Abstract
Aging of the heart is associated with a blunted response to sympathetic stimulation, reduced contractility, and increased propensity for arrhythmias, with the risk of sudden cardiac death significantly increased in the elderly population. The altered cardiac structural and functional phenotype, as well as age-associated prevalent comorbidities including hypertension and atherosclerosis, predispose the heart to atrial fibrillation, heart failure, and ventricular tachyarrhythmias. At the cellular level, perturbations in mitochondrial function, excitation-contraction coupling, and calcium homeostasis contribute to this electrical and contractile dysfunction. Major determinants of cardiac contractility are the intracellular release of Ca2+ from the sarcoplasmic reticulum by the ryanodine receptors (RyR2), and the following sequestration of Ca2+ by the sarco/endoplasmic Ca2+-ATPase (SERCa2a). Activity of RyR2 and SERCa2a in myocytes is not only dependent on expression levels and interacting accessory proteins, but on fine-tuned regulation via post-translational modifications. In this paper, we review how aberrant changes in intracellular Ca2+ cycling via these proteins contributes to arrhythmogenesis in the aged heart.
Keywords: calcium signaling, cardiac arrhythmia, ageing, ryanodine receptor, sarco/endoplasmic Ca2+-ATPase
1. Introduction
Heart disease remains the leading cause of death in the US [1], in part due to the aging population. There is a significantly increased incidence of cardiovascular disease and arrhythmia in the elderly [2,3], and over 130 million adults are projected to have a form of cardiac disease by 2035, with costs to the economy expected to top $1 trillion [1]. An understanding of the molecular and cellular mechanisms underlying arrhythmogenesis in aging remains paramount for the development of targeted therapeutics that may reduce this burden.
At the cellular level, the cardiac disease phenotype is a culmination of altered response to β-adrenergic stimulation [4,5,6], mitochondrial dysfunction [7], increased reactive oxygen species (ROS) emission [8,9], and dysregulated Ca2+ homeostasis [10]. This causes impaired systolic and diastolic function, impaired relaxation, and cardiac arrhythmia.
Contraction and relaxation of the cardiomyocyte is driven by precise, coordinated excitation-contraction coupling, which is the process linking electrical excitability and intracellular Ca2+ homeostasis. Triggering action potentials depolarize the cell and open L-type Ca2+ channels (LTCCs) at the sarcolemma, which initiates Ca2+ release from the sarcoplasmic reticulum (SR) Ca2+ release channels and the cardiac ryanodine receptors (RyR2s). Global increases in intracellular Ca2+ concentration ([Ca2+]i) activate contractile machinery, which initiates cardiac systole. Relaxation and diastole ensue when Ca2+ is extruded from the cytosol via the Na+/Ca2+ exchanger (NCX1, major cardiac isoform) and sequestered into the SR via the sarco/endoplasmic reticulum Ca2+-ATPase (SERCa2a, major cardiac isoform). Any perturbations in intracellular Ca2+ handling can, therefore, alter contractility and electrical stability of the heart. It is well established that excitation-contraction coupling becomes dysfunctional with age, which drives increased propensity for cardiac arrhythmias in the elderly population [10,11].
We and others have comprehensively reviewed the function, post-translational modifications, and role of excitation-contraction coupling proteins in the development of cardiac arrhythmia [12,13,14,15,16]. In this case, we provide a more concentrated focus on aberrant intracellular Ca2+ release and mechanisms of arrhythmogenesis that occur in the aged heart.
2. Intracellular Ca2+ Homeostasis in the Aged Heart
2.1. Ryanodine Receptor
Much of the intracellular Ca2+ required for cardiac contraction comes via the major intracellular SR Ca2+ release channel, RyR2 [17]. Within junctional SR, RyR2 channels are in close proximity of LTCCs to facilitate rapid and coordinated Ca2+ transport upon sarcolemma depolarization. A small LTCC-mediated influx of Ca2+ into the cytosol activates single clusters of RyR2 channels. The subsequent increase in local [Ca2+]i, known as a Ca2+ spark [18], activates other clusters of RyR2 channels and triggers a much larger Ca2+-induced Ca2+ release (CICR) from the SR across the cardiomyocyte [19]. The summation of Ca2+ sparks results in a global increase in [Ca2+]i, or Ca2+ transient, which initiates muscle contraction.
The open probability of RyR2 channels is finite, which leads to small and unsynchronized SR Ca2+ release events during diastole known as SR Ca2+ leak [20]. Some of this leak can be visualized as Ca2+ sparks, while some is effectively invisible due to current detection limits. Although leak plays an important physiological role in determining SR Ca2+ content, an enhanced leak can be detrimental to cardiac function [13,16,21]. Increased spontaneous Ca2+ leak during diastole promotes an untimely inward depolarizing current via NCX1, which leads to early and delayed afterdepolarizations (EADs and DADs, respectively) and the initiation of triggered activity. A reduction of SR Ca2+ release during systole also contributes to diminished contractile function, which predisposes the heart to arrhythmogenesis [14,21,22].
Leak is elevated in the failing heart [23], as well as in conditions characterized by gain-of-function mutations in proteins of the RyR2 macromolecular complex, such as catecholaminergic polymorphic ventricular tachycardia (CPVT) [24,25]. It is also evident that Ca2+ leak is elevated in the aging heart [9,26,27,28]. The propensity for diastolic Ca2+ sparks and waves was shown to be significantly increased in aged mice, while stabilization of interdomain interactions in RyR2 via application of therapeutic dantrolene could reduce excessive SR Ca2+ leak and attenuate these pro-arrhythmic events [28]. In aged rat ventricular myocytes, Zhu et al. 2005 reported a decreased Ca2+ transient amplitude and a reduced SR Ca2+ content, as well as an increased spontaneous Ca2+ spark frequency with a reduced average amplitude [27]. Single channel studies revealed that the open probability of RyR2 isolated from aged ventricular myocytes was increased, but with a shorter mean open time, which explains the increase in spark activity. The authors posited that posttranslational modifications of RyR2 may increase the sensitivity to activating Ca2+.
Dynamic and reversible posttranslational modifications of RyR2 are key to modulating channel open probability and grading SR Ca2+ release during changing metabolic demands. Phosphorylation of cardiac ion channels and proteins during β-adrenergic stimulation, including RyR2, is well established to increase positive chronotropic and inotropic effects on cardiac function [29,30]. Associated kinases of the RyR2 complex include protein kinase A (PKA), Ca2+/calmodulin-dependent kinase II (CaMKII), and associated phosphatases include protein phosphatase 1, 2A, and 2B (PP1, PP2A, and PP2B, respectively). The altered phosphorylation status of RyR2 and changes in the activity of associated kinases and phosphatases have long been implicated in aberrant Ca2+ cycling, and this has been well documented in many animal models of cardiac disease, as well as in humans [29,30]. Our current understanding is that both maximum phosphorylation and incomplete dephosphorylation of RyR2 can result in increased activity of the channel [30]. An augmented SR Ca2+ leak that is worsened by these modifications can initiate triggered activity and premature ventricular contractions (PVCs), which may degenerate into polymorphic/bidirectional ventricular tachycardia/fibrillation. Since β-adrenergic signaling is deranged in the aging heart [6], one might expect to see reduced PKA-mediated RyR2 phosphorylation. Cooper et al. (2013) reported unchanged RyR2 phosphorylation at PKA-specific sites Serine-2808 and Serine-2031 under basal conditions in aged ventricular myocytes from female rabbits [9], and there are limited data regarding phosphatase association and activity on RyR2 in cardiac aging. The relevance of PKA-mediated phosphorylation of RyR2 to an enhanced SR Ca2+ leak remains debated [21,31,32,33].
On the other hand, there is substantial evidence to support the role of CaMKII-mediated phosphorylation in modulating RyR2 channel function in both health and disease [34,35,36,37,38]. Cooper et al. (2013) also demonstrated that, in the presence of β-adrenergic agonist isoproterenol, RyR2 phosphorylation at CaMKII site Serine-2814 was significantly increased in ventricular myocytes from old rabbit hearts (four to six years old) in comparison to young (five to nine months old) [9]. A significantly increased activity of stress response kinase JNK2 was reported to activate CaMKII and, thus, upregulate RyR2-mediated diastolic SR Ca2+ leak in aged mouse atria (24–32 months old) [39]. Guo et al. (2014) observed increased RyR2 phosphorylation at CaMKII-specific site S2814 in aged mouse atrial myocytes, which increased aberrant intracellular waves and facilitated AF initiation [40]. This was in conjunction with increased oxidation of the channel.
As another possible posttranslational modification of RyR2, oxidation destabilizes interdomain interactions [41,42,43] and increases open probability of the channel [44,45]. Accelerated SR Ca2+ leak via oxidized RyR2s in diseased hearts results in diminished systolic Ca2+ release, due to substantial depletion of SR Ca2+ content during diastole. At faster pacing rates, this can lead to the occurrence of Ca2+-dependent action potential duration alternans. These beat-to-beat fluctuations can contribute as a substrate for arrhythmia [46,47,48,49]. Increased RyR2 activity, in conjunction with accelerated SERCa2a-mediated SR Ca2+ uptake during β-adrenergic stimulation, also enhances the propensity for the generation of proarrhythmic, diastolic Ca2+ waves [36,45,50,51,52,53,54,55]. Spontaneous Ca2+ waves that are large and/or fast enough can evoke NCX1 current that is large enough to reach a threshold for the generation of an extrasystolic action potential, which underlies triggered activity at the organ level [56]. We have previously demonstrated that the age-associated increase of ROS production by mitochondria in aged rabbit ventricular myocytes leads to thiol-oxidation of RyR2, which underlies channel hyperactivity [9]. The formation of advanced glycation end products (AGEs) on proteins is an additional posttranslational modification, first reported to modulate RyR2 in a model of chronic diabetes [57]. Glycation of RyR2 has also recently been documented in myocytes in aged mice (>20 months old) and atrial appendages of elderly patients (>75 years old) [58] and might contribute to enhanced SR Ca2+ leak. Increased Ca2+ leak due to an altered refractory period for RyR2-mediated SR Ca2+ release [51,59,60] contributes to the pathogenesis of triggered activity. Impaired refractoriness and reduced subsequent Ca2+ transient amplitude in aged myocytes may also facilitate the onset of cardiac alternans that can drive arrhythmogenesis in the elderly [46,47,48,49].
Expression levels of RyR2 in aging are generally reported as unchanged [9,27,61,62,63]. However, as large macromolecular complexes, RyR2 can modulate the altered expression and activity of multiple accessory proteins such as calmodulin (CaM), calsequestrin (CSQ2), triadin, and junctin [64,65]. Most studies to date report no changes in CSQ2 protein expression levels or transcriptional levels [27,62,66], but, more recently, significantly decreased CSQ2 protein levels were observed in aged human atrial myocytes (>75 years old) in conjunction with significantly reduced SERCa2a expression and SR Ca2+ content [67]. Reduced CSQ2-mediated buffering of Ca2+ within the SR has been reported to cause spontaneous activation of RyR2 [68,69]. The expression and function of other accessory proteins in cardiac aging remain to be explored.
