Abstract
Gap junction (GJ) channels in invertebrates have been used to understand cell-to-cell communication in vertebrates. GJs are a common form of intercellular communication channels which connect the cytoplasm of adjacent cells. Dysregulation and structural alteration of the gap junction-mediated communication have been proven to be associated with a myriad of symptoms and tissue-specific pathologies. Animal models relying on the invertebrate nervous system have exposed a relationship between GJs and the formation of electrical synapses during embryogenesis and adulthood. The modulation of GJs as a therapeutic and clinical tool may eventually provide an alternative for treating tissue formation-related diseases and cell propagation. This review concerns the similarities between Hirudo medicinalis innexins and human connexins from nucleotide and protein sequence level perspectives. It also sets forth evidence of computational techniques applied to the study of proteins, sequences, and molecular dynamics. Furthermore, we propose machine learning techniques as a method that could be used to study protein structure, gap junction inhibition, metabolism, and drug development.
Keywords: connexin, innexin, gap junctions, leech, central nervous system, machine learning
1. Introduction
Gap junctions (GJs) are intercellular cytoplasmic channels composed of an arrangement of transmembrane proteins particular to metazoans. These proteins form hemichannels which, while unpaired, provide a leakage passage for cytosolic molecules (like glutamate and ATP) into the extracellular medium [1]. These channels allow the exchange of intracellular ions, second messengers, and small metabolites (1 to 2 kDa) and the passage of direct current across adjacent cells [2,3,4]. Thus, current-transferring conduits provide a fundamental path for distributing electrical synapses and coordinating cellular signaling [5,6].
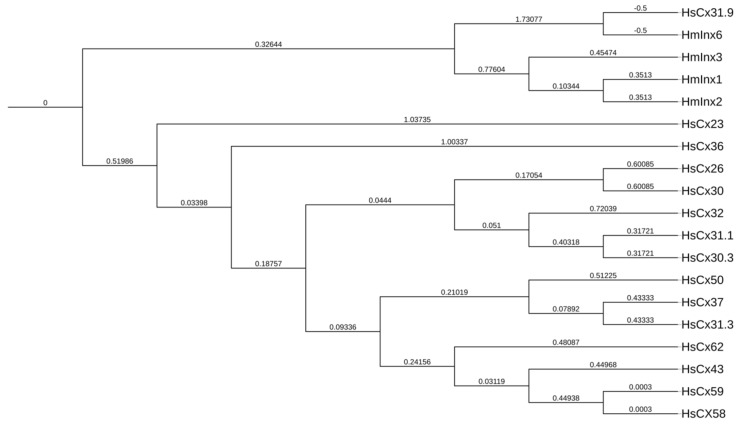
Currently, three main members of the gene superfamily make up these hemi-channels: connexins, pannexins, and innexins. While genetic orthology may not be evident in relating the vertebrate gap-junction connexins with their invertebrate counterpart (innexins), sequence identity analysis has identified protein homologues within the genome of several vertebrates: pannexins [2,7,8,9]. Although the junctional role of pannexins has remained questionable, their permeability to ATP, as well as their specific distribution throughout erythrocytes which do not form GJs, indicates an alternative role to that of direct intercellular communication [1,10]. However, structure-wise, the three families encode a similar topology composed of four transmembrane domains forming two extracellular loops and one intracellular loop, as well as a cytoplasmic N- and C- terminus [11,12,13]. Figure 1 is a nucleotide sequence alignment that shows the similarity between innexins and connexins using a dendrogram to categorize the relationship between those proteins. The human connexin Cx31.9 (GJA11) presented the highest level of similarity with leech innexins. Cx31.9 is expressed in several human tissues, as well as in muscle [14,15], and it exhibits very low unitary conductance and low sensitivity to transjunctional voltage [16,17]. Additionally, it has been reported that Cx31.9 plays no role in AV-nodal impulse delay or conduction elsewhere in the human heart [16].
Figure 1.
Innexin and connexin relationship dendrogram (nucleotide sequence alignment) using ClustalW. Cx31.9 presented the highest level of similarity with leech innexins. It is found on chromosome 17 and expressed in several vital organs, such as the cerebral cortex, heart, liver, and lungs. Cx31.9 presents some unique functional properties and voltage behaviors.
In the case of connexins and innexins, large genetic families have been identified throughout several animal models. In mammals, out of the 20 connexin genes present in mice (Mus musculus), 19 can be arranged as orthologous pairs with the 21 connexins present in humans [18,19]. Meanwhile, in zebrafish (Danio rerio), up to 37 connexin genes have been characterized. This is the largest connexin gene family described thus far [19,20]. For invertebrates such as the fruit fly (Drosophila melanogaster), eight innexins encoding different loci have been identified with multiple splice isoforms [21,22]. In the nematode (Caenorhabditis elegans) and in the medicinal leech (Hirudo medicinalis), up to 25 and 21 innexins have been determined and localized, respectively [12,13,23].
In this review, we focused on the leech nervous system as a biological model to understand the human nervous system. Then, we described the morphological comparison of the molecular constituents of vertebrate and invertebrate gap junctions (connexins and innexins), as well as a description of different techniques used for inhibiting cell-to-cell communication and blocking individual channels. We propose computational methods that could be used to study protein structure, gap junction inhibition, metabolism, and drug development.
