| NMDA | N-methyl-D-aspartate |
| OB | olfactory bulb |
| RT | room temperature |
| TERT-tg mice | telomerase reverse transcriptase-overexpressing transgenic mice |
| TOAD-64 | turned on after division 64 |
| TUC-4 | TOAD-64/Ulip-1/CRMP4 |
| Ulip-1 | Ulip-1 |
| UV | ultrasonic vocalization |
| vGluT1 | vesicular glutamate transporter 1 |
| WT | wild type |
Funding
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (JSPS; Grant Numbers 16K07034), and partially supported by Research Center for Biomedical Engineering in Toyo University.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Charrier E., Reibel S., Rogemond V., Aguera M., Thomasset N., Honnorat J. Collapsin response mediator proteins (CRMPs): Involvement in nervous system development and adult neurodegenerative disorders. Mol. Neurol. 2003;28:51–64. doi: 10.1385/MN:28:1:51. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E.F., Strittmatter S.M. The CRMP family of proteins and their role in Sema3A signaling. Adv. Exp. Med. Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quach T.T., Honnorat J., Kolattukudy P.E., Khanna R., Duchemin A.M. CRMPs: Critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol. Psychiatry. 2015;20:1037–1045. doi: 10.1038/mp.2015.77. [DOI] [PubMed] [Google Scholar]
- 4.Hensley K., Venkova K., Christov A., Gunning W., Park J. Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita N., Takahashi A., Takao K., Yamamoto T., Kolattukudy P., Miyakawa T., Goshima Y. Mice lacking collapsin response mediator protein 1 manifest hyperactivity, impaired learning and memory, and impaired prepulse inhibition. Front. Behav. Neurosci. 2013;7:216. doi: 10.3389/fnbeh.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Joo J., Nah S.S., Kim J.W., Kim H.K., Kwon J.T., Lee H.Y., Kim Y.O., Kim H.J. Changes in Dpysl2 expression are associated with prenatally stressed rat offspring and susceptibility to schizophrenia in humans. Int. J. Mol. Med. 2015;35:1574–1586. doi: 10.3892/ijmm.2015.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham X., Song G., Lao S., Goff L., Zhu H., Valle D., Avramopoulos D. The DPYSL2 gene connects mTOR and schizophrenia. Transl. Psychiatry. 2016;6:e933. doi: 10.1038/tp.2016.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsutiya A., Nakano Y., Hansen-Kiss E., Kelly B., Nishihara M., Goshima Y., Corsmeier D., White P., Herman G.E., Ohtani-Kaneko R. Human CRMP4 mutation and disrupted Crmp4 expression in mice are associated with ASD characteristics and sexual dimorphism. Sci. Rep. 2017;7:16812. doi: 10.1038/s41598-017-16782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y., Ohshima T., Sasaki Y., Suzuki H., Yanai S., Yamashita N., Nakamura F., Takei K., Ihara Y., Mikoshiba K., et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 10.Toba J., Nikkuni M., Ishizeki M., Yoshii A., Watamura N., Inoue T., Ohshima T. PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem. Biophys. Res. Commun. 2016;473:1039–1044. doi: 10.1016/j.bbrc.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim A.E., Kang P., Bucelli R.C., Ferguson C.J., Schmidt R.E., Varadhachary A.S., Day G.S. Autoimmune encephalitis with multiple autoantibodies: A diagnostic and therapeutic challenge. Neurologist. 2018;23:55–59. doi: 10.1097/NRL.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa H., Ohtani-Kaneko R., Naiki M., Okada T., Masuko K., Yudoh K., Suematsu N., Okamoto K., Nishioka K., Kato T. Involvement of post-translational modification of neuronal plasticity-related proteins in hyperalgesia revealed by a proteomic analysis. Proteomics. 2008;8:1706–1719. doi: 10.1002/pmic.200700928. [DOI] [PubMed] [Google Scholar]
- 13.Piekarz A.D., Due M.R., Khanna M., Wang B., Ripsch M.S., Wang R., Meroueh S.O., Vasko M.R., White F.A., Khanna R. CRMP-2 peptide mediated decrease of high and low voltage-activated calcium channels, attenuation of nociceptor excitability, and anti-nociception in a model of AIDS therapy-induced painful peripheral neuropathy. Mol. Pain. 2012;8:54. doi: 10.1186/1744-8069-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada S., Matsuura W., Takano M., Tokuyama S. Proteomic profiling in the spinal cord and sciatic nerve in a global cerebral ischemia-induced mechanical allodynia mouse model. Biol. Pharm. Bull. 2016;39:230–238. doi: 10.1248/bpb.b15-00647. [DOI] [PubMed] [Google Scholar]