2.2. Sarcoplasmic Reticulum Ca2+-ATP-ase
After intracellular Ca2+ release, [Ca2+]i must be sufficiently reduced for muscle relaxation. Sequestration of Ca2+ from the cytosol back into the SR occurs via SERCa2a, which is an ATP-dependent process that restores SR Ca2+ content. The affinity of SERCa2a for Ca2+ and, thus, its pumping activity is negatively regulated by association with inhibitory protein phospholamban (PLB). Phosphorylation of PLB by PKA or CaMKII relieves this inhibition, which enhances SERCa2a Ca2+ affinity and stimulates SR Ca2+ uptake [70]. An age-associated reduction in SERCA2a-mediated SR Ca2+ uptake is documented in most studies, but is not universal [9,61,71,72]. Reduced pump activity can be due to either reduced expression levels or reduced phosphorylation of the SERCa2a/PLB complex [61,62,66,70,73,74]. Since the SR Ca2+ load is a critical factor of cardiac alternans [46], depressed SERCa2a activity in cardiac aging can cause this phenomena, as well as triggered activity, due to increase Ca2+ extrusion via NCX1 [75].
Protein expression levels of SERCa2a in aged vs. young myocytes are reported as unchanged [9,53,74] or significantly reduced [66,76,77,78]. A decreased SR Ca2+ content and depressed Ca2+ transient amplitude was associated with a decrease in SERCa2a and CSQ2 levels in human atrial myocytes (>75 years old) [67]. The expression of PLB is purported to progressively increase with age [79], in which reduces the activity of SERCa2a that may be associated with increased pump inhibition. Schmidt et al. (2005) reported a 40% decrease in SERCa2a protein content in senescent myocardium of rats compared to adult (26 months old vs. 6 months old), with reduced activity [76]. Given that Ca2+ efflux into the SR in an ATP-dependent process, SERCa2a overexpression could increase energy requirements in the aged heart. Consumption of ATP by SERCa2a accounts for ~15% of cardiac energy usage [80] and the decreased energy reserve may also contribute to contractile dysfunction in the aged heart. Although SERCa2a overexpression could increase energy requirements of the aged heart, restoration of protein levels by adenoviral gene transfer was shown to normalize diastolic function in vivo, which restores the contractile reserve in aged rat myocardium [76]. Attenuating reduced SERCa2a-mediated Ca2+ buffering capacity by gene transfer of parvalbumin also improved diastolic function and force frequency relationship in aged rats [76,81].
As β-adrenergic signaling is deficient in the aged heart, this leads to a markedly reduced PKA-dependent phosphorylation of PLB [10,82]. Xu and Narayanan (1998) suggested that an age-related decline in SERCa2a activity may be attributed to altered CaMKII-mediated phosphorylation, given the reduced expression of CaMKII δ isoform in aged rats (26–28 months old) [61]. This would reduce endogenous CaMKII-mediated PLB/SERCa2a phosphorylation, which also reduces pump activity. Altered phosphatase activity may also modulate the phosphorylation status of the SERCa2a/PLB complex, with dephosphorylation of PLB regulated by PP1. Significantly elevated activity of PP1 has been reported in models of heart failure, which is attributable, in part, to reduced levels of its endogenous inhibitor protein I-1 [83,84,85,86,87]. It was shown in the aged mouse heart (20 months old) that inducible expression of constitutively active I-1 increased phosphorylation of PLB at Serine-16/Threonine-17, and this maneuver potentially offset enhanced CaMKII-mediated RyR2 phosphorylation, since incidences of arrhythmia did not increase with age [88].
Much like RyR2, SERCa2a is also susceptible to redox modification by free radicals and reactive oxygen species. Knyushko et al. (2005) reported significant accumulation of 3-nitrotyrosine in SERCa2a of aged rat hearts (26 months old) [89]. Declining activity of SERCa2a in aged rat ventricular myocytes could not be explained by differences in protein expression levels of the pump or of PLB, but increased oxidative damage of these proteins was indicated by reduced free sulfhydryl groups [90]. More direct evidence of this modification was shown by Qin et al. [91], whereby hydrogen-peroxide mediated oxidation of SERCa2a at Cysteine-674 contributed to depressed SERCA2a and impaired relaxation of ventricular myocytes in the senescent murine heart (21 months old). Adenoviral overexpression of SERCa2a with this residue mutated to a serine partially preserved SERCa2a activity during H2O2 exposure.
2.3. L-Type Ca2+ Channel
Influx of Ca2+ into the cytosol serves as the initiating step of intracellular Ca2+ release, which activates RyR2 channels by CICR. This occurs at the sarcolemma through LTCCs, which are voltage-dependent and activated during action potential. Regulation of LTCC activity is complex, whereby CaMKII-mediated phosphorylation of C-terminal bound CaM can lessen Ca2+-dependent inactivation. Furthermore, channels can be modulated by β-adrenergic stimulation via PKA. Altered activation and inactivation properties of LTCCs can widen the window of current, which increases the propensity of reactivation during late phases of action potential. This can enhance triggered activity in cardiac disease [92]. There are disparate results regarding expression and activity of LTCCs in aging, depending on sex, animal species, and the chamber of the heart [11].
A delayed activation of LTCCs has been suggested to contribute to increased action potential duration in aged rats (24–>27 months old) [93,94,95], as well as greater amplitude of transient outward K+ currents (Ito) [93]. Delayed inactivation and a reduced peak ICa density was reported in senescent rat ventricular myocytes in another study (>27 months old) [94]. In ventricular myocytes of aged vs. young male mice (24 months old vs. 7 months old), a significant reduction in peak ICa density as well as significantly slower Ca2+-dependent activation was demonstrated, but not in females [96]. Salameh et al. (2010) reported a significant reduction in LTCC maximal conductance and ICa per cell volume in aged rabbit ventricular myocytes (26 months old) [72]. This effect was compensated by a positive shift in steady state inactivation, which enhanced the late ICa component. This can induce higher SR Ca2+ loading. Decreased ICa has been observed in atria of aged dogs (>8 years old) [97,98,99,100].
In ventricular myocytes isolated from aged sheep hearts (>8 years old), Dibb et al. (2004) reported a larger peak inward ICa, as well as increased fractional SR Ca2+ release and a larger systolic Ca2+ transient [101]. No change in excitation-contraction coupling gain (coupling of LTCCs to the release of Ca2+ from the SR) was observed in this model, which is suggestive that changes in systolic Ca2+ transient arose via increased peak/integrated ICa rather than due to altered SR content or prolonged action potential duration. This increased ICa provided increased trigger for RyR2-mediated CICR, while maintaining SR Ca2+ content, to sustain cardiac output during increased vessel stiffness and demands of the aged myocardium.
Differences in atrial vs. ventricular LTCC activity with age are evident from a more recent paper by Clarke et al. (2017), whereby atrial myocytes from aged female sheep (>8 years old) showed a decreased peak ICa [102]. This served to offset an age-associated increase in SR Ca2+ content, while enhanced intracellular Ca2+ buffering in this model could explain the reduction in Ca2+ transient amplitude and the rate of decay of systolic Ca2+. Aged sheep are vulnerable for the development of AF, and this is associated with cardiac alternans [103].
Reduced expression levels of LTCCs can promote cardiac alternans because of reduced fidelity of coupling with RyR2 channels [104]. Herraiz-Martínez et al. 2015 reported diminished expression levels of LTCC pore forming subunit α1c and, thus, reduced ICa in aged human atrial myocytes (>75 years old) [67]. However, reduced protein expression levels do not always result in altered ICa. In a study of healthy and failing human hearts, similar ICa was demonstrated in heart failure despite a significant decrease in expression levels of α1c, which is likely due to enhanced phosphorylation of LTCCs [105]. In cardiac aging, defective β-adrenergic signaling may lead to reduced stimulation of ICa through PKA [4].
The fidelity of LTCC coupling with RyR2 channels and the efficiency of Ca2+ release initiation is not only dependent on expression levels, but on the distribution of channels within the myocyte. Primarily located in T-tubules in junctional SR, LTCCs form clusters that oppose clusters of RyR2 channels, and changes in T-tubule structure and function have been implicated in impaired contractility observed in cardiac disease [106,107,108,109]. In ventricular myocytes of aged mice (24 months old), Kong et al. (2018) recently reported reduced ICa density at the T-tubules, but not at the sarcolemma [110], which further highlights the complexity of LTCC regulation in the aging heart.
2.4. Na+/Ca2+ Exchanger
Although SERCa2a sequesters Ca2+ from the cytosol and replenishes SR Ca2+ stores, efflux of Ca2+ from the cytosol also occurs via NCX1, which is the main route of extrusion from the cardiomyocyte. Working in reversal mode during the early stage of action potential, intracellular Ca2+ influx ‘primes’ RyR2 channels and improves the efficiency of CICR [111]. During the late phases of action potential, NCX1 works in a forward mode to extrude Ca2+ from the cytosol. Given its electrogenic properties, NCX1 serves as a regulator of action potential during late phases of repolarization and decay of the intracellular Ca2+ transient. In the failing heart, upregulation of NCX1 in heart failure contributes to prolongation of action potential duration, reactivation of LTCCs, and the generation of triggered events [56,112,113]. Increased levels of intracellular [Na+] in heart failure also enhances reverse mode activity, which may increase [Ca2+]i and, thus, RyR2 activity. It is well established that aging also prolongs action potential and diastole [10], which implicates deranged NCX1 activity. However, reports of NCX1 expression levels and activity in aged myocardium vary.
Reduced expression levels of NCX1 were reported in the hearts of aged male rats and mice (24 months old) [114,115], while others demonstrated unchanged levels of the protein [62,72,116,117]. Mace et al. (2003) demonstrated that, under conditions of controlled [Ca2+] and [Na+], advancing age in ventricular myocytes of male rats (27–31 months old) was associated with increased forward NCX1 activity, which contributes to prolongation of action potential duration and arrhythmogenesis [116]. In ventricular myocytes, which are isolated from aged sedentary female rats (24 months old), both caffeine-induced Ca2+ transients and integrated NCX1 current were increased in comparison to young controls, contributing to diminished contractile function [118].
2.5. Sexual Dimorphism of Intracellular Ca2+ Release
While the risk for cardiovascular disease grows with aging in both sexes, there remains a predisposition to different cardiovascular disease between men and women, with evidence of sex-specific variation in cardiac remodeling [119]. This variation is not only in the morphological structure and vasculature of the aged heart [120], but in Ca2+ homeostasis at the cellular level [11]. For an extensive review of sexual differences in cardiac aging and excitation-contraction coupling, see Feridooni et al. [11].
Most aging studies described in this review utilized male rodents, whereby the systolic Ca2+ transient amplitude was generally reported to decline [27,79,91,96,121]. However, this is not universal [28,122]. Conversely, the Ca2+ transient amplitude does not appear to decline with age in female rodents [9,96,121,123], and is demonstrated to increase in aged female sheep [101]. Age-related alterations in excitation-contraction coupling, including peak ICa density, fractional shortening, and SR Ca2+ content were demonstrated to be more prominent with age in male mouse hearts vs. female [96,121]. This sexual dimorphism may aid in explaining why myocardial contractility appears to be better preserved in females in comparison to age-matched males [124]. Age-associated changes in cardiac excitation-contraction coupling proteins are summarized in Table 1. Although differences in intracellular Ca2+ homeostasis between aged males and females are apparent, an increase in mitochondrial ROS has been demonstrated in the senescent heart of both sexes [125].
Table 1.