2. The Leech Nervous System: A Chain of Possibilities
As with most other annelids, the basic nervous system of the medicinal leech (Hirudo spp.) consists of a single nerve cord which runs along the ventral side of the body [24,25]. Amid both peripheral ganglia lie 21 segmental ganglia, each possessing approximately 400 neurons arranged in a tubular fashion around a central glial neuropil that provides nourishment and structure to the ganglion. Any individual neuron within the ganglion may contain the neurotransmitters acetylcholine, octopamine, gamma-aminobutyric acid (GABA), serotonin, and dopamine, as well several neuropeptides, such as met-enkephalin (mENK), FMRF-amide, bombesin, vasoactive intestinal polypeptide (VIP), and substance P [26,27,28]. The coordination of these signaling molecules alongside segmental ganglia provides the fundamental basis of hierarchical behavior patterns (feeding, swimming, and crawling) [25,29,30,31]. In addition, out of 21 innexins identified in H. medicinalis, 15 have been found to be exclusively expressed across the nervous system in both neurons and glial cells [13,32,33]. Moreover, during H. medicinalis development, specific innexins are highly expressed at certain age stage or tissue type [33].
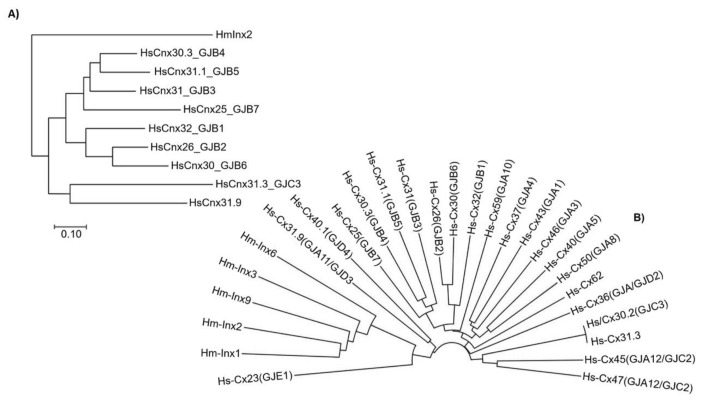
With the purpose of studying and describing GJs’ coupling patterns, as well as their relation to synaptic transmission, numerous models have been proposed and assayed. However, few have proven to be as efficient and practical as the medicinal leech nervous system, in part due to the similarity between human connexins and leech innexins [25,28]. Figure 2 shows the taxa relationships between human connexins and leech innexins. A comparison between HmInx2 and its closest human connexins is shown in Figure 2a, while a full comparison is shown in Figure 2b. As several studies have indicated, gap junction regulation may serve as a recognition mechanism that mediates the formation of electrical synapses during the embryonic development of H. medicinalis [12,32,34]. This suggests that electrical coupling not only precedes chemical synaptogenesis but may, in fact, lay the foundation through which transient neuronal circuits formed by interactions of complementary synaptic targets are eventually rectified through the emergence of chemical synapses during development [13,35,36]. Not restricted to synaptic coupling, this signaling mechanism has been proven to regulate and allocate glial network formation through the expression of particular innexin hemichannels [13,33,34].
Figure 2.
Evolutionary relationships of taxa (protein sequence alignment). Comparing (A) HmInx2 vs. human connexins. (B) HmInx1, 2, 3, 6, 9, and 14 vs. human connexins. This amino acid sequence alignment shows that Cx31.9 (GJA11) and Cx23 (GJB1) are closest to the H. medicinalis innexins family.
For research focused around the underlying mechanisms of synaptic transmission and neurochemistry, as well as neuronal development and regeneration, the medicinal leech may be a suitable model for analyzing cell-to-cell adhesion and communication. It may also be an elegant approach to behavioral neuroscience beyond the capabilities of the discrete, yet overly simplistic, neuronal circuits of models such as D. melanogaster or C. elegans [25]. Given that numerous response mechanisms have been identified and described to be associated with a limited population of identifiable neurons whose innexin profiles have been determined within the leech, ethological research value has been placed on GJs and their neuronal wiring capabilities [25,30,37].
3. The Molecular Structure of Connexins/Innexins
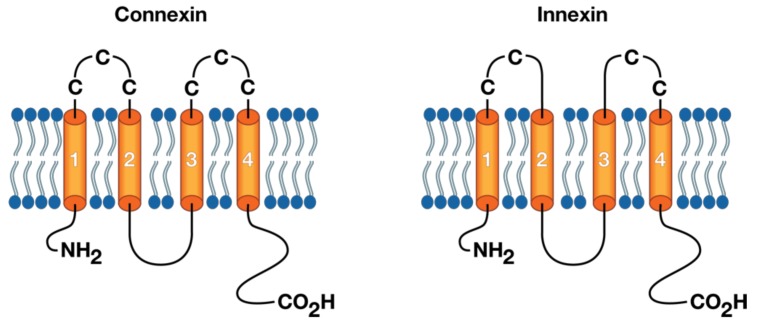
As previously stated, hemichannel composition consists mainly of a hexameric arrangement of connexin/innexin monomers into a cylindrical channel with a central axial pore, the coupling of which produces a functional intercellular gap junction. Though every innexin isoform varies to a certain degree in terms of sequence identity, at a structural level they appear consistent in terms of the following features: tetra spanning α-helical transmembrane segments (TM1–TM4), one cytoplasmic loop (CL), and two extracellular loops (E1 and E2) [4,8]. Figure 3 depicts a general connexin/innexin structure showing the cysteine residues in the extracellular loops. Amino- and carboxy-termini reside within the intracellular face of the junctional membrane, where they assemble into cytoplasmic domains that confer multiple gating and selectivity properties to the gap junction. Though the junctional membrane’s significance in cell recognition may not be immediately evident at the monomer level, together with the C-terminus and the cytoplasmic loop, it provides the highest degree of size variation among the monomer isoforms [37,38,39,40].
Figure 3.