- 15.Lawal M.F., Olotu F.A., Agoni C., Soliman M.E. Exploring the C-Terminal Tail Dynamics: Structural and Molecular Perspectives into the Therapeutic Activities of Novel CRMP-2 Inhibitors, Naringenin and Naringenin-7-O-glucuronide, in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2018;15:e1800437. doi: 10.1002/cbdv.201800437. [DOI] [PubMed] [Google Scholar]
- 16.Kolodkin A.L., Matthes D.J., O’Connor T.P., Patel N.H., Admon A., Bentley D., Goodman C.S. Fasciclin IV: Sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y., Raible D., Raper J.A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-L. [DOI] [PubMed] [Google Scholar]
- 18.Raper J.A. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 2000;10:88–94. doi: 10.1016/S0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 19.Fenstermaker V., Chen Y., Ghosh A., Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J. Neurobiol. 2004;58:403–412. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- 20.Pascual M., Pozas E., Soriano E. Role of class 3 semaphorins in the development and maturation of the septohippocampal pathway. Hippocampus. 2005;15:184–202. doi: 10.1002/hipo.20040. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y. Semaphorin signaling in vertebrate neural circuit assembly. Front. Mol. Neurosci. 2012;5:71. doi: 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goshima Y., Nakamura F., Strittmatter P., Strittmatter S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 23.Minturn J.E., Fryer H.J., Geschwind D.H., Hockfield S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J. Neurosci. 1995;15:6757–6766. doi: 10.1523/JNEUROSCI.15-10-06757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minturn J.E., Geschwind D.H., Fryer H.J., Hockfield S. Early postmitotic neurons transiently express TOAD-64, a neural specific protein. J. Comp. Neurol. 1995;355:369–379. doi: 10.1002/cne.903550304. [DOI] [PubMed] [Google Scholar]
- 25.Byk T., Dobransky T., Cifuentes-Diaz C., Sobel A. Identification and molecular characterization of Unc-33-like phosphoprotein (Ulip), a putative mammalian homolog of the axonal guidance-associated unc-33 gene product. J. Neurosci. 1996;16:688–701. doi: 10.1523/JNEUROSCI.16-02-00688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamajima N., Matsuda K., Sakata S., Tamaki N., Sasaki M., Nonaka M. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene. 1996;180:157–163. doi: 10.1016/S0378-1119(96)00445-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita N., Uchida Y., Ohshima T., Hirai S., Nakamura F., Taniguchi M., Mikoshiba K., Honnorat J., Kolattukudy P., Thomasset N., et al. Collapsin response mediator protein 1 mediates reelin signaling in cortical neuronal migration. J. Neurosci. 2006;26:13357–13362. doi: 10.1523/JNEUROSCI.4276-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alabed Y.Z., Poolm M., Tone S.O., Sutherland C., Fournier A.E. GSK3 beta regulates myelin-dependent axon outgrowth inhibition through CRMP4. J. Neurosci. 2010;30:5635–5643. doi: 10.1523/JNEUROSCI.6154-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charrier E., Mosinger B., Meissirel C., Aguera M., Rogemond V., Reibel S., Salin P., Chounlamountri N., Perrot V., Belin M.F., et al. Transient alterations in granule cell proliferation, apoptosis and migration in postnatal developing cerebellum of CRMP1−/− mice. Genes Cells. 2006;11:1337–1352. doi: 10.1111/j.1365-2443.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita N., Morita A., Uchida Y., Nakamura F., Usui H., Ohshima T., Taniguchi M., Honnorat J., Thomasset N., Takei K., et al. Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J. Neurosci. 2007;27:12546–12554. doi: 10.1523/JNEUROSCI.3463-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su K.Y., Chien W.L., Fu W.M., Yu I.S., Huang H.P., Huang P.H., Lin S.R., Shih J.Y., Lin Y.L., Hsueh Y.P., et al. Mice deficient in collapsin response mediator protein-1 exhibit impaired long-term potentiation and impaired spatial learning and memory. J. Neurosci. 2007;27:2513–2524. doi: 10.1523/JNEUROSCI.4497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita N., Goshima Y. Collapsin response mediator proteins regulate neuronal development and plasticity by switching their phosphorylation status. Mol. Neurobiol. 2012;45:234–246. doi: 10.1007/s12035-012-8242-4. [DOI] [PubMed] [Google Scholar]