Changes in excitation-contraction coupling proteins during cardiac aging. Abbreviations: mo—months old, yrs—years old.
| Change in Function | Species | Sex | Ages Studied | Myocyte Type | Comments | Reference |
|---|---|---|---|---|---|---|
| Ryanodine Receptor (RyR2) | ||||||
| ↑ | Mouse | Both | 6 vs. 24 mo (young adult vs. old) | Ventricular | Increased spontaneous Ca2+ spark activity | [26] |
| Mouse | Unreported | 6 vs. 24 mo (young adult vs. old) | Ventricular | Increased single channel open probability, increased spontaneous Ca2+ spark activity | [27] | |
| Mouse | Male | 3–4 vs. 24–26 mo (young vs. old) | Ventricular | Increased RyR2-mediated diastolic sparks and waves | [28] | |
| Rabbit | Female | 5–9 mo vs. 4–6 yrs (young adult vs. old) | Ventricular | Increased oxidation and SR Ca2+ leak, unchanged PKA- but increased CaMKII-mediated phosphorylation | [9] | |
| Mouse | Male | 4–6 vs. >20 mo (young adult vs. old) | Atrial | Increased glycation | [58] | |
| Mouse | Male | 2–2.5 vs. 24–32 mo (young vs. old) | Atrial | Increased JNK2/CaMKII activity, enhanced SR Ca2+ leak | [39] | |
| Mouse | Male | 4–5 vs. 24 mo (young adult vs. old) | Atrial | Increased CaMKII-mediated phosphorylation and oxidation | [40] | |
| Human | Both | <75 vs. >75 yrs (adult vs. old) | Atrial | Increased glycation | [58] | |
| Human | Both | <55, 55–74, >75 yrs (young, middle aged, old) | Atrial | Reduced CSQ2 expression and increased spontaneous RyR2 activity | [67] | |
| Sarco-endoplasmic reticulum Ca2+-ATPase (SERCa2a) | ||||||
| ↔ | Rabbit | Female | 5–9 mo vs. 4–6 yrs (young adult vs. old) | Ventricular | Unchanged protein levels | [9] |
| Rat | Unreported | 3 vs. 6 mo (young vs. adult) | Ventricular | Unchanged protein levels | [53] | |
| Rat | Male | 5, 15, 26 mo (young adult, adult, old) | Ventricular | Unchanged mRNA levels | [74] | |
| Rabbit | Male | 6 vs. 26 mo (young vs. adult) | Atrial and ventricular | No change in protein expression | [72] | |
| ↓ | Rat | Male | 6 vs. 26 mo (young vs. adult) | Ventricular | Reduced protein expression and pump activity | [76] |
| Rat | Male | 6–8 vs. 26–28 mo (adult vs. old) | Ventricular | Reduced PKA-dependent PLB phosphorylation | [82] | |
| Mouse | Male | 5, 24, and 34 mo (young adult, old, senescent) | Ventricular | Increased PLB expression | [79] | |
| Rat | Male | 6–8 vs. 26–28 mo (adult vs. old) | Ventricular | Depressed activity associated with reduced CaMKII expression | [61] | |
| Rat | Male | 5 vs. 26 mo (young adult vs. old) | Ventricular | Increased 3-Nitrotyrosine modification | [89] | |
| Rat | Male | 2–26 mo (young-old) | Whole heart | Oxidative damage associated with reduced activity | [90] | |
| Mouse | Unreported | 5 vs. 21 mo (adult vs. old) | Ventricular | Increased SERCa2a oxidation | [91] | |
| Human | Both | <55, 55–74, >75 yrs (young, middle aged, old) | Atrial | Decreased expression levels associated with reduced SR Ca2+ content | [67] | |
| L-Type Ca2+ Channel (LTCC) | ||||||
| ↔ | Mouse | Female | 7 vs. 24 mo (adult vs. old) | Ventricular | No alterations in activation or peak ICa | [96] |
| Rabbit | Female | 5–9 mo vs. 4–6 yrs (young adult vs. old) | Ventricular | No change in peak ICa, reduced responsiveness to β-adrenegic stimulation | [9] | |
| ↓ | Mouse | Male | 3 vs. 24 mo (young vs. old) | Ventricular | Reduced ICa density at T-tubules | [110] |
| Rat | Male | 2–3, 8–9, 25–26 mo (young, middle aged, old) | Ventricular | Delayed activation | [93] | |
| Rat | Male | 6 vs. >27 mo (adult vs. old) | Ventricular | Delayed inactivation and reduced peak ICa density | [94] | |
| Rat | Male | 3, 6–8, 24 mo (young, adult, old) | Ventricular | Delayed activation | [95] | |
| Mouse | Male | 7 vs. 24 mo (adult vs. old) | Ventricular | Slower activation and reduced peak ICa | [96] | |
| Rabbit | Male | 6 vs. 26 mo (young vs. adult) | Ventricular | Reduced ICa and maximal conductance, enhanced late component | [72] | |
| Sheep | Female | 18 mo vs. >8 yrs (young vs. old) | Ventricular | Increased peak/integrated ICa | [101] | |
| Dog | Unreported | 2–5, >8 yrs (adult vs. old) | Atrial | Reduced ICa, increased Ito | [97] | |
| Dog | Both | 2–5, >8 yrs (adult vs. old) | Atria | Decreased mRNA and protein expression levels, reduced ICa | [98] | |
| Dog | Unreported | 1–3, >8 yrs (adult vs old) | Atria | Decreased mRNA and protein expression levels, lower peak ICa density | [99,100] | |
| Sheep | Female | 18 mo vs. >8yrs (young adult vs. old) | Atrial | Decreased peak ICa | [102] | |
| Human | Both | <55, 55–74, >75 yrs (young, middle aged, old) | Atrial | Decreased peak ICa | [67] | |
| Na+/Ca+ exchanger (NCX1) | ||||||
| ↔ | Rats | Male | 6 vs. 26 mo (adult vs. old) | Ventricular | Unchanged expression levels | [62] |
| Rabbits | Male | 6 vs. 26 mo (young vs. adult) | Atrial and ventricular | No change in protein expression | [72] | |
| Rabbits | Female | 5–9 mo vs. 4–6 yrs (young adult vs. old) | Ventricular | No change in protein expression | [9] | |
| Rat | Male | 14–15 vs. 27–31 mo (adult vs. old) | Atrial and ventricular | No change in protein expression | [116] | |
| Mice | Unreported | 3 vs. 26–28 mo (young vs. old) | Ventricular | No change in protein levels | [117] | |
| ↑ | Rat | Male | 14–15 vs. 27–31 mo (middle aged vs. old) | Atrial and ventricular | Increased forward activity | [116] |
| Rat | Female | 3 vs. 24 mo (young vs. old) | Ventricular | Increased integrated current | [118] | |
| ↓ | Rat | Male | 4 vs. 24 mo (young vs. old) | Ventricular | Reduced protein expression levels | [114,115] |
2.6. Effects of Mitochondria on Intracellular Ca2+ Release
The heart is particularly vulnerable to mitochondrial dysfunction given the huge energetic needs of the contracting myocardium [126]. Cardiac aging is associated with a decline in mitochondrial function, a diminished capacity to maintain redox balance, and an increased emission of ROS [127]. Mitochondria-derived ROS are well established to alter the function of multiple ion channels [29,128] including RyR2 [9,36,41,129], SERCa2a [91,130], LTCCs [131], and NCX1 [132].
As evident from the previous discussion, an altered redox status of excitation-contraction coupling proteins appears to be a common mechanism of dysfunction in cardiac aging. It is well established that mitochondrial-mediated oxidative stress contributes to the derangement of cardiac Ca2+ homeostasis outlined in this review and it is implicated in the development of both atrial [133,134] and ventricular fibrillation [135]. Scavenging mitochondrial ROS or improving antioxidant activity, therefore, remains an attractive therapeutic strategy in cardiac disease [136]. Mitochondrial-targeted scavenger XJB-5-131 attenuated age-induced loss of cardio-protection and enhanced resistance to IR injury in aged rats (29 months old) [137], while mitoTEMPO reduced oxidative stress in both senescent rat (24 months old) and rabbit (4 to 6 years old) ventricular myocytes [9,138]. Genetic enhancement of mitochondrial antioxidant activity via overexpression of catalase reduced oxidative modifications and attenuated age-related changes in excitation-contraction coupling protein expression [139,140]. Electron and ROS scavenging or inhibition of mitochondrial oxidase has been shown to improve intracellular Ca2+ handling and reduce arrhythmic potential in other disease models as well as aging, including heart failure and diabetic cardiomyopathy [9,12,45,141,142].
Diminished mitochondrial function and enhanced ROS emission has been attributed to changes in the mitochondrial matrix [Ca2+] ([Ca2+]m). Both increased and decreased [Ca2+]m are reported as deleterious to cardiac function [143,144,145,146,147,148,149,150]. Discrepancies in the data may stem from different methods used by experimentalists to measure [Ca2+]m, including harsh procedures to isolate mitochondria, or loading myocytes with membrane-permeable dyes that can impair membrane integrity. The development of mitochondrial-targeted genetic Ca2+ probes and in vivo delivery methods should help resolve ongoing controversies [55,143,151,152,153,154]. Utilizing one such probe, we recently demonstrated that enhancement of mitochondrial Ca2+ accumulation increased mitochondrial ROS production and enhanced proarrhythmic spontaneous Ca2+ release in a rat model of hypertrophy [55]. On the contrary, inhibition of mitochondrial Ca2+ influx attenuated pro-arrhythmic activity in this model and reduced mitochondrial ROS emission. In pathophysiology, it would be rational to reduce mitochondrial Ca2+ influx, despite some evidence that SR-mitochondria communication may be diminished in aging [58,63,155]. This reduction may be viewed as an adaptive mechanism to reduce mitochondrial [Ca2+] and, thereby, limit deleterious mitochondrial ROS production in the senescent myocardium.
3. Perspective
Since an explosive growth in the elderly population is expected over the next 20 years [1], it is critical to develop therapies for age-associated cardiovascular disease and to reduce prevalence of sudden cardiac death. It is well established that intracellular Ca2+ homeostasis is perturbed in the aged heart, which contributes to increased arrhythmogenesis [10,11]. However, current findings are disparate, depending on species, stage, and sex. These differences must be addressed in future studies and in larger animal models of aging, as well as human tissues. Furthermore, for an improved understanding of the mechanisms that drive Ca2+-dependent cardiac dysfunction in the elderly, it is necessary to investigate other proteins that modulate intracellular Ca2+ handling including associated accessory proteins, kinases, and phosphatases.