General connexin/innexin structures forming gap junctions and “hemi-channels”. Vertebrates connexins have three cysteine residues (left) in each of their extracellular loops, while invertebrates innexins have only two cysteines per loop (right). Both connexins and innexins have four transmembrane domains (orange tubes) connected by one cytoplasmic loop (black curve), and have both NH2 and CO2H terminals in the cytosol.
Extracellular loops, located between TM1 and TM2 and TM3 and TM4, have been shown to function as docking sites between complementary GJs through the disulfide bonding of three cysteines in each connexin loop or two in each innexin loop [40,41,42]. Structurally, both loops possess a highly conserved amino acid sequence among connexins (except Cx31), with a [C–X6–C–X3–C] pattern for E1 and [C–X5–C–X5–C] pattern for E2 [18,42]. Therefore, docking specificity is not thought to arise from sequence-specific coupling, but from a complex arrangement of antiparallel β sheets connected by disulfide bonds into concentric β barrels [42,43]. This structural hypothesis translates accordingly into innexins, where β-sheet “hairpins” accumulate around E2, while E1 creates a constriction ring around the axial pore through a small α-helix [44]. To properly understand how these molecular structures provide recognition, gating, and flexibility, a higher-order analysis must be performed from the monomer into the hexameric hemichannel or, better yet, into the dodecameric oligopeptide that is the gap junction.
4. The Molecular Structure of Gap Junction Proteins
Throughout the hemichannel configuration, the orientation of α-helices surrounding the axial pore aligns predominantly in a clockwise fashion, with only a couple of right-handed segments lining the pore into a crisscrossed, tilted pattern [42,45]. Several models have aspired to predict an ion permeability mechanism based on this tilting, where structural occlusion of the pore would occur through the twisting constriction of these helices in a manner similar to that of an iris diaphragm in a camera [43,45,46] However, recent X-ray analysis performed on connexin-mediated GJs revealed no structural variation between Ca2+-bound and Ca2+-free channels, suggesting the existence of a cation exclusion mechanism based on electrostatic interactions instead [47].
Once properly assembled through the alignment of bundled transmembrane segments, hemichannel functionality depends on the coordinated interaction of numerous molecular domains [44]. Within the cytosol, N-terminal regions form a funnel-like structure around the pore entrance at the transmembrane region, restricting its diameter and effectively determining permeability properties, such as molecular cutoff size and charge selectivity. It also determines channel activity properties, such as transjunctional voltage gating [44,47,48]. Amino acid residues located at the first cytoplasmic positions have revealed a sensor-like role in determining conductance and channel polarization, allowing for a highly sensitive voltage-dependent gating. This is known as fast-gating [39,48,49]. Simultaneously, a cytoplasmic dome composed of the CL and C-terminal domain creates an entrance that acts as a harness for the N-terminal loops, associating structural functionality with the pore funnel through the intercalating habit of N-terminal α-helix and TM1 [44]. The structure of gap junction channels and the differences between connexins and innexin GJs have been reviewed [50].
5. Inhibition of Gap Junction Communication
GJs can be inhibited at a certain level by modifying the proteins of innexins and connexins or their corresponding RNA [51]. Different methods can be used to target the proteins with chemical agents or with antibodies, and other methods target and block the mRNA to cut the translation to proteins [52,53]. The results and characteristics of some techniques are described below.
5.1. Chemical Mechanisms
Chemical agents have been used to block and uncouple gap junction communication (GJC) between cells. Octanol, heptanol, and arachidonic acid are efficient reagents used to block GJC in different organisms with a variety of biological purposes [51,54]. These compounds block action potentials in the membrane by increasing junctional resistance [55].
There is evidence of GJC inhibition achieved using heptanol and arachidonic acid. GJC was blocked using heptanol in Planaria [54], the sea anemone [56], and mice [57]. GJC was also blocked using arachidonic acid in leeches [58] and with both heptanol and arachidonic acid in the sea anemones [59].
When using octanol for inhibiting GJC, connexon downregulation was demonstrated in different insects, like Oncopeltus, Hyalophora, Drosophila, Xylocopa, and Periplaneta [60,61,62,63]. In addition, GJC was inhibited in rodents to better understand the function of proteins and transcriptional regulators, as well as the mechanism of function of these inhibitors on cell communication [64,65,66]. A review of gap junction blockers in animal models in relation to seizures which includes a comprehensive list of inhibitors, can be found in [51].
Chemical mechanisms excel at reversibly blocking GJC, although these blockers are non-specific for different innexins and connexins and need a precise concentration to properly inhibit GJC [65].
5.2. RNA Interference
Another technique that is used to inhibit GJC is RNA interference (RNAi). RNAi decreases or eliminates a target mRNA by injecting the cell with a specific double-stranded RNA (dsRNA), thus preventing the translation of mRNA into a protein [67]. Due to the protein nature of GJs, RNA interference is a suitable approach to inhibiting GJC by knocking down innexin or connexin genes [25].
Previous results conclude that connexin mimetic peptides represent the only specific inhibitors for gap junction channel function, except for small interfering RNA [68]. Nevertheless, their use has been limited due to their low efficacy of inhibition (40–50%) and slow onset of action, while a faster action has been observed in paired oocytes when peptides were applied to single oocytes before the pairing. However, the most updated literature reports some cases where the use of siRNA has a higher percentage of inhibition. For example, Cx43 expression and, thus, channel functionality have been successfully suppressed (around 70%) using RNAi-based approaches in human bone marrow stromal cells [69]. The same Cx43 was suppressed by approximately 60% in human bronchial fibroblasts [69], 90% in human pulmonary endothelial cells [70], and 90% in mouse 3T3 fibroblasts and HL-1 cardiomyocytes [71]. In all these experiments the gap junction was significantly decreased by 50%. Other cases have been reported where the inhibition of connexins using siRNA is not so high. For example, Cx37, Cx40, and Cx43 were inhibited using siRNA in human umbilical vein endothelial cells, representing 40%, 31.5%, and 32.7% of maximal inhibition, respectively [72].