- 33.Arimura N., Inagaki N., Chihara K., Ménager C., Nakamura N., Amano M., Iwamatsu A., Goshima Y., Kaibuchi K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J. Biol. Chem. 2000;275:23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- 34.Arimura N., Ménager C., Kawano Y., Yoshimura T., Kawabata S., Hattori A., Fukata Y., Amano M., Goshima Y., Inagaki M., et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Cole A.R., Causeret F., Yadirgi G., Hastie C.J., McLauchlan H., McManus E.J., Hernández F., Eickholt B.J., Nikolic M., Sutherland C. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J. Biol. Chem. 2006;281:16591–16598. doi: 10.1074/jbc.M513344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alabed Y.Z., Pool M., Ong Tone S., Fournier A.E. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J. Neurosci. 2007;27:1702–1711. doi: 10.1523/JNEUROSCI.5055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka H., Morimura R., Ohshima T. Dpysl2 (CRMP2) and Dpysl3 (CRMP4) phosphorylation by Cdk5 and DYRK2 is required for proper positioning of Rohon-Beard neurons and neural crest cells during neurulation in zebrafish. Dev. Biol. 2012;370:223–236. doi: 10.1016/j.ydbio.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Morimura R., Nozawa K., Tanaka H., Ohshima T. Phosphorylation of Dpsyl2 (CRMP2) and Dpsyl3 (CRMP4) is required for positioning of caudal primary motor neurons in the zebrafish spinal cord. Dev. Neurobiol. 2013;73:911–920. doi: 10.1002/dneu.22117. [DOI] [PubMed] [Google Scholar]
- 40.Kowara R., Chen Q., Milliken M., Chakravarthy B. Calpain-mediated truncation of dihydropyrimidinase-like 3 protein (DPYSL3) in response to NMDA and H2O2 toxicity. J. Neurochem. 2005;95:466–474. doi: 10.1111/j.1471-4159.2005.03383.x. [DOI] [PubMed] [Google Scholar]
- 41.Kowara R., Moraleja K.L., Chakravarthy B. Involvement of nitric oxide synthase and ROS-mediated activation of L-type voltage-gated Ca2+ channels in NMDA-induced DPYSL3 degradation. Brain Res. 2006;1119:40–49. doi: 10.1016/j.brainres.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 42.Kowara R., Moraleja K.L., Chakravarthy B. PLA(2) signaling is involved in calpain-mediated degradation of synaptic dihydropyrimidinase-like 3 protein in response to NMDA excitotoxicity. Neurosci. Lett. 2008;430:197–202. doi: 10.1016/j.neulet.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Liu W., Zhou X.W., Liu S., Hu K., Wang C., He Q., Li M. Calpain-truncated CRMP-3 and -4 contribute to potassium deprivation-induced apoptosis of cerebellar granule neurons. Proteomics. 2009;9:3712–3728. doi: 10.1002/pmic.200800979. [DOI] [PubMed] [Google Scholar]
- 44.Quinn C.C., Chen E., Kinjo T.G., Kelly G., Bell A.W., Elliott R.C., McPherson P.S., Hockfield S. TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J. Neurosci. 2003;23:2815–2823. doi: 10.1523/JNEUROSCI.23-07-02815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuasa-Kawada J., Suzuki R., Kano F., Ohkawara T., Murata M., Noda M. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur. J. Neurosci. 2003;17:2329–2343. doi: 10.1046/j.1460-9568.2003.02664.x. [DOI] [PubMed] [Google Scholar]
- 46.Tan M., Cha C., Ye Y., Zhang J., Li S., Wu F., Gong S., Guo G. CRMP4 and CRMP2 interact to coordinate cytoskeleton dynamics, regulating growth cone development and axon elongation. Neural. Plast. 2015:947423. doi: 10.1155/2015/947423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J. Neurosci. Res. 2002;70:327–334. doi: 10.1002/jnr.10387. [DOI] [PubMed] [Google Scholar]