Although many differences are reported regarding the expression and function of excitation-contraction coupling proteins in the aged heart, virtually universal findings include deficient β-adrenergic signaling, mitochondrial dysfunction, and increased ROS emission, as well as a reduction in intrinsic antioxidant defenses and enhancement of RyR2 activity, regardless of sex [9,26,27,28,40,127,133,134,136]. These universal findings are summarized in Figure 1. Reactive oxygen species have long been identified to play a pathophysiological role in aging, with the theory of free radicals as a primary driving force in determining lifespan introduced in 1956 [156]. It has since been well established that altered redox balance modulates cardiac excitation-contraction coupling [9,36,41,129,130,131,132]. Targeting of mitochondrial ROS and, thus, hyperactive RyR2, therefore, remains an attractive therapeutic target for arrhythmogenesis in cardiac disease and aging [9,55,129,136]. It has been demonstrated that ROS scavenger MitoQ can attenuate ischemia-reperfusion induced cardiac injury [157], hypertrophy [158], and aortic stiffness [159] in animal models of cardiac disease and aging. MitoQ was also shown to improve vascular endothelial function in healthy, older adults [160]. By reducing ROS formation at the mitochondrial respiratory chain, antioxidant peptide SS-31 prevented pressure-overload heart failure in mice [161,162], and many clinical trials with this drug are currently underway (www.clinicaltrials.gov, drug named Elamipretide or MT-131). While these tools hold promise, limited success of ROS scavenging strategies have been reported in most clinical studies [136], which is likely due to insufficient targeting and poor cellular distribution of drugs [136,163]. Given that the balance of ROS production and detoxification is essential to cell function in physiology [164], it also remains unclear as to what level of ROS may be beneficial or detrimental in pathophysiology [136], with some evidence that increased ROS can be beneficial for the function of cardiovascular endothelial cells, depending on source and subcellular localization [165].
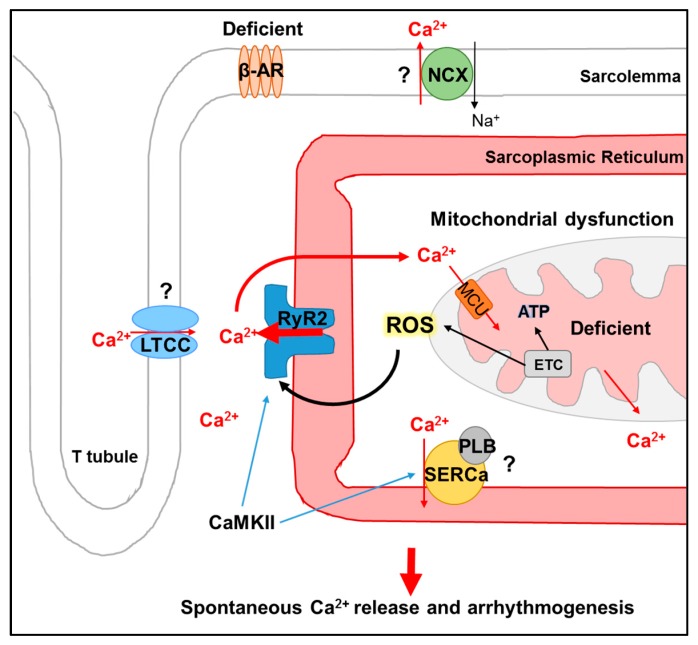
Figure 1.
Schematic summarizing the effects of cardiac aging on intracellular Ca2+ release in senescent myocytes, via a mitochondrial ROS-RyR2 axis. Virtually universal findings in aged myocytes include (1) deficient signaling through β-adrenergic receptors (β-AR) and (2) mitochondrial dysfunction, including diminished activity of the electron transport chain (ETC) and ATP production, as well an increased ROS emission. (3) It also includes enhanced activity of RyR2, due to oxidation by ROS. CaMKII phosphorylation may also increase RyR2 activity. Increased spontaneous intracellular Ca2+ release via oxidized RyR2s underlies arrhythmogenesis. Question marks indicate disparate findings regarding the effects of aging on LTCC, NCX, and SERCa2a/PLB function, whereby activity has been reported as unchanged, increased, or decreased. There is some evidence that mitochondrial Ca2+ levels may be diminished in cardiac aging even though this remains to be fully explored.
An alternative approach to modulating redox balance in aged myocytes is the normalization of mitochondrial Ca2+ homeostasis. Oxidative phosphorylation and generation of ATP in the mitochondria is highly dependent on [Ca2+]m, and there is evidence that mitochondrial Ca2+ cycling is altered in cardiac disease [143,144,145,146,147,148,149,150]. Whether reduction or enhancement of [Ca2+]m holds therapeutic potential remains controversial [55,143,146,166,167,168], as does the overall contribution of mitochondrial Ca2+ to cardiac excitation-contraction coupling [13,169,170,171,172,173]. While we have demonstrated that enhancement of mitochondrial Ca2+ accumulation augments RyR2 function and SR Ca2+ leak via increased ROS emission [55], there also remains a question as to the reverse: does enhanced SR Ca2+ leak via RyR2 directly modulate [Ca2+]m and mitochondrial function? Using phosphomimetic mutations of Serine-2808 to modulate RyR2 function, Santulli et al. (2015) suggested that [Ca2+]m increases as a result of SR Ca2+ leak results in mitochondrial dysfunction in mice [145]. The topology of RyR2 facing the dyad to prime for CICR, rather than the SR-mitochondrial cleft, means only a small amount of Ca2+ is likely to be taken up by mitochondria [174], so how much this can be increased under conditions of enhanced leak is unclear. The sensitivity of the mitochondrial Ca2+ uniporter (MCU) complex to Ca2+ is not thought to be affected by aging [63], and as of yet, there have been few studies investigating mitochondrial Ca2+ homeostasis in the senescent heart.
The impact of exercise and physical activity on the progression of cardiac aging is also an active area of research. There is evidence in both healthy animal models and models of other cardiac disease phenotypes that exercise can induce alterations in cardiac Ca2+ cycling [175,176,177]. Endurance exercise attenuated increased Ca2+ spark frequency, CaMKII-mediated RyR2 phosphorylation at Serine-2814, and Ca2+ alternans in a dog model of sudden cardiac death, which reduced ischemically-induced VF [175]. Long-term interval training also reduced CaMKII-dependent phosphorylation of RyR2 and the incidence of VT in a mouse model of CPVT [176]. In spontaneously hypertensive rats, endurance exercise training reversed increased RyR2 expression and attenuated the proarrhythmic increase in Ca2+ spark activity in left ventricular myocytes [177]. In the context of the aging heart, research on the effects of exercise on intracellular Ca2+ homeostasis remains limited. There is some evidence that exercise training improved Ca2+ cycling and contractility, which is associated with increased expression of SERCA2a in aged rodents (28 months old) [178], while others report no change in the expression or function of excitation-contraction coupling proteins in exercise-trained aged rodents [179,180]. In clinical trials, there is evidence that training can improve diastolic function and systolic reserve capacity in the elderly [181,182]. Two years of exercise training in middle-aged adults was recently shown to improve maximal oxygen uptake and decrease arterial stiffness, with authors suggesting this may protect against future cardiac pathologies that are attributable to sedentary aging [183]. There is also modest evidence that exercise improves the blunted response of the aged heart to β-adrenergic stimulation and increases cardiac reserve [184,185]. However, there are several studies that have shown that, while exercise training can improve exercise capacity of the heart, it does not significantly alter cardiac aging phenotypes [185,186,187,188].
Calorific restriction and fasting remains the only strategy demonstrated to significantly increase one’s health span and lifespan, both in animal models and humans [189]. The efficacy of calorific restriction highlights that mitochondrial dysfunction and metabolic derangement may contribute significantly to cardiac contractile dysfunction in the elderly. It has been suggested that sirtuins, which is a subgroup of deacetylases that are expressed in many tissues including the heart, mediate the anti-aging effects of calorific restriction [190]. Protein acetylation is a post-translational regulatory mechanism known to play roles in autophagy, ROS emission, and cell death [191,192]. Reduced expression of sirtuins and increased protein acetylation can result in enzymatic dysfunction and this is associated with multiple chronic and cardiac diseases [193,194,195,196]. Extensive lysine acetylation of mitochondrial proteins has been observed in mouse models of heart failure, as well as in the end-stage human failing heart [197]. Acetylation of excitation-contraction coupling proteins and downstream effects remains relatively unexplored. Application of Sirtuin 1 activator resveratrol enhanced the expression and activity of SERCa2a, which attenuated depressed contractile function in both a mouse model of diabetic cardiomyopathy and a rat model of hypertrophy [198,199]. Acetylation of SERCa2a was recently demonstrated to directly modulate pump function, with elevated acetylation observed in both animal and human failing hearts [200]. There remains limited knowledge of the effects of acetylation and sirtuins on intracellular Ca2+ handling proteins and arrhythmogeneses in the aged heart, but this is worthy of future investigation.
Abbreviations
| AF | Atrial fibrillation |
| CaM | Calmodulin |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CPVT | Catecholaminergic polymorphic ventricular tachycardia |
| CSQ | Calsequestrin |
| DAD | Delayed afterdepolarization |
| EAD | Early afterdepolarization |
| LTCC | L-type Ca2+ channel |
| NCX1 | Na+/Ca2+ exchanger |
| PKA | Protein kinase A |
| PP1 | Protein phosphatase 1 |
| PP2A | Protein phosphatase 2A |
| PP2B | Protein phosphatase 2B |
| ROS | Reactive oxygen species |
| RyR2 | Ryanodine receptor, type 2 |
| SERCa2a | Sarco/endoplasmic reticulum Ca2+-ATPase, type 2a |
| VF | Ventricular fibrillation |
| VT | Ventricular tachycardia |
Author Contributions
Conceptualization, S.H. and D.T. Writing—original draft preparation, S.H. Writing—review and editing, S.H. and D.T. Funding acquisition, S.H. and D.T.