In leeches, RNAi was used to decrease the expression of the innexin Hm-inx1, reducing gap junction expression in individual neurons by more than 80% [52]. In the desert locust (Schistocerca gregaria), a decrease of expression levels in Inx1 (74%), Inx2 (85%), Inx3 (95%), and Inx4 (65%) genes was obtained compared to the controls [73]. In Anopheles mosquitoes, gene knockdown of innexin AGAP001476 mRNA gene was achieved with dsRNA at a 60% level [74].
In the mosquito Aedes aegypti, using dsRNA resulted in a significant knockdown of Inx1 (32%), Inx2 (69%), Inx3 (51%), Inx4 (71%), and Inx7 (86%), as well as a very low percentage in Inx8 knockdown [75]. Similar to the latter study, an Aedes aegypti injection of Inx2 dsRNA resulted in a reduction of 73% in mRNA expression, producing a different reduction level in different tissues. There was a knockdown of Inx2 in the midgut (95%), ovaries (89%), fat body (91%), and malpighian tubules (45%) [76].
Contrary to the chemical mechanisms, RNAi has the advantage of being specific for a given innexin target due to the specificity of the dsRNA to the mRNA. Nonetheless, the reduction percentage in the expression of the genes can vary in different innexins, organisms, and even tissues.
5.3. Anti-Peptide Antibodies
Anti-peptide antibodies (ApepA) are specific antibodies that are used to target specific portions of the connexin proteins in the cell [77]. Antibodies can additionally inhibit hemichannels without affecting gap junction function because the antibodies cannot access all the proteins that form GJs [78].
Polyclonal antibodies have been used in rat hearts to study cell-to-cell communication. Antibodies were constructed to bind intracellular amino acid sequences 5–7, 314–322, and 363–382 of protein Cx43. The first two antibodies did not interfere with cell-to-cell communication, but the third was able to block coupling in 50% of the injected cells [79]. In a similar work, antibodies to amino acids 113–123, 241–260, 283–298, and 346–360 were studied for their effect on the phosphorylation of Cx43 [80]. Two different antibodies were made to target the last 23 amino acids (360–382) of Cx43, resulting in the inhibition of gap junction uncoupling, an increase in the channels’ open time, and a change in their selectivity [81]. In addition, ApepA were used against the extracellular loops of Cx proteins in human cells. This resulted in the total inhibition of the hemichannels, without affecting cell-to-cell coupling [82].
ApepA against Inx2 of Drosophila blocked the GJCs between oocytes and follicle cells in the intracellular C-terminus and the intracellular loop [83]. Previous works used antibodies to localize the expression of innexins in tissue [84], where they showed that the gap junction protein innexin-2 is expressed in a small group of nerve cells in the lower body column of invertebrates and that an anti-innexin-2 antibody binds to gap junctions in the same region. However, they did not use gene shutdown or innexin inhibition.
5.4. Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) are short DNA sequences specifically designed to target the mRNA transcripts of specific proteins in order to decrease or abolish the expression of such proteins [85,86]. Direct applications of the use of ASOs in studying neurodegenerative diseases have been reviewed [85,86,87].
ASOs have been used with a Pluronic F-127 gel delivery system to regulate specific connexin expression. They are injected directly into tissues, resulting in connexin knockdown for 24–48 h [88]. The role of Cx43 was investigated using ASODs in a rodent model of optic nerve damage with and without modulation expression, resulting in a knockdown of Cx43 production and a decrease of new GJs, which reduced cell death and optic nerve oedema [89]. A similar study used a model of corneal wound healing to estimate the effect of Cx43 using ASOs. The knockdown of Cx43 resulted in faster wound closure and more uniform repair [90].
ASOs are effective when administered throughout the transcription of connexin genes and have no effect blocking connexin channels that already exist. In addition, ASOs need to be around 18–30 bases long. If they are longer, cell penetration is not possible. If they are shorter, they will be less specific. The advantages of ASOs are that they are easy to use, dose controllable, and low-cost compared to other gene knockout protocols [88].
5.5. Mimetic Peptides
Mimetic peptides (MPs) are synthetic peptides that assume a configuration compatible with a specific protein and inhibit channel formation by imitating connexin–connexin binding. Nonetheless, MPs are able to create channels on their own [91,92].
It was confirmed that MPs specific to the second extracellular region of connexins can inhibit specific types of gap junction channels [93]. MPs have been used to interrupt GJC in endothelial muscle and homocellular muscle culture systems. In addition, it was proven that peptides are reversible following a washout treatment [94].
Although MPs can inhibit gap junction channels, some evidence suggests a lack of inhibition of channel currents (less than 30%) [68]. Likewise, connexin MPs seem to inhibit pannexin channels, which are different in sequence from connexins [53]. Based on the results from those studies, MPs are not suitable for the straightforward inhibition of gap junction channels. MPs can only block the formation of new GJs but do not affect connections that are already formed.
6. Computational Models
Computational techniques have been useful in studying biological processes [95,96]. Recent evidence discusses the use of various computational approaches to simulate the molecular flux through connexin hemichannels using the structure of the pores obtained by X-ray crystallography and assuming Brownian dynamics for the molecules in flux [97,98,99,100]. In addition, an automated fluorescence microscope technique was developed to quantify gap junction communication using the values for nucleus number, cytoplasm area, cell perimeter, and fluorescence intensity from each cell in order to be able to recognize gap junction blockers [101]. Furthermore, a computational model was created to demonstrate the degradation of GJs inside the ischemic area in cardiac cells [102].