- 48.Cnops L., Hu T.T., Burnat K., Van der Gucht E., Arckens L. Age-dependent alterations in CRMP2 and CRMP4 protein expression profiles in cat visual cortex. Brain Res. 2006;1088:109–119. doi: 10.1016/j.brainres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Tsutiya A., Ohtani-Kaneko R. Postnatal alteration of collapsin response mediator protein 4 mRNA expression in the mouse brain. J Anat. 2012;221:341–351. doi: 10.1111/j.1469-7580.2012.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koide T., Aleksic B., Ito Y., Usui H., Yoshimi A., Inada T., Suzuki M., Hashimoto R., Takeda M., Iwata N., et al. A two-stage case-control association study of the dihydropyrimidinase-like 2 gene (DPYSL2) with schizophrenia in Japanese subjects. J. Hum. Genet. 2010;55:469–472. doi: 10.1038/jhg.2010.38. [DOI] [PubMed] [Google Scholar]
- 51.Bader V., Tomppo L., Trossbach S.V., Bradshaw N.J., Prikulis I., Leliveld S.R., Lin C.Y., Ishizuka K., Sawa A., Ramos A., et al. Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits. Hum. Mol. Genet. 2012;21:4406–4418. doi: 10.1093/hmg/dds273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins-de-Souza D., Cassoli J.S., Nascimento J.M., Hensley K., Guest P.C., Pinzon-Velasco A.M., Turck C.W. The protein interactome of collapsin response mediator protein-2 (CRMP2/DPYSL2) reveals novel partner proteins in brain tissue. Proteomics Clin. Appl. 2015;9:817–831. doi: 10.1002/prca.201500004. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Pham X., Zhang L., Chen P.L., Burzynski G., McGaughey D.M., He S., McGrath J.A., Wolyniec P., Fallin M.D., et al. Functional variants in DPYSL2 sequence increase risk of schizophrenia and suggest a link to mTOR signaling. G3. 2014;5:61–72. doi: 10.1534/g3.114.015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura H., Yamashita N., Kimura A., Kimura Y., Hirano H., Makihara H., Kawamoto Y., Jitsuki-Takahashi A., Yonezaki K., Takase K., et al. Comprehensive behavioral study and proteomic analyses of CRMP2-deficient mice. Genes Cells. 2016;21:1059–1079. doi: 10.1111/gtc.12403. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura H., Takahashi-Jitsuki A., Makihara H., Asano T., Kimura Y., Nakabayashi J., Yamashita N., Kawamoto Y., Nakamura F., Ohshima T., et al. Proteome and behavioral alterations in phosphorylation-deficient mutant Collapsin Response Mediator Protein2 knock-in mice. Neurochem. Int. 2018;119:207–217. doi: 10.1016/j.neuint.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H., Kang E., Wang Y., Yang C., Yu H., Wang Q., Chen Z., Zhang C., Christian K.M., Song H., et al. Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat. Commun. 2016;1:7. doi: 10.1038/ncomms11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller B.H., Zeier Z., Xi L., Lanz T.A., Deng S., Strathmann J., Willoughby D., Kenny P.J., Elsworth J.D., Lawrence M.S., et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E., et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsutiya A., Watanabe H., Nakano Y., Nishihara M., Goshima Y., Ohtani-Kaneko R. Deletion of collapsin response mediator protein 4 results in abnormal layer thickness and elongation of mitral cell apical dendrites in the neonatal olfactory bulb. J. Anat. 2016;228:792–804. doi: 10.1111/joa.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diagnostic and Statistical Manual of Mental Disorders. 5th ed (DSM-V) American Psychiatric Association; Philadelphia, PA, USA: 2013. [Google Scholar]
- 61.Tsutiya A., Nishihara M., Goshima Y., Ohtani-Kaneko R. Mouse pups lacking collapsin response mediator protein 4 manifest impaired olfactory function and hyperactivity in the olfactory bulb. Eur. J. Neurosci. 2015;42:2335–2345. doi: 10.1111/ejn.12999. [DOI] [PubMed] [Google Scholar]