Funding
The American Heart Association, grant number 18POST33960456 (SH), and the National Institutes of Health National Heart Lung and Blood Institute, grant number HL121796 (DT), funded this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Steenman M., Lande G. Cardiac aging and heart disease in humans. Biophys. Rev. 2017;9:131–137. doi: 10.1007/s12551-017-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakatta E.G., Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 4.Xiao R.P., A Spurgeon H., O’Connor F., Lakatta E.G. Age-associated changes in beta-adrenergic modulation on rat cardiac excitation-contraction coupling. J. Clin. Investig. 1994;94:2051–2059. doi: 10.1172/JCI117559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao R.P., Tomhave E.D., Wang D.J., Ji X., O Boluyt M., Cheng H., Lakatta E.G., Koch W.J. Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J. Clin. Investig. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lucia C., Eguchi A., Koch W.J. New Insights in Cardiac β-Adrenergic Signaling during Heart Failure and Aging. Front. Pharmacol. 2018;9:904. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tocchi A., Quarles E.K., Basisty N., Gitari L., Rabinovitch P.S. Mitochondrial Dysfunction in Cardiac Ageing. Biochim. Biophys. Acta (BBA) Bioenerg. 2015;1847:1424–1433. doi: 10.1016/j.bbabio.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorov D.B. Reactive Oxygen Species (ROS)-induced ROS Release: A New Phenomenon Accompanying Induction of the Mitochondrial Permeability Transition in Cardiac Myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper L.L., Li W., Lu Y., Centracchio J., Terentyeva R., Koren G., Terentyev D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J. Physiol. 2013;591:5895–5911. doi: 10.1113/jphysiol.2013.260521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janczewski A.M., Lakatta E.G. Modulation of sarcoplasmic reticulum Ca(2+) cycling in systolic and diastolic heart failure associated with aging. Heart Fail. Rev. 2010;15:431–445. doi: 10.1007/s10741-010-9167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feridooni H.A., Dibb K.M., Howlett S.E. How cardiomyocyte excitation, calcium release and contraction become altered with age. J. Mol. Cell. Cardiol. 2015;83:62–72. doi: 10.1016/j.yjmcc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton S., Terentyev D. Proarrhythmic Remodeling of Calcium Homeostasis in Cardiac Disease; Implications for Diabetes and Obesity. Front. Physiol. 2018;9:1517. doi: 10.3389/fphys.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner D.A., Caldwell J.L., Kistamás K., Trafford A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landstrom A.P., Dobrev D., Wehrens X.H. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bers D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Zima A.V., Bovo E., Mazurek S.R., Rochira J.A., Li W., Terentyev D. Ca handling during Excitation-Contraction Coupling in Heart Failure. Pflugers Arch. Eur. J. Physiol. 2014;466:1129–1137. doi: 10.1007/s00424-014-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fill M., Copello J.A. Ryanodine Receptor Calcium Release Channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Pt 1Am. J. Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 19.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassani R.A., Bers D.M., Bers D. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys. J. 1995;68:2015–2022. doi: 10.1016/S0006-3495(95)80378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bers D.M. Cardiac Sarcoplasmic Reticulum Calcium Leak: Basis and Roles in Cardiac Dysfunction. Annu. Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 22.Boyden P.A., Smith G.L. Ca2+ leak-What is it? Why should we care? Can it be managed? Heart Rhythm. 2018;15:607–614. doi: 10.1016/j.hrthm.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon T.R., Pogwizd S.M., Bers D.M. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 24.Uehara A., Murayama T., Yasukochi M., Fill M., Horie M., Okamoto T., Matsuura Y., Uehara K., Fujimoto T., Sakurai T., et al. Extensive Ca2+ leak through K4750Q cardiac ryanodine receptors caused by cytosolic and luminal Ca2+ hypersensitivity. J. Gen. Physiol. 2017;149:199–218. doi: 10.1085/jgp.201611624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalova Z., Gyorke I., Terentyeva R., Vedamoorthyrao S., Blom N.A., Valle G., et al. Abnormal Interactions of Calsequestrin with the Ryanodine Receptor Calcium Release Channel Complex Linked to Exercise-Induced Sudden Cardiac Death. Circ. Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 26.Howlett S.E., Grandy S.A., Ferrier G.R. Calcium spark properties in ventricular myocytes are altered in aged mice. Am. J. Physiol. Circ. Physiol. 2006;290:H1566–H1574. doi: 10.1152/ajpheart.00686.2005. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X., Altschafl B.A., Hajjar R.J., Valdivia H.H., Schmidt U. Altered Ca2+ sparks and gating properties of ryanodine receptors in aging cardiomyocytes. Cell Calcium. 2005;37:583–591. doi: 10.1016/j.ceca.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Domeier T.L., Roberts C.J., Gibson A.K., Hanft L.M., McDonald K.S., Segal S.S. Dantrolene suppresses spontaneous Ca2+ release without altering excitation-contraction coupling in cardiomyocytes of aged mice. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H818–H829. doi: 10.1152/ajpheart.00287.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niggli E., Ullrich N.D., Gutierrez D., Kyrychenko S., Poláková E., Shirokova N. Posttranslational modifications of cardiac ryanodine receptors: Ca(2+) signaling and EC-coupling. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:866–875. doi: 10.1016/j.bbamcr.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terentyev D., Hamilton S. Regulation of sarcoplasmic reticulum Ca2+ release by serine-threonine phosphatases in the heart. J. Mol. Cell. Cardiol. 2016;101:156–164. doi: 10.1016/j.yjmcc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houser S.R. Role of RyR2 phosphorylation in heart failure and arrhythmias: Protein kinase A-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ. Res. 2014;114:1320–1327. doi: 10.1161/CIRCRESAHA.114.300569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bovo E., Huke S., Blatter L.A., Zima A.V. The effect of PKA-mediated phosphorylation of ryanodine receptor on SR Ca2+ leak in ventricular myocytes. J. Mol. Cell. Cardiol. 2017;104:9–16. doi: 10.1016/j.yjmcc.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobrev D., Wehrens X.H. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ai X., Curran J.W., Shannon T.R., Bers D.M., Pogwizd S.M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 35.Curran J., Brown K.H., Santiago D.J., Pogwizd S., Bers D.M., Shannon T.R. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J. Mol. Cell. Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belevych A.E., Terentyev D., Terentyeva R., Nishijima Y., Sridhar A., Hamlin R.L., Carnes C.A., Györke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: Role of altered ryanodine receptor function. Cardiovasc. Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattiazzi A., Bassani R.A., Escobar A.L., Palomeque J., Valverde C.A., Petroff M.V., Bers D.M. Chasing cardiac physiology and pathology down the CaMKII cascade. Am. J. Physiol. Circ. Physiol. 2015;308:H1177–H1191. doi: 10.1152/ajpheart.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegyi B., Bers D.M., Bossuyt J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2019;127:246–259. doi: 10.1016/j.yjmcc.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Yan J., Zhao W., Thomson J.K., Gao X., Demarco D.M., Carrillo E., Chen B., Wu X., Ginsburg K.S., Bakhos M., et al. Stress Signaling JNK2 Crosstalk with CaMKII Underlies Enhanced Atrial Arrhythmogenesis. Circ. Res. 2018;122:821–835. doi: 10.1161/CIRCRESAHA.117.312536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X., Yuan S., Liu Z., Fang Q. Oxidation- and CaMKII-mediated sarcoplasmic reticulum Ca(2+) leak triggers atrial fibrillation in aging. J. Cardiovasc. Electrophysiol. 2014;25:645–652. doi: 10.1111/jce.12395. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki M., Yano M., Oda T., Tateishi H., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 42.Zima A.V., Mazurek S.R. Reviews of Physiology, Biochemistry and Pharmacology. Volume 171. Springer; Cham, Switzerland: 2016. Functional Impact of Ryanodine Receptor Oxidation on Intracellular Calcium Regulation in the Heart; pp. 39–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikolaienko R., Bovo E., Zima A.V. Redox Dependent Modifications of Ryanodine Receptor: Basic Mechanisms and Implications in Heart Diseases. Front. Physiol. 2018;9:1775. doi: 10.3389/fphys.2018.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boraso A., Williams A.J. Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Pt 2Am. J. Physiol. 1994;267:H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- 45.Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards J.N., Blatter L.A. Cardiac alternans and intracellular calcium cycling. Clin. Exp. Pharmacol. Physiol. 2014;41:524–532. doi: 10.1111/1440-1681.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobie E.A., Song L.S., Lederer W.J. Restitution of Ca(2+) release and vulnerability to arrhythmias. J. Cardiovasc. Electrophysiol. 2006;17(Suppl. 1):S64–S70. doi: 10.1111/j.1540-8167.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nivala M., Qu Z. Calcium alternans in a couplon network model of ventricular myocytes: Role of sarcoplasmic reticulum load. Am. J. Physiol. Circ. Physiol. 2012;303:H341–H352. doi: 10.1152/ajpheart.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Lacalle E., Cantalapiedra I.R., Peñaranda A., Cinca J., Hove-Madsen L., Echebarria B., Cantalapiedra I.R. Dependency of Calcium Alternans on Ryanodine Receptor Refractoriness. PLoS ONE. 2013;8:e55042. doi: 10.1371/journal.pone.0055042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belevych A.E., Terentyev D., Viatchenko-Karpinski S., Terentyeva R., Sridhar A., Nishijima Y., Wilson L.D., Cardounel A.J., Laurita K.R., Carnes C.A., et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc. Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belevych A.E., Terentyev D., Terentyeva R., Ho H.T., Gyorke I., Bonilla I.M., Carnes C.A., Billman G.E., Györke S. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ. Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho H.-T., Stevens S.C.W., Terentyeva R., Carnes C.A., Terentyev D., Györke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J. Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kandilci H.B., Tuncay E., Zeydanli E.N., Sozmen N.N., Turan B. Age-related regulation of excitation–contraction coupling in rat heart. J. Physiol. Biochem. 2011;67:317–330. doi: 10.1007/s13105-011-0077-3. [DOI] [PubMed] [Google Scholar]
- 54.Bovo E., Lipsius S.L., Zima A.V. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J. Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton S., Terentyeva R., Kim T.Y., Bronk P., Clements R.T., O-Uchi J., Csordás G., Choi B.R., Terentyev D. Pharmacological Modulation of Mitochondrial Ca2+ Content Regulates Sarcoplasmic Reticulum Ca2+ Release via Oxidation of the Ryanodine Receptor by Mitochondria-Derived Reactive Oxygen Species. Front. Physiol. 2018;9:1831. doi: 10.3389/fphys.2018.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pogwizd S.M., Qi M., Yuan W., Samarel A.M., Bers D.M. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 1999;85:1009–1019. doi: 10.1161/01.RES.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 57.Bidasee K.R., Nallani K., Yu Y., Cocklin R.R., Zhang Y., Wang M., Dincer Ü.D., Besch H.R. Chronic Diabetes Increases Advanced Glycation End Products on Cardiac Ryanodine Receptors/Calcium-Release Channels. Diabetes. 2003;52:1825–1836. doi: 10.2337/diabetes.52.7.1825. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Meana M., Minguet M., Bou-Teen D., Miro-Casas E., Castans C., Castellano J., Bonzon-Kulichenko E., Igual A., Rodriguez-Lecoq R., Vazquez J., et al. Ryanodine Receptor Glycation Favors Mitochondrial Damage in the Senescent Heart. Circulation. 2019;139:949–964. doi: 10.1161/CIRCULATIONAHA.118.035869. [DOI] [PubMed] [Google Scholar]