Machine learning is one of the fields of artificial intelligence that focuses on providing tools for data analysis. Those tools are often fast and scalable, but a large data set is expected to permit greater learning and prediction [103,104]. Machine learning has been used in engineering and in the natural sciences (physics, chemistry, and biology) and can help in the life sciences by providing useful computational models for neuroscience. The data used can diversify from small molecules to omic data (e.g., genomic, proteomic, transcriptomic, metabolomic) [105,106]. Nevertheless, further applications of machine learning to the study of gap junction channels is necessary due to the lack of research involving computational approaches in the study of innexin and connexin channels.
Support vector machines (SVMs) have been used to predict the different types of proteins as they have considerable accuracy for differentiating type I transmembrane, type II transmembrane, multipass transmembrane, lipid-chain anchored membrane, and GPI-anchored membrane [107]. An SVM model was generated to predict the secondary structure of proteins [108]. The same method was used to create a detector of membrane activity in α-helical peptide sequences [109] and to create a classifier to differentiate the redox states in molecular dynamics of proteins [110]. In addition, machine learning approaches regarding the study of proteins have been developed to predict DNA-protein binding sites [111], protein ligand biding affinity [112], the relationship between primary and secondary structure of globular proteins [113], protein sorting signals based on the sequence of amino acids [114], and many more.
There is a lack of machine learning applications regarding the inhibition or blockage of GJs. Nevertheless, in studies where confocal microscopy was used to study effects of ApepA in GJs from the myocardium [115], inverted microscopy was used to analyze infarct reduction by gap junction inhibition with octanol [116]. Fluorescence microscopy images were taken to study the effects of gap junction blockade in the suppression of central nervous system diseases [117]. There is benefit in the use of machine learning techniques, specifically convolutional neural networks, to process images. Convolutional neural networks have been successful in pattern classification and detection in natural images [104].
Related to metabolic applications, machine learning was used to develop a model to discriminate between related genotypes using metabolome analysis data [118]. An algorithm was developed to determine the metabolism and toxicity of new compounds [119]. The prediction of the biological function of compounds of metabolic pathways was achieved to predict what metabolic pathway a molecule belongs to [120]. A machine learning tool was trained to classify between essential and non-essential reactions using topologic, genomic, and transcriptomic features [121]. In addition, a machine learning method was used to predict metabolic pathways based on genome data [122] and to study drug metabolic process using gene cancer data [123].
Machine learning approaches are of particular interest in drug development due to their applicability in several steps of drug discovery methodology [124]. An SVM was used to predict the cleavability of oligopeptides to HIV proteases [125]. A machine learning approach was used to discern between substrates from an inhibitor of carrier proteins to be used in drug development [126]. Lastly, the SVM was trained with energy terms from docking sites to predict binding affinity and its application to drug biding affinity [127].
Cell-to-cell communication has a significant role in tumor differentiation and proliferation. Connexins containing GJs, tunneling nanotubes, and hemichannels are part of that type of communication. However, new approaches (such as machine learning) could provide new insights to the study of those signals between connected cells [128]. Nonetheless, there is little evidence of the use of machine learning techniques in cell-to-cell communication studies.
A machine learning strategy for studying Cx39 hemichannel permeability to certain molecules has been developed. Since the net charge, size, and shape are insufficient properties for determining pore affinity to certain molecules, the model consisted of 11 descriptors belonging to six categories: electronegativity, ionization potential, polarizability, size and geometry, topological flexibility, and valence [129].
7. Discussion and Future Work
Gap junction channels allow for the exchange of different metabolites and currents among cells. GJs are formed from proteins encoded by three types of genes: innexins in invertebrates and connexins and pannexins in vertebrates. Innexins and connexins have been identified throughout several animal models, where different species share similarities in the biological functions performed by the proteins encoded by those genes.
The medicinal leech is a suitable biological model for studying the nervous system, partly due to the similarities between human connexins and leech innexins [130]. Research has focused on the mechanisms of synaptic transmission and neurochemistry. In this regard, the leech seems to be an appropriate model for studying the behaviors of human connexins in a simpler living model.
Several studies have used rodents to study the functions of innexins or connexins using different methods aimed to inhibit the formation of GJs. Those methods vary in their specificity toward the entire protein or small sequences of amino acids. Other methods use different molecules to target protein mRNA and inhibit the translation of the targeted gene. Thus, the selection of a method to block GJs should be specific to the expected results.
The applications of machine learning to biological data and problems extends over several areas related to the study of GJs. There are different applications of machine learning techniques to study protein structure and interactions with other molecules. Research involving innexin and connexin proteins could be strengthened with the use of machine learning in order to improve structural and functional studies. In addition, the development of novel drugs that target gap junction genes or proteins could benefit from algorithms that predict the binding affinity of some molecules to other molecules and predict how a molecule will function in a specific metabolic pathway. Furthermore, given that several biological studies benefit from the use of microscopic images, these studies could use image processing algorithms, like convolutional neural networks, to detect patterns or classify images of biological tissue.
Even though in vivo and in vitro experimentation are the traditional ways to do research, computational approaches have been successful in different applications in biological fields, creating an important new arena for machine learning techniques. This gives scientists an opportunity to use different computational techniques to study gap junction related behavior. In silico experimentation is an advantageous approach for studying different biological processes as it reduces the time and resource consumption needed for in vitro experimentation.