- 62.Takarae Y., Sablich S.R., White S.P., Sweeney J.A. Neurophysiological hyperresponsivity to sensory input in autism spectrum disorders. J. Neurodev. Disord. 2016;8:29. doi: 10.1186/s11689-016-9162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takarae Y., Sweeney J. Neural Hyperexcitability in Autism Spectrum Disorders. Brain Sci. 2017;7:129. doi: 10.3390/brainsci7100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spencer C.M., Alekseyenko O., Hamilton S.M., Thomas A.M., Serysheva E., Yuva-Paylor L.A., Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: Genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C.X., Cantu D.A., Mantri S.S., Zeiger W.A., Goel A., Portera-Cailliau C. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J. Neurosci. 2017;37:6475–6487. doi: 10.1523/JNEUROSCI.0651-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ethridge L.E., White S.P., Mosconi M.W., Wang J., Byerly M.J., Sweeney J.A. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl. Psychiatry. 2016;6:e787. doi: 10.1038/tp.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmeisser M.J., Ey E., Wegener S., Bockmann J., Stempel A.V., Kuebler A., Janssen A.L., Udvardi P.T., Shiban E., Spilker C., et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 68.Won H., Lee H.R., Gee H.Y., Mah W., Kim J.I., Lee J., Ha S., Chung C., Jung E.S., Cho Y.S., et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 69.Ko H.G., Oh S.B., Zhuo M., Kaang B.K. Reduced acute nociception and chronic pain in Shank2−/− mice. Mol. Pain. 2016;4:12. doi: 10.1177/1744806916647056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scattoni M.L., Crawley J., Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van der Gucht E., Clerens S., Cromphout K., Vandesande F., Arckens L. Differential expression of c-fos in subtypes of GABAergic cells following sensory stimulation in the cat primary visual cortex. Eur. J. Neurosci. 2002;16:1620–1626. doi: 10.1046/j.1460-9568.2002.02226.x. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan S.L., Ressler K.J., Buck L.B. Spatial patterning and information coding in the olfactory system. Curr. Opin. Genet. Dev. 1995;5:516–523. doi: 10.1016/0959-437X(95)90057-N. [DOI] [PubMed] [Google Scholar]
- 73.Mombaerts P., Wang F., Dulac C., Chao S.K., Nemes A., Mendelsohn M., Edmondson J., Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/S0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 74.Salcedo E., Zhang C., Kronberg E., Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem. Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- 75.Pathania M., Davenport E.C., Muir J., Sheehan D.F., López-Doménech G., Kittler J.T. The autism and schizophrenia associated gene CYFIP1 is critical for the maintenance of dendritic complexity and the stabilization of mature spines. Transl. Psychiatry. 2014;4:e374. doi: 10.1038/tp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagaoka A., Takehara H., Hayashi-Takagi A., Noguchi J., Ishii K., Shirai F., Yagishita S., Akagi T., Ichiki T., Kasai H. Abnormal intrinsic dynamics of dendritic spines in a fragile X syndrome mouse model in vivo. Sci. Rep. 2016;6:26651. doi: 10.1038/srep26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng N., Alshammari F., Hughes E., Khanbabaei M., Rho J.M. Dendritic overgrowth and elevated ERK signaling during neonatal development in a mouse model of autism. PLoS ONE. 2017;12:e0179409. doi: 10.1371/journal.pone.0179409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montani C., Ramon-Brossier M., Ponzoni L., Gritti L., Cwetsch A.W., Braida D., Saillour Y., Terragni B., Mantegazza M., Sala M., et al. The X-linked intellectual disability protein IL1RAPL1 regulates dendrite complexity. J. Neurosci. 2017;37:6606–6627. doi: 10.1523/JNEUROSCI.3775-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niisato E., Nagai J., Yamashita N., Abe T., Kiyonari H., Goshima Y., Ohshima T. CRMP4 suppresses apical dendrite bifurcation of CA1 pyramidal neurons in the mouse hippocampus. Dev. Neurobiol. 2012;72:1447–1457. doi: 10.1002/dneu.22007. [DOI] [PubMed] [Google Scholar]
- 80.Niisato E., Nagai J., Yamashita N., Nakamura F., Goshima Y., Ohshima T. Phosphorylation of CRMP2 is involved in proper bifurcation of the apical dendrite of hippocampal CA1 pyramidal neurons. Dev. Neurobiol. 2013;73:142–151. doi: 10.1002/dneu.22048. [DOI] [PubMed] [Google Scholar]