- 59.Terentyev D., Viatchenko-Karpinski S., Valdivia H.H., Escobar A.L., Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 2002;91:414–420. doi: 10.1161/01.RES.0000032490.04207.BD. [DOI] [PubMed] [Google Scholar]
- 60.Sobie E.A., Song L.S., Lederer W.J. Local recovery of Ca2+ release in rat ventricular myocytes. Pt 2J. Physiol. 2005;565:441–447. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu A., Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am. J. Physiol. 1998;275:H2087–H2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt U., del Monte F., Miyamoto M.I., Matsui T., Gwathmey J.K., Rosenzweig A., Hajjar R.J. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi: 10.1161/01.CIR.101.7.790. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Sanz C., Ruiz-Meana M., Miro-Casas E., Nunez E., Castellano J., Loureiro M., Barba I., Poncelas M., Rodriguez-Sinovas A., Vazquez J., et al. Defective sarcoplasmic reticulum–mitochondria calcium exchange in aged mouse myocardium. Cell Death Dis. 2014;5:e1573. doi: 10.1038/cddis.2014.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gyorke I., Hester N., Jones L.R., Györke S. The Role of Calsequestrin, Triadin, and Junctin in Conferring Cardiac Ryanodine Receptor Responsiveness to Luminal Calcium. Biophys. J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meissner G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017;149:1065–1089. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taffet G.E., Tate C.A. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Pt 2Am. J. Physiol. Circ. Physiol. 1993;264:H1609–H1614. doi: 10.1152/ajpheart.1993.264.5.H1609. [DOI] [PubMed] [Google Scholar]
- 67.Herraiz-Martínez A., Álvarez-García J., Llach A., Molina C.E., Fernandes J., Ferrero-Gregori A., Rodríguez C., Vallmitjana A., Benítez R., Padró J.M., et al. Ageing is associated with deterioration of calcium homeostasis in isolated human right atrial myocytes. Cardiovasc. Res. 2015;106:76–86. doi: 10.1093/cvr/cvv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terentyev D., Viatchenko-Karpinski S., Györke I., Volpe P., Williams S.C., Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knollmann B.C., Chopra N., Hlaing T., Akin B., Yang T., Ettensohn K., Knollmann B.E., Horton K.D., Weissman N.J., Holinstat I., et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Investig. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacLennan D.H., Kranias E.G. Phospholamban: A crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 71.Isenberg G., Borschke B., Rueckschloss U. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell Calcium. 2003;34:271–280. doi: 10.1016/S0143-4160(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 72.Salameh A., Dhein S., Fleischmann B., Grohe C., Hescheler J., Linz K.W., Meyer R. The aging heart: Changes in the pharmacodynamic electrophysiological response to verapamil in aged rabbit hearts. J. Physiol. Pharmacol. 2010;61:141–151. [PubMed] [Google Scholar]
- 73.Froehlich J. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J. Mol. Cell. Cardiol. 1978;10:427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan P., Jurkovicova D., Babusikova E., Hudecova S., Racay P., Sirova M., Lehotsky J., Drgova A., Dobrota D., Krizanova O. Effect of aging on the expression of intracellular Ca(2+) transport proteins in a rat heart. Mol. Cell. Biochem. 2007;301:219–226. doi: 10.1007/s11010-007-9414-9. [DOI] [PubMed] [Google Scholar]
- 75.O’Rourke B., Kass D.A., Tomaselli G.F., Kääb S., Tunin R., Marbán E., O’Rourke B., Kaab S., Marban E. Mechanisms of Altered Excitation-Contraction Coupling in Canine Tachycardia-Induced Heart Failure, I. Circ. Res. 1999;84:562–570. doi: 10.1161/01.RES.84.5.562. [DOI] [PubMed] [Google Scholar]
- 76.Zhu X., Huq F., Schmidt U., Lebeche D., Guerrero J.L., Hajjar R.J. In vivo gene transfer of parvalbumin improves diastolic function in aged rat hearts. Cardiovasc. Res. 2005;66:318–323. doi: 10.1016/j.cardiores.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Lompre A.M., Lambert F., Lakatta E.G., Schwartz K. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ. Res. 1991;69:1380–1388. doi: 10.1161/01.RES.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 78.Cain B.S., Meldrum D.R., Joo K.S., Wang J.F., Meng X., Cleveland J.C., Jr., Banerjee A., Harken A.H. Human SERCA2a levels correlate inversely with age in senescent human myocardium. J. Am. Coll. Cardiol. 1998;32:458–467. doi: 10.1016/S0735-1097(98)00233-2. [DOI] [PubMed] [Google Scholar]
- 79.Lim C.C., Liao R., Varma N., Apstein C.S. Impaired lusitropy-frequency in the aging mouse: Role of Ca(2+)-handling proteins and effects of isoproterenol. Am. J. Physiol. 1999;277:H2083–H2090. doi: 10.1152/ajpheart.1999.277.5.H2083. [DOI] [PubMed] [Google Scholar]
- 80.Tian R., Halow J.M., Meyer M., Dillmann W.H., Figueredo V.M., Ingwall J.S., Camacho S.A. Thermodynamic limitation for Ca2+ handling contributes to decreased contractile reserve in rat hearts. Am. J. Physiol. 1998;275:H2064–H2071. doi: 10.1152/ajpheart.1998.275.6.H2064. [DOI] [PubMed] [Google Scholar]
- 81.Michele D.E., Szatkowski M.L., Albayya F.P., Metzger J.M. Parvalbumin gene delivery improves diastolic function in the aged myocardium in vivo. Mol. Ther. 2004;10:399–403. doi: 10.1016/j.ymthe.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Jiang M.T., Moffat M.P., Narayanan N. Age-related alterations in the phosphorylation of sarcoplasmic reticulum and myofibrillar proteins and diminished contractile response to isoproterenol in intact rat ventricle. Circ. Res. 1993;72:102–111. doi: 10.1161/01.RES.72.1.102. [DOI] [PubMed] [Google Scholar]
- 83.Neumann J., Eschenhagen T., Jones L.R., Linck B., Schmitz W., Scholz H., Zimmermann N. Increased Expression of Cardiac Phosphatases in Patients with End-stage Heart Failure. J. Mol. Cell. Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 84.Carr A.N., Schmidt A.G., Suzuki Y., Del Monte F., Sato Y., Lanner C., Breeden K., Jing S.-L., Allen P.B., Greengard P., et al. Type 1 Phosphatase, a Negative Regulator of Cardiac Function. Mol. Cell. Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta R.C., Mishra S., Rastogi S., Imai M., Habib O., Sabbah H.N. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 86.Pamminger T., Ditz D., El-Armouche A., Zolk O., Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc. Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Haghighi K., Bidwell P., Kranias E.G. Phospholamban Interactome in Cardiac Contractility and Survival: A New Vision of an OLD Friend. J. Mol. Cell. Cardiol. 2014;77:160–167. doi: 10.1016/j.yjmcc.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pritchard T.J., Kawase Y., Haghighi K., Anjak A., Cai W., Jiang M., Nicolaou P., Pylar G., Karakikes I., Rapti K., et al. Active Inhibitor-1 Maintains Protein Hyper-Phosphorylation in Aging Hearts and Halts Remodeling in Failing Hearts. PLoS ONE. 2013;8:e80717. doi: 10.1371/journal.pone.0080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knyushko T.V., Sharov V.S., Williams T.D., Schöneich C., Bigelow D.J. 3-Nitrotyrosine Modification of SERCA2a in the Aging Heart: A Distinct Signature of the Cellular Redox Environment†. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 90.Babušíková E., Lehotský J., Dobrota D., Račay P., Kaplán P. Age-associated changes in Ca(2+)-ATPase and oxidative damage in sarcoplasmic reticulum of rat heart. Physiol. Res. 2012;61:453–460. doi: 10.33549/physiolres.932320. [DOI] [PubMed] [Google Scholar]
- 91.Qin F., Siwik D.A., Lancel S., Zhang J., Kuster G.M., Luptak I., Wang L., Tong X.Y., Kang Y.J., Cohen R.A., et al. Hydrogen Peroxide–Mediated SERCA Cysteine 674 Oxidation Contributes to Impaired Cardiac Myocyte Relaxation in Senescent Mouse Heart. J. Am. Heart Assoc. 2013;2:000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weiss J.N., Garfinkel A., Karagueuzian H.S., Chen P.-S., Qu Z. Early Afterdepolarizations and Cardiac Arrhythmias. Heart Rhythm. 2010;7:1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lakatta E.G., Houser S.R., E Walker K. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc. Res. 1993;27:1968–1977. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- 94.Liu S.J., Wyeth R.P., Melchert R.B., Kennedy R.H. Aging-associated changes in whole cell K+ and L-type Ca2+ currents in rat ventricular myocytes. Am. J. Physiol. 2000;279:H889–H900. doi: 10.1152/ajpheart.2000.279.3.H889. [DOI] [PubMed] [Google Scholar]
- 95.Josephson I.R., Guia A., Stern M.D., Lakatta E.G. Alterations in Properties of L-Type Ca Channels in Aging Rat Heart. J. Mol. Cell. Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- 96.Grandy S.A., Howlett S.E. Cardiac excitation-contraction coupling is altered in myocytes from aged male mice but not in cells from aged female mice. Am. J. Physiol. Circ. Physiol. 2006;291:H2362–H2370. doi: 10.1152/ajpheart.00070.2006. [DOI] [PubMed] [Google Scholar]
- 97.Dun W., Yagi T., Rosen M.R., A Boyden P. Calcium and potassium currents in cells from adult and aged canine right atria. Cardiovasc. Res. 2003;58:526–534. doi: 10.1016/S0008-6363(03)00288-8. [DOI] [PubMed] [Google Scholar]
- 98.Gan T.-Y., Qiao W., Xu G.-J., Zhou X.-H., Tang B.-P., Song J.-G., Li Y.-D., Zhang J., Li F.-P., Mao T., et al. Aging-associated changes in L-type calcium channels in the left atria of dogs. Exp. Ther. Med. 2013;6:919–924. doi: 10.3892/etm.2013.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu G.-J., Gan T.-Y., Zhang Y., Tang B.-P., Chen Z.-H., Mahemuti A., Jiang T., Song J.-G., Guo X., Li Y.-D., et al. Alterations in the expression of atrial calpains in electrical and structural remodeling during aging and atrial fibrillation. Mol. Med. Rep. 2013;8:1343–1352. doi: 10.3892/mmr.2013.1684. [DOI] [PubMed] [Google Scholar]
- 100.Xu G.-J., Gan T.-Y., Tang B.-P., Chen Z.-H., Jiang T., Song J.-G., Guo X., Li J.-X. Age-related changes in cellular electrophysiology and calcium handling for atrial fibrillation. J. Cell. Mol. Med. 2013;17:1109–1118. doi: 10.1111/jcmm.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dibb K., Rueckschloss U., Eisner D., Isenberg G., Trafford A. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J. Mol. Cell. Cardiol. 2004;37:1171–1181. doi: 10.1016/j.yjmcc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Clarke J.D., Caldwell J.L., Pearman C.M., Eisner D.A., Trafford A.W., Dibb K.M. Increased Ca buffering underpins remodelling of Ca2+ handling in old sheep atrial myocytes. J. Physiol. 2017;595:6263–6279. doi: 10.1113/JP274053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pearman C.M., Madders G.W., Radcliffe E.J., Kirkwood G.J., Lawless M., Watkins A., Smith C.E., Trafford A.W., Eisner D.A., Dibb K.M. Increased Vulnerability to Atrial Fibrillation Is Associated with Increased Susceptibility to Alternans in Old Sheep. J. Am. Heart Assoc. 2018;7:009972. doi: 10.1161/JAHA.118.009972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harvey R.D., Hell J.W. CaV1.2 signaling complexes in the heart. J. Mol. Cell. Cardiol. 2013;58:143–152. doi: 10.1016/j.yjmcc.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen X., Piacentino V., 3rd, Furukawa S., Goldman B., Margulies K.B., Houser S.R. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 2002;91:517–524. doi: 10.1161/01.RES.0000033988.13062.7C. [DOI] [PubMed] [Google Scholar]