Machine learning offers new opportunities in studying connexins and innexins, GJs, membrane flux of molecules, GJ blockers, etc., particularly to complement and expand what is already known in the field. Multidisciplinary research is the key to new developments involving applications of novel computational approaches to the understanding of current biological questions. Therefore, this research improves the quality and reach of these studies and scientific publications.
Acknowledgments
We would like to thank the COMEXUS Fulbright-García Robles program for providing funding to D.-L.F. (PS00275077) and the Consejo Nacional de Ciencia y Tecnología for the financial support to C.C. (Grant No. 763840) and D.-L.F. (Grant No. 175415).
Abbreviations
| GJs | Gap junctions |
| CJC | Gap junction communication |
| RNAi | RNA interference |
| dsRNA | Double-stranded RNA |
| ApepA | Anti-peptide antibodies |
| ASOs | Antisense oligonucleotides |
| MPs | Mimetic peptides |
| SVM | Support vector machine |
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Scemes E., Spray D.C., Meda P. Connexins, pannexins, innexins: Novel roles of “hemi-channels”. Pflug. Arch. Eur. J. Physiol. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panchin Y.V. Evolution of gap junction proteins—The pannexin alternative. J. Exp. Biol. 2005:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 3.Dykes I.M. Molecular Basis of Gap Junctional Communication in the CNS of the Leech Hirudo medicinalis. J. Neurosci. 2004;24:886–894. doi: 10.1523/JNEUROSCI.3676-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshima A., Matsuzawa T., Nishikawa K., Fujiyoshi Y. Oligomeric structure and functional characterization of caenorhabditis elegans innexin-6 gap junction protein. J. Biol. Chem. 2013;288:10513–10521. doi: 10.1074/jbc.M112.428383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen M.S., Axelsen L.N., Sorgen P.L., Verma V., Delmar M., Holstein-Rathlou N.H. Gap junctions. Compr. Physiol. 2012;2:1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohman A.W., Isakson B.E. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588:1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Yen M.R., Saier M.H. Gap junctional proteins of animals: The innexin/pannexin superfamily. Prog. Biophys. Mol. Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fushiki D., Hamada Y., Yoshimura R., Endo Y. Phylogenetic and bioinformatic analysis of gap junction-related proteins, innexins, pannexins and connexins. Biomed. Res. 2010;31:133–142. doi: 10.2220/biomedres.31.133. [DOI] [PubMed] [Google Scholar]
- 10.Locovei S., Wang J., Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann C., Lechner H., Lo B., Knieps M., Herrmann S., Famulok M., Bauer R., Hoch M. eteromerization of Innexin Gap Junction Proteins Regulates Epithelial Tissue Organization in Drosophila. Mol. Biol. Cell. 2006;17:1676–1685. doi: 10.1091/mbc.e05-11-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker M.W., Macagno E.R. Gap Juntion Proteins and the Wiring (Rewiring) of Neural Circuits. Dev. Neurobiol. 2016:1–23. doi: 10.1002/dneu. [DOI] [PubMed] [Google Scholar]
- 13.Yazdani N., Firme C.P., Macagno E.R., Baker M.W. Expression of a dominant negative mutant innexin in identified neurons and glial cells reveals selective interactions among gap junctional proteins. Dev. Neurobiol. 2013;73:571–586. doi: 10.1002/dneu.22082. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen P.A., Beahm D.L., Giepmans B.N., Baruch A., Hall J.E., Kumar N.M. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J. Biol. Chem. 2002;277:38272–38283. doi: 10.1074/jbc.M205348200. [DOI] [PubMed] [Google Scholar]
- 15.White T., Srinivas M., Ripps H., Trovato-Salinaro A., Condorelli D., Bruzzone R. Virtual cloning, functional expression, and gating analysis of human connexin31.9. Am. J. Physiol. Cell Physiol. 2002;283:C960–C970. doi: 10.1152/ajpcell.00163.2002. [DOI] [PubMed] [Google Scholar]
- 16.Kreuzberg M., Söhl G., Kim J.S., Verselis V., Willecke K., Bukauskas F. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ. Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukauskas F., Kreuzberg M., Rackauskas M., Bukauskiene A., Bennett M., Verselis V., Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: Implications for atrioventricular conduction in the heart. Proc. Natl. Acad. Sci. USA. 2006;103:9726–9733. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Eastman S.D., Chen T.H., Falk M.M., Mendelson T.C., Iovine M.K. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics. 2006;87:265–274. doi: 10.1016/j.ygeno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cruciani V., Mikalsen S.O. Evolutionary selection pressure and family relationships among connexin genes. Biol. Chem. 2007;388:253–264. doi: 10.1515/BC.2007.028. [DOI] [PubMed] [Google Scholar]
- 21.Tao W., Zhang S., Turenchalk G.S., Stewart R.A., John M.A.R.S., Chen W., Xu T. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nature. 1999;21:177–181. doi: 10.1038/5960. [DOI] [PubMed] [Google Scholar]
- 22.Stebbings L.A., Todman M.G., Phelan P., Bacon J.P., Davies J.A. Two Drosophila Innexins Are Expressed in Overlapping Domains and Cooperate to Form Gap-Junction Channels. Mol. Biol. Cell. 2000;11:2459–2470. doi: 10.1091/mbc.11.7.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starich T., Sheehan M., Jadrich J., Shaw J. Innexins in C. elegans. Cell Commun. Adhes. 2001;8:311–314. doi: 10.3109/15419060109080744. [DOI] [PubMed] [Google Scholar]