- 81.Cha C., Zhang J., Ji Z., Tan M., Li S., Wu F., Chen K., Gong S., Guo G., Lin H. CRMP4 regulates dendritic growth and maturation via the interaction with actin cytoskeleton in cultured hippocampal neurons. Brain Res. Bull. 2016;124:286–294. doi: 10.1016/j.brainresbull.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Takaya R., Nagai J., Piao W., Niisato E., Nakabayashi T., Yamazaki Y., Nakamura F., Yamashita N., Kolattukudy P., Goshima Y., et al. CRMP1 and CRMP4 are required for proper orientation of dendrites of cerebral pyramidal neurons in the developing mouse brain. Brain Res. 2017;1655:161–167. doi: 10.1016/j.brainres.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Eissa N., Al-Houqani M., Sadeq A., Ojha S.K., Sasse A., Sadek B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 2018;12:304. doi: 10.3389/fnins.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horder J., Petrinovic M.M., Mendez M.A., Bruns A., Takumi T., Spooren W., Barker G.J., Künnecke B., Murphy D.G. Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry. 2018;8:106. doi: 10.1038/s41398-018-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carlson C.G. Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012;100:850–854. doi: 10.1016/j.pbb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim K.C., Cho K.S., Yang S.M., Gonzales E.L., Valencia S., Eun P.H., Choi C.S., Mabunga D.F., Kim J.W., Noh J.K., et al. Sex differences in autism-like behavioral phenotypes and postsynaptic receptors expression in the prefrontal cortex of TERT transgenic mice. Biomol. Ther. 2017;25:374–382. doi: 10.4062/biomolther.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fung L.K., Hardan A.Y. Developing medications targeting glutamatergic dysfunction in autism: Progress to date. CNS Drugs. 2015;29:453–463. doi: 10.1007/s40263-015-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silverman J.L., Tolu S.S., Barkan C.L., Crawley J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta M.V., Gandal M.J., Siegel S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE. 2011;6:e26077. doi: 10.1371/journal.pone.0026077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gatto C.L., Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front. Synaptic. Neurosci. 2010;2:4. doi: 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubenstein J.L. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr. Opin. Neurol. 2010;23:118–123. doi: 10.1097/WCO.0b013e328336eb13. [DOI] [PubMed] [Google Scholar]
- 92.Jamain S., Betancur C., Quach H., Philippe A., Fellous M., Giros B., Gillberg C., Leboyer M., Bourgeron T. Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naaijen J., Bralten J., Poelmans G., Glennon J.C., Franke B., Buitelaar J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry. 2017;7:e999. doi: 10.1038/tp.2016.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Werling D.M., Geschwind D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013;26:146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Werling D.M., Geschwind D.H. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol. Autism. 2015;6:27. doi: 10.1186/s13229-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubenstein E., Wiggins L.D., Lee L.C. A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. J. Dev. Phys. Disabil. 2015;27:119–139. doi: 10.1007/s10882-014-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen C., Van Horm J.D. GENDAAR Research Consortium. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 2017;11:38–61. doi: 10.1007/s11682-015-9504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu J., He X., Yao D., Li Z., Li H., Zhao Z. A sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with autism spectrum disorders in a Chinese Han cohort. Behav. Brain Funct. 2011;7:13. doi: 10.1186/1744-9081-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Landini M., Merelli I., Raggi M.E., Galluccio N., Ciceri F., Bonfanti A., Camposeo S., Massagli A., Villa L., Salvi E., et al. Association Analysis of Noncoding Variants in Neuroligins 3 and 4X Genes with Autism Spectrum Disorder in an Italian Cohort. Int. J. Mol. Sci. 2016;17:1765. doi: 10.3390/ijms17101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ey E., Torquet N., Le Sourd A.M., Leblond C.S., Boeckers T.M., Faure P., Bourgeron T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav. Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 101.Kim K.C., Kim P., Go H.S., Choi C.S., Park J.H., Kim H.J., Jeon S.J., Dela Pena I.C., Han S.H., Cheong J.H., et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neurochem. 2013;124:832–843. doi: 10.1111/jnc.12147. [DOI] [PubMed] [Google Scholar]
- 102.Schneider T., Roman A., Basta-Kaim A., Kubera M., Budziszewska B., Schneider K., Przewłockia R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Konopko M.A., Densmore A.L., Krueger B.K. Sexually Dimorphic Epigenetic Regulation of Brain-Derived Neurotrophic Factor in Fetal Brain in the Valproic Acid Model of Autism Spectrum Disorder. Dev. Neurosci. 2017;39:507–518. doi: 10.1159/000481134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicolini C., Fahnestock M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018;299:217–227. doi: 10.1016/j.expneurol.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 105.Ju A., Hammerschmidt K., Tantra M., Krueger D., Brose N., Ehrenreich H. Juvenile manifestation of ultrasound communication deficits in the neuroligin-4 null mutant mouse model of autism. Behav. Brain Res. 2014;270:159–164. doi: 10.1016/j.bbr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 106.Iwakura T., Sakoh M., Tsutiya A., Yamashita N., Ohtani A., Tsuda M.C., Ogawa S., Tsukahara S., Nishihara M., Shiga T., et al. Collapsin response mediator protein 4 affects the number of tyrosine hydroxylase-immunoreactive neurons in the sexually dimorphic nucleus in female mice. Dev. Neurobiol. 2013;73:502–517. doi: 10.1002/dneu.22076. [DOI] [PubMed] [Google Scholar]
- 107.Sumida H., Nishizuka M., Kano Y., Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci. Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-R. [DOI] [PubMed] [Google Scholar]
- 108.Ferri S.L., Abel T., Brodkin E.S. Sex differences in autism spectrum disorder: A review. Curr. Psychiatry Rep. 2018;20:9. doi: 10.1007/s11920-018-0874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Knickmeyer R.C., Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J. Child. Neurol. 2006;21:825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- 110.Auyeung B., Baron-Cohen S., Ashwin E., Knickmeyer R., Taylor K., Hackett G. Fetal testosterone and autistic traits. Br. J. Psychol. 2009;100:1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- 111.Auyeung B., Taylor K., Hackett G., Baron-Cohen S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol. Autism. 2010;1:11. doi: 10.1186/2040-2392-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Auyeung B., Ahluwalia J., Thomson L., Taylor K., Hackett G., O’Donnell K.J., Baron-Cohen S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol. Autism. 2012;3:17. doi: 10.1186/2040-2392-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baron-Cohen S., Auyeung B., Nørgaard-Pedersen B., Hougaard D.M., Abdallah M.W., Melgaard L., Cohen A.S., Chakrabarti B., Ruta L., Lombardo M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cherskov A., Pohl A., Allison C., Zhang H., Payne R.A., Baron-Cohen S. Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Transl. Psychiatry. 2018;8:136. doi: 10.1038/s41398-018-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mong J.A., Glaser E., McCarthy M.M. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J. Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCarthy M.M., Wright C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry. 2017;81:402–410. doi: 10.1016/j.biopsych.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Werling D.M., Parikshak N.N., Geschwind D.H. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun. 2016;7:10717. doi: 10.1038/ncomms10717. [DOI] [PMC free article] [PubMed] [Google Scholar]