- 106.Wei J.Y., Spurgeon H.A., Lakatta E.G. Excitation-contraction in rat myocardium: Alterations with adult aging. Am. J. Physiol. Circ. Physiol. 1984;246:H784–H791. doi: 10.1152/ajpheart.1984.246.6.H784. [DOI] [PubMed] [Google Scholar]
- 107.Crossman D.J., Young A.A., Ruygrok P.N., Nason G.P., Baddelely D., Soeller C., Cannell M.B. t-tubule disease: Relationship between t-tubule organization and regional contractile performance in human dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2015;84:170–178. doi: 10.1016/j.yjmcc.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scardigli M., Ferrantini C., Crocini C., Pavone F.S., Sacconi L. Interplay Between Sub-Cellular Alterations of Calcium Release and T-Tubular Defects in Cardiac Diseases. Front. Physiol. 2018;9:1474. doi: 10.3389/fphys.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones P.P., Macquaide N., Louch W.E. Dyadic Plasticity in Cardiomyocytes. Front. Physiol. 2018;9:1773. doi: 10.3389/fphys.2018.01773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong C.H.T., Bryant S.M., Watson J.J., Gadeberg H.C., Roth D.M., Patel H.H., Cannell M.B., Orchard C.H., James A.F. The Effects of Aging on the Regulation of T-Tubular ICa by Caveolin in Mouse Ventricular Myocytes. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:711–719. doi: 10.1093/gerona/glx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ñeco P., Rose B., Huynh N., Zhang R., Bridge J.H., Philipson K.D., Goldhaber J.I. Sodium-Calcium Exchange Is Essential for Effective Triggering of Calcium Release in Mouse Heart. Biophys. J. 2010;99:755–764. doi: 10.1016/j.bpj.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Despa S., Bers D.M. Na⁺ transport in the normal and failing heart—Remember the balance. J. Mol. Cell. Cardiol. 2013;61:2–10. doi: 10.1016/j.yjmcc.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ottolia M., Torres N., Bridge J.H.B., Philipson K.D., Goldhaber J.I. Na/Ca exchange and contraction of the heart. J. Mol. Cell. Cardiol. 2013;61:28–33. doi: 10.1016/j.yjmcc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janapati V., Wu A., Davis N., Derrico C.A., Levengood J., Schummers J., Colvin R.A. Post-transcriptional regulation of the Na+/Ca2+ exchanger in aging rat heart. Mech. Ageing Dev. 1995;84:195–208. doi: 10.1016/0047-6374(95)01656-2. [DOI] [PubMed] [Google Scholar]
- 115.Assayag P., Charlemagne D., Marty I., De Leiris J., Lompré A.M., Boucher F., Valère P.-E., Lortet S., Swynghedauw B., Besse S. Effects of sustained low-flow ischemia on myocardial function and calcium-regulating proteins in adult and senescent rat hearts. Cardiovasc. Res. 1998;38:169–180. doi: 10.1016/S0008-6363(97)00283-6. [DOI] [PubMed] [Google Scholar]
- 116.Mace L.C., Palmer B.M., Brown D.A., Jew K.N., Lynch J.M., Glunt J.M., Parsons T.A., Cheung J.Y., Moore R.L. Influence of age and run training on cardiac Na+/Ca2+ exchange. J. Appl. Physiol. 2003;95:1994–2003. doi: 10.1152/japplphysiol.00551.2003. [DOI] [PubMed] [Google Scholar]
- 117.Li Q., Wu S., Li S.-Y., Lopez F.L., Du M., Kajstura J., Anversa P., Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 118.Ozturk N., Olgar Y., Er H., Kucuk M., Ozdemir S. Swimming exercise reverses aging-related contractile abnormalities of female heart by improving structural alterations. Cardiol. J. 2017;24:85–93. doi: 10.5603/CJ.a2016.0069. [DOI] [PubMed] [Google Scholar]
- 119.Keller K.M., Howlett S.E. Sex Differences in the Biology and Pathology of the Aging Heart. Can. J. Cardiol. 2016;32:1065–1073. doi: 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 120.Kane A.E., Howlett S.E. Differences in Cardiovascular Aging in Men and Women. Adv. Exp. Med. Biol. 2018;1065:389–411. doi: 10.1007/978-3-319-77932-4_25. [DOI] [PubMed] [Google Scholar]
- 121.Howlett S.E. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H659–H670. doi: 10.1152/ajpheart.00214.2009. [DOI] [PubMed] [Google Scholar]
- 122.Farrell S.R., Howlett S.E. The effects of isoproterenol on abnormal electrical and contractile activity and diastolic calcium are attenuated in myocytes from aged Fischer 344 rats. Mech. Ageing Dev. 2007;128:566–573. doi: 10.1016/j.mad.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Ross J.L., Howlett S.E. Age and Ovariectomy Abolish Beneficial Effects of Female Sex on Rat Ventricular Myocytes Exposed to Simulated Ischemia and Reperfusion. PLoS ONE. 2012;7:38425. doi: 10.1371/journal.pone.0038425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Claessens T.E., Rietzschel E.R., De Buyzere M.L., De Bacquer D., De Backer G., Gillebert T.C., Verdonck P.R., Segers P., Gillebert T. Noninvasive assessment of left ventricular and myocardial contractility in middle-aged men and women: Disparate evolution above the age of 50? Am. J. Physiol. Heart Circ. Physiol. 2007;292:H856–H865. doi: 10.1152/ajpheart.00759.2006. [DOI] [PubMed] [Google Scholar]
- 125.Sanz A., Hiona A., Kujoth G.C., Seo A.Y., Hofer T., Kouwenhoven E., Kalani R., Prolla T., Barja G., Leeuwenburgh C., et al. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp. Gerontol. 2007;42:173–182. doi: 10.1016/j.exger.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y., Li Y., He C., Gou B., Song M. Mitochondrial regulation of cardiac aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018 doi: 10.1016/j.bbadis.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 127.Chaudhary K.R., El-Sikhry H., Seubert J.M. Mitochondria and the aging heart. J. Geriatr. Cardiol. 2011;8:159–167. doi: 10.3724/SP.J.1263.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blatter L.A., Zima A.V. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 129.Kim T.Y., Terentyeva R., Roder K.H., Li W., Liu M., Greener I., Hamilton S., Polina I., Murphy K.R., Clements R.T., et al. SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovasc. Res. 2017;113:343–353. doi: 10.1093/cvr/cvx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ying J., Sharov V., Xu S., Jiang B., Gerrity R., Schöneich C., Cohen R.A. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic. Biol. Med. 2008;45:756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muralidharan P., Szappanos H.C., Ingley E., Hool L.C. The cardiac L-type calcium channel alpha subunit is a target for direct redox modification during oxidative stress-the role of cysteine residues in the alpha interacting domain. Clin. Exp. Pharmacol. Physiol. 2017;44:46–54. doi: 10.1111/1440-1681.12750. [DOI] [PubMed] [Google Scholar]
- 132.Liu T., O’Rourke B. Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J. Biol. Chem. 2013;288:31984–31992. doi: 10.1074/jbc.M113.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie W., Santulli G., Reiken S.R., Yuan Q., Osborne B.W., Chen B.-X., Marks A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015;5:11427. doi: 10.1038/srep11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoo S., Aistrup G., Shiferaw Y., Ng J., Mohler P.J., Hund T.J., Waugh T., Browne S., Gussak G., Gilani M., et al. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight. 2018;3:120728. doi: 10.1172/jci.insight.120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morita N., Sovari A.A., Xie Y., Fishbein M.C., Mandel W.J., Garfinkel A., Lin S.-F., Chen P.-S., Xie L.-H., Chen F., et al. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1594–H1605. doi: 10.1152/ajpheart.00579.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dietl A., Maack C. Targeting Mitochondrial Calcium Handling and Reactive Oxygen Species in Heart Failure. Heart Fail. Rep. 2017;14:338–349. doi: 10.1007/s11897-017-0347-7. [DOI] [PubMed] [Google Scholar]
- 137.Escobales N., Nuñez R.E., Jang S., Parodi-Rullan R., Ayala-Peña S., Sacher J.R., Skoda E.M., Wipf P., Frontera W., Javadov S. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J. Mol. Cell. Cardiol. 2014;77:136–146. doi: 10.1016/j.yjmcc.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olgar Y., Degirmenci S., Durak A., Billur D., Can B., Mutlu G.K., Inan E.A., Turan B. Aging related functional and structural changes in the heart and aorta: MitoTEMPO improves aged-cardiovascular performance. Exp. Gerontol. 2018;110:172–181. doi: 10.1016/j.exger.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 139.Dai D.-F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J., Gollahon K., Martin G.M., Loeb L.A., Ladiges W.C., et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao C., Behring J.B., Shao D., Sverdlov A.L., Whelan S.A., Elezaby A., Yin X., Siwik D.A., Seta F., Costello C.E., et al. Overexpression of Catalase Diminishes Oxidative Cysteine Modifications of Cardiac Proteins. PLoS ONE. 2015;10:0144025. doi: 10.1371/journal.pone.0144025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Umanskaya A., Santulli G., Xie W., Andersson D.C., Reiken S.R., Marks A.R. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc. Natl. Acad. Sci. USA. 2014;111:15250–15255. doi: 10.1073/pnas.1412754111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Valdés Á., Treuer A.V., Barrios G., Ponce N., Fuentealba R., Dulce R.A., González D.R. NOX Inhibition Improves β-Adrenergic Stimulated Contractility and Intracellular Calcium Handling in the Aged Rat Heart. Int. J. Mol. Sci. 2018;19:2404. doi: 10.3390/ijms19082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu T., Takimoto E., Dimaano V.L., DeMazumder D., Kettlewell S., Smith G., Sidor A., Abraham T.P., O’Rourke B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sloan R.C., Moukdar F., Frasier C.R., Patel H.D., Bostian P.A., Lust R.M., Brown D.A. Mitochondrial permeability transition in the diabetic heart: Contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J. Mol. Cell. Cardiol. 2012;52:1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 145.Santulli G., Xie W., Reiken S.R., Marks A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luongo T.S., Lambert J.P., Gross P., Nwokedi M., Lombardi A.A., Shanmughapriya S., Carpenter A.C., Kolmetzky D., Gao E., van Berlo J.H., et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature. 2017;545:93–97. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paillard M., Tubbs E., Thiebaut P.-A., Gomez L., Fauconnier J., Da Silva C.C., Teixeira G., Mewton N., Belaidi E., Durand A., et al. Depressing Mitochondria-Reticulum Interactions Protects Cardiomyocytes from Lethal Hypoxia-Reoxygenation Injury. Circulation. 2013;128:1555–1565. doi: 10.1161/CIRCULATIONAHA.113.001225. [DOI] [PubMed] [Google Scholar]
- 148.Griffiths E.J., Balaska D., Cheng W.H. The ups and downs of mitochondrial calcium signalling in the heart. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010;1797:856–864. doi: 10.1016/j.bbabio.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 149.Uchi J., Jhun B.S., Xu S., Hurst S., Raffaello A., Liu X., Yi B., Zhang H., Gross P., Mishra J., et al. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid. Redox Signal. 2014;21:863–879. doi: 10.1089/ars.2013.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Suárez J., Cividini F., Scott B.T., Lehmann K., Díaz-Juárez J., Diemer T., Dai A., Suarez J.A., Jain M., Dillmann W.H. Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J. Biol. Chem. 2018;293:8182–8195. doi: 10.1074/jbc.RA118.002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kettlewell S., Cabrero P., Nicklin S.A., Dow J.A., Davies S., Smith G.L. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J. Mol. Cell. Cardiol. 2009;46:891–901. doi: 10.1016/j.yjmcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 152.Kaestner L., Scholz A., Tian Q., Ruppenthal S., Tabellion W., Wiesen K., Katus H.A., Müller O.J., Kotlikoff M.I., Lipp P. Genetically encoded Ca2+ indicators in cardiac myocytes. Circ. Res. 2014;114:1623–1639. doi: 10.1161/CIRCRESAHA.114.303475. [DOI] [PubMed] [Google Scholar]
- 153.Jones J.S., Small D.M., Nishimura N. In Vivo Calcium Imaging of Cardiomyocytes in the Beating Mouse Heart with Multiphoton Microscopy. Front. Physiol. 2018;9:969. doi: 10.3389/fphys.2018.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bovo E., Nikolaieko R., Bhayani S., Kahn D., Cao Q., Martin J.L., Kuo I.Y., Robia S., Zima A.V. Novel approach for quantification of endoplasmic reticulum Ca2+ transport. Am. J. Physiol. Heart Circ. Physiol. 2019 doi: 10.1152/ajpheart.00031.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.No M.-H., Heo J.-W., Yoo S.-Z., Jo H.-S., Park D.-H., Kang J.-H., Seo D.-Y., Han J., Kwak H.-B. Effects of aging on mitochondrial hydrogen peroxide emission and calcium retention capacity in rat heart. J. Exerc. Rehabil. 2018;14:920–926. doi: 10.12965/jer.1836550.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harman D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 157.Adlam V.J., Harrison J.C., Porteous C.M., James A.M., Smith R.A.J., Murphy M.P., Sammut I. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 158.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cochemé H.M., Murphy M., Dominiczak A.F. Mitochondria-Targeted Antioxidant MitoQ10 Improves Endothelial Function and Attenuates Cardiac Hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 159.Gioscia-Ryan R.A., Battson M.L., Cuevas L.M., Eng J.S., Murphy M.P., Seals D.R. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J. Appl. Physiol. (1985) 2018;124:1194–1202. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rossman M.J., Santos-Parker J.R., Steward C.A., Bispham N.Z., Cuevas L.M., Rosenberg H.L., Woodward K.A., Chonchol M., Gioscia-Ryan R.A., Murphy M.P., et al. Chronic Supplementation with a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71:1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nickel A.G., Von Hardenberg A., Hohl M., Löffler J.R., Kohlhaas M., Becker J., Reil J.-C., Kazakov A., Bonnekoh J., Stadelmaier M., et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015;22:472–484. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 162.Dai D.-F., Hsieh E.J., Chen T., Menendez L.G., Basisty N.B., Tsai L., Beyer R.P., Crispin D.A., Shulman N.J., Szeto H.H., et al. Global Proteomics and Pathway Analysis of Pressure-overload Induced Heart Failure and Its Attenuation by Mitochondrial Targeted Peptides. Circ. Heart Fail. 2013;6:1067–1076. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai D.-F., Chiao Y.A., Marcinek D.J., Szeto H.H., Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aon M., Cortassa S., O’Rourke B. Redox-optimized ROS balance: A unifying hypothesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aldosari S., Awad M., Harrington E.O., Sellke F.W., Abid M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants. 2018;7:14. doi: 10.3390/antiox7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.García-Rivas G.D.J., Carvajal K., Correa F., Zazueta C., García-Rivas G.J. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br. J. Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schweitzer M.K., Wilting F., Sedej S., Dreizehnter L., Dupper N.J., Tian Q., Moretti A., My I., Kwon O., Priori S.G., et al. Suppression of Arrhythmia by Enhancing Mitochondrial Ca2+ Uptake in Catecholaminergic Ventricular Tachycardia Models. JACC Basic Transl. Sci. 2017;2:737–747. doi: 10.1016/j.jacbts.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie A., Zhou A., Liu H., Shi G., Liu M., Boheler K.R., Dudley S.C., Jr. Mitochondrial Ca2+ flux modulates spontaneous electrical activity in ventricular cardiomyocytes. PLoS ONE. 2018;13:e0200448. doi: 10.1371/journal.pone.0200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dedkova E.N., Blatter L.A. Mitochondrial Ca2+ and the heart. Cell Calcium. 2008;44:77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 170.O’Rourke B., Blatter L.A. Mitochondrial Ca2+ uptake: Tortoise or hare? J. Mol. Cell. Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hohendanner F., Ljubojević S., MacQuaide N., Sacherer M., Sedej S., Biesmans L., Wakula P., Platzer D., Sokolow S., Herchuelz A., et al. Intracellular dyssynchrony of diastolic cytosolic [Ca2+] decay in ventricular cardiomyocytes in cardiac remodeling and human heart failure. Circ. Res. 2013;113:527–538. doi: 10.1161/CIRCRESAHA.113.300895. [DOI] [PubMed] [Google Scholar]
- 172.Williams G.S., Boyman L., Chikando A.C., Khairallah R.J., Lederer W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu X., Ginsburg K.S., Kettlewell S., Bossuyt J., Smith G.L., Bers D.M. Measuring local gradients of intramitochondrial [Ca(2+)] in cardiac myocytes during sarcoplasmic reticulum Ca(2+) release. Circ. Res. 2013;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De La Fuente S., Lambert J.P., Nichtova Z., Sanz C.F., Elrod J.W., Sheu S.-S., Csordás G. Spatial Separation of Mitochondrial Calcium Uptake and Extrusion for Energy-Efficient Mitochondrial Calcium Signaling in the Heart. Cell Rep. 2018;24:3099–3107.e4. doi: 10.1016/j.celrep.2018.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bonilla I.M., Belevych A.E., Sridhar A., Nishijima Y., Ho H.T., He Q., Kukielka M., Terentyev D., Terentyeva R., Liu B., et al. Endurance exercise training normalizes repolarization and calcium-handling abnormalities, preventing ventricular fibrillation in a model of sudden cardiac death. J. Appl. Physiol. (1985) 2012;113:1772–1783. doi: 10.1152/japplphysiol.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manotheepan R., Danielsen T.K., Sadredini M., Anderson M.E., Carlson C.R., Lehnart S.E., Sjaastad I., Stokke M.K. Exercise training prevents ventricular tachycardia in CPVT1 due to reduced CaMKII-dependent arrhythmogenic Ca2+ release. Cardiovasc. Res. 2016;111:295–306. doi: 10.1093/cvr/cvw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carneiro-Júnior M.A., Quintão-Júnior J.F., Drummond L.R., Lavorato V.N., Drummond F.R., Amadeu M.A., Oliveira E.M., Felix L.B., Cruz J.S., Mill J.G., et al. Effect of exercise training on Ca2+ release units of left ventricular myocytes of spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2014;47:960–965. doi: 10.1590/1414-431X20144063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Groban L., Jobe H., Lin M., Houle T., Kitzman D.A., Sonntag W. Effects of Short-Term Treadmill Exercise Training or Growth Hormone Supplementation on Diastolic Function and Exercise Tolerance in Old Rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008;63:911–920. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thomas M.M., Vigna C., Betik A.C., Tupling A.R., Hepple R.T. Cardiac calcium pump inactivation and nitrosylation in senescent rat myocardium are not attenuated by long-term treadmill training. Exp. Gerontol. 2011;46:803–810. doi: 10.1016/j.exger.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 180.Walton R.D., Jones S.A., Rostron K.A., Kayani A.C., Close G.L., McArdle A., Lancaster M.K. Interactions of Short-Term and Chronic Treadmill Training with Aging of the Left Ventricle of the Heart. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:1005–1013. doi: 10.1093/gerona/glv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Edelmann F., Gelbrich G., Düngen H.D., Fröhling S., Wachter R., Stahrenberg R., Binder L., Töpper A., Lashki D.J., Schwarz S., et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 182.Spina R.J., Turner M.J., A Ehsani A. Beta-adrenergic-mediated improvement in left ventricular function by exercise training in older men. Am. J. Physiol. 1998;274:H397–H404. doi: 10.1152/ajpheart.1998.274.2.H397. [DOI] [PubMed] [Google Scholar]
- 183.Howden E.J., Sarma S., Lawley J.S., Opondo M., Cornwell W., Stoller D., Urey M.A., Adams-Huet B., Levine B.D. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications for Heart Failure Prevention. Circulation. 2018;137:1549–1560. doi: 10.1161/CIRCULATIONAHA.117.030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leosco D., Parisi V., Femminella G.D., Formisano R., Petraglia L., Allocca E., Bonaduce D., Femminella D.G. Effects of exercise training on cardiovascular adrenergic system. Front. Physiol. 2013;4:348. doi: 10.3389/fphys.2013.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roh J., Rhee J., Chaudhari V., Rosenzweig A. The Role of Exercise in Cardiac Aging: From Physiology to Molecular Mechanisms. Circ. Res. 2016;118:279–295. doi: 10.1161/CIRCRESAHA.115.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McGuire D.K., Levine B.D., Williamson J.W., Snell P.G., Blomqvist C.G., Saltin B., Mitchell J.H. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104:1358–1366. doi: 10.1161/hc3701.096099. [DOI] [PubMed] [Google Scholar]
- 187.Fujimoto N., Prasad A., Hastings J.L., Arbab-Zadeh A., Bhella P.S., Shibata S., Palmer D., Levine B.D. Cardiovascular Effects of 1 Year of Progressive and Vigorous Exercise Training in Previously Sedentary Individuals Older Than 65 Years of Age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haykowsky M.J., Brubaker P.H., Stewart K.P., Morgan T.M., Eggebeen J., Kitzman D.W. Effect of Endurance Training on the Determinants of Peak Exercise Oxygen Consumption in Elderly Patients with Stable Compensated Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Most J., Tosti V., Redman L.M., Fontana L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zullo A., Simone E., Grimaldi M., Musto V., Mancini F.P. Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle. Int. J. Mol. Sci. 2018;19:928. doi: 10.3390/ijms19040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tanno M., Kuno A., Horio Y., Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012;107:273. doi: 10.1007/s00395-012-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Verdin E., Ott M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 193.Parodi-Rullán R.M., Chapa-Dubocq X.R., Javadov S. Acetylation of Mitochondrial Proteins in the Heart: The Role of SIRT3. Front. Physiol. 2018;9:1094. doi: 10.3389/fphys.2018.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marcelli S., Corbo M., Iannuzzi F., Negri L., Blandini F., Nisticò R., Feligioni M. The Involvement of Post-Translational Modifications in Alzheimer’s Disease. Curr. Res. 2018;15:313–335. doi: 10.2174/1567205014666170505095109. [DOI] [PubMed] [Google Scholar]
- 195.Peleg S., Feller C., Ladurner A.G., Imhof A. The Metabolic Impact on Histone Acetylation and Transcription in Ageing. Trends Biochem. Sci. 2016;41:700–711. doi: 10.1016/j.tibs.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 196.Treviño-Saldaña N., García-Rivas G. Regulation of Sirtuin-Mediated Protein Deacetylation by Cardioprotective Phytochemicals. Oxidative Med. Cell. Longev. 2017;2017:1–16. doi: 10.1155/2017/1750306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Horton J.L., Martin O.J., Riley N.M., Richards A.L., Vega R.B., Leone T.C., Bedi K.C., Margulies K.B., Lai L., Pagliarini D.J., et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;1:e84897. doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sulaiman M., Matta M.J., Sunderesan N.R., Gupta M.P., Periasamy M., Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dong Q., Wu Z., Li X., Yan J., Zhao L., Yang C., Lu J., Deng J., Chen M. Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of Ca(2+) cycling proteins. J. Transl. Med. 2014;12:323. doi: 10.1186/s12967-014-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gorski P.A., Jang S.P., Jeong D., Lee A., Lee P., Oh J.G., Chepurko V., Yang D.K., Kwak T.H., Eom S.H., et al. Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+-ATPase in Heart Failure. Circ. Res. 2019;124:e63–e80. doi: 10.1161/CIRCRESAHA.118.313865. [DOI] [PMC free article] [PubMed] [Google Scholar]