- 24.Leake L.D. The leech as a scientific tool. Endeavour. 1983;7:88–93. doi: 10.1016/S0160-9327(83)80008-8. [DOI] [PubMed] [Google Scholar]
- 25.Wagenaar D.A. A classic model animal in the 21st century: Recent lessons from the leech nervous system. J. Exp. Biol. 2015;218:3353–3359. doi: 10.1242/jeb.113860. [DOI] [PubMed] [Google Scholar]
- 26.Sargent P.B., Nicholls G. Extrasynaptic Receptors on Cell Bodies of Neurons in Central Nervous System of the Leech. J. Neurophysiol. 1977;40 doi: 10.1152/jn.1977.40.2.446. [DOI] [PubMed] [Google Scholar]
- 27.Leake L.D., Crowe R., Burnstock G. Localisation of substance P-, somatostatin-, vasoactive intestinal polypeptide- and met-enkephalin-immunoreactive nerves in the peripheral and central nervous systems of the leech (Hirudo medicinalis) Cell Tisue Res. 1986:345–351. doi: 10.1007/BF00251050. [DOI] [Google Scholar]
- 28.Nicholls D.G., Sihra T.S., Sanchez-Prieto J. Calcium-Dependent and-Independent Release of Glutamate from Synaptosomes Monitored by Continuous Fluorometry. J. Neurochem. 1987;49:50–57. doi: 10.1111/j.1471-4159.1987.tb03393.x. [DOI] [PubMed] [Google Scholar]
- 29.Kristan W.B., Stent G.S., Ort C.A. Neuronal Control of Swimming in the Medicinal Leech. J. Comp. Physiol. A. 1974;119:97–119. doi: 10.1007/BF00617837. [DOI] [Google Scholar]
- 30.Lent C.M., Dickinson M.H. Serotonin integrates the feeding behavior of the medicinal leech. J. Comp. Physiol. A. 1984:457–471. doi: 10.1007/BF00610161. [DOI] [Google Scholar]
- 31.Misell L.M., Shaw B.K., Kristan W.B., Jr. Behavioral hierarchy in the medicinal leech, Hirudo medicinalis: Feeding as a dominant behavior. Behav. Brain Res. 1998;90:13–21. doi: 10.1016/S0166-4328(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 32.Dykes I.M., Macagno E.R. Molecular characterization and embryonic expression of innexins in the leech Hirudo medicinalis. Dev. Genes Evol. 2006;216:185–197. doi: 10.1007/s00427-005-0048-1. [DOI] [PubMed] [Google Scholar]
- 33.Kandarian B., Sethi J., Wu A., Baker M., Yazdani N., Kym E., Sanchez A., Edsall L., Gaasterland T., Macagno E. The medicinal leech genome encodes 21 innexin genes: Different combinations are expressed by identified central neurons. Dev. Genes Evol. 2012;222:29–44. doi: 10.1007/s00427-011-0387-z. [DOI] [PubMed] [Google Scholar]
- 34.Firme C.P., Natan R.G., Yazdani N., Macagno E.R., Baker M.W. Ectopic Expression of Select Innexins in Individual Central Neurons Couples Them to Pre-Existing Neuronal or Glial Networks That Express the Same Innexin. J. Neurosci. 2012;32:14265–14270. doi: 10.1523/JNEUROSCI.2693-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo T.M. Transient Electrical Coupling Delays the Onset of Chemical Neurotransmission at Developing Synapses. J. Neurosci. 2004;24:112–120. doi: 10.1523/JNEUROSCI.4336-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin-Burgin A., Eisenhart F.J., Kristan W.B., Jr., French K.A. Embryonic electrical connections appear o prefigure a behavioral circuit in the leech CNS. J. Comp. Physiol. A. 2006:123–133. doi: 10.1007/s00359-005-0055-8. [DOI] [PubMed] [Google Scholar]
- 37.Kristan W.B., Wittenberg G., Nusbaum M.P., Stern-Tomlinson W. Multifunctional interneurons in behavioral circuits of the medicinal leech. Experientia. 1988;44:383–389. doi: 10.1007/BF01940531. [DOI] [PubMed] [Google Scholar]
- 38.Beyer E.C., Paul D.L., Goodenough D.A. Connexin Family of Gap Junction Proteins. J. Membr. Biol. 1990;194:187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- 39.Harris A.L. Voltage-sensing and Substate Rectification: Moving Parts of Connexin Channels. J. Gen. Physiol. 2002;119 doi: 10.1085/jgp.119.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglesias R., Spray D.C., Scemes E. Mefloquine Blockade of Pannexin1 Currents: Resolution of a Conflict. Cell Commun. Adhes. 2010;16:131–137. doi: 10.3109/15419061003642618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar N.M., Gilula N.B. The Gap Juntion Communication Channel. Cell. 1996;84:381–388. doi: 10.1016/S0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 42.Foote C.I., Zhou L., Zhu X., Nicholson B.J. The Pattern of Disulfide Linkages in the Extracellular Loop Regions of Connexin 32 Suggests a Model for the Docking Interface of Gap Junctions. J. Cell Biol. 1998;140:1187–1198. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sosinsky G.E., Nicholson B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Oshima A., Tani K., Fujiyoshi Y. Atomic structure of the innexin-6 gap junction channel determined by cryo-EM. Nat. Commun. 2016;7:1–8. doi: 10.1038/ncomms13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unwin P.N.T., Zamphigi G. Structure of the juntion between communicating cells. Nature. 1980;283:545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- 46.Müller D.J., Hand G.M., Engels A., Sosinsky G.E. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett B.C., Purdy M.D., Baker K.A., Acharya C., Mcintire W.E., Stevens R.C., Zhang Q., Harris A.L., Abagyan R., Yeager M. An electrostatic mechanism for Ca2+-mediated regulation of gap junction channels. Nat. Commun. 2016;7:1–12. doi: 10.1038/ncomms9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh S., Rivkin S., Tang Q., Verselis V.K., Bargiello T.A. Determinants of Gating Polarity of a Connexin 32 Hemichannel. Biophys. J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bargiello T.A., Tang Q., Oh S., Kwon T. Voltage-dependent conformational changes in connexin channels. Biochim. Biophys. Acta Biomembr. 2012;1818:1807–1822. doi: 10.1016/j.bbamem.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skerrett I.M., Williams J.B. A structural and functional comparison of gap junction channels composed of onnexins and innexins. Dev. Neurobiol. 2017;77:522–547. doi: 10.1002/dneu.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manjarrez-Marmolejo J., Franco-Pérez J. Gap Junction Blockers: An Overview of their Effects on Induced Seizures in Animal Models. Curr. Neuropharmacol. 2016:759–771. doi: 10.2174/1570159X14666160603115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todd K.L., Kristan W.B., French K.A. Gap Junction Expression Is Required for Normal Chemical Synapse Formation. J. Neurosci. 2010;30:15277–15285. doi: 10.1523/JNEUROSCI.2331-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl G. Gap Junction—Mimetic Peptides do Work, but in Unexpected Ways. Cell Commun. Adhes. 2009:9061. doi: 10.1080/15419060801891018. [DOI] [PubMed] [Google Scholar]
- 54.Nogi T., Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev. Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Johnston M., Simon S., Ramón F. Interaction of anesthetics with electrical synapses. Nature. 1980;286:498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- 56.Allaire K.M., Watson G.M. Rho participates in chemoreceptor-induced changes in morphology to hair bundle mechanoreceptors of the sea anemone, Nematostella vectensis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013;165:139–148. doi: 10.1016/j.cbpa.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Tse G., Yeo J.M., Tse V., Kwan J., Sun B. Gap junction inhibition by heptanol increases ventricular rhythmogenicity by reducing conduction velocity without affecting repolarization properties or myocardial refractoriness in Langendorff-perfused mouse hearts. Mol. Med. Rep. 2016;14:4069–4074. doi: 10.3892/mmr.2016.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samuels S.E., Lipitz J.B., Wang J., Dahl G., Muller K.J. Arachidonic acid closes innexin/pannexin hannels and thereby inhibits microglia cell movement to a nerve injury. Dev. Neurobiol. 2013;73:621–631. doi: 10.1002/dneu.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mire P., Nasse J., Venable-thibodeaux S. Gap junctional communication in the vibration-sensitive response of sea anemones. Hear. Res. 2000;144:109–123. doi: 10.1016/S0378-5955(00)00047-2. [DOI] [PubMed] [Google Scholar]
- 60.Adler E.L., Woodruff R.I., Chester W. Varied Effects of 1-Octanol on Gap Junctional Communication Between Ovarian Epithelial Cells and Oocytes of Oncopeltus fasciatus, Hyalophora cecropia, and Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2000;43:22–32. doi: 10.1002/(SICI)1520-6327(200001)43:1<22::AID-ARCH4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 61.Anderson K.L., Woodruff R.I. A gap junctionally transmitted epithelial cell signal regulates endocytic yolk uptake in Oncopeltus fasciatus. Dev. Biol. 2001;239:68–78. doi: 10.1006/dbio.2001.0433. [DOI] [PubMed] [Google Scholar]
- 62.Brooks R.A., Woodruff R.I. Calmodulin transmitted through gap junctions stimulates endocytic incorporation of yolk precursors in insect oocytes. Dev. Biol. 2004;271:339–349. doi: 10.1016/j.ydbio.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 63.Yoshimura R., Suetsugu T., Endo Y. Serotonergic transmission and gap junctional coupling in proventricular muscle cells in the American cockroach, Periplaneta americana. J. Insect Physiol. 2017;99:122–129. doi: 10.1016/j.jinsphys.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Talaverón R., Fernández P., Escamilla R., Pastor A.M., Hayley S. Neural progenitor cells isolated from the subventricular zone present hemichannel activity and form functional gap junctions with glial cells. Front. Cell. Neurosci. 2015;9:1–10. doi: 10.3389/fncel.2015.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagiava A., Theophilidis G., Sargiannidou I., Kyriacou K., Kleopa K.A. Oxaliplatin-induced neurotoxicity is mediated through gap junction channels and hemichannels and can be prevented by octanol. Neuropharmacology. 2015;97:289–305. doi: 10.1016/j.neuropharm.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 66.Takano K., Ogawa M., Kawabe K., Moriyama M. Inhibition of Gap Junction Elevates Glutamate Uptake in Cultured Astrocytes. Neurochem. Res. 2017 doi: 10.1007/s11064-017-2316-7. [DOI] [PubMed] [Google Scholar]
- 67.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Lett. Nat. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 68.Wang J., Ma M., Locovei S., Keane R.W., Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: Size matters. Am. J. Physiol. Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 69.Talbot J., Brion R., Lamora A., Mullard M., Morice S., Heymann D., Verrecchia F. Connexin43 intercellular communication drives the early differentiation of human bone marrow stromal cells into osteoblasts. J. Cell. Physiol. 2018;233:946–957. doi: 10.1002/jcp.25938. [DOI] [PubMed] [Google Scholar]
- 70.O’Donnell J.J., III, Birukova A.A., Beyer E.C., Birukov K.G. Gap junction protein connexin43 exacerbates lung vascular permeability. PLoS ONE. 2014;9:e100931. doi: 10.1371/journal.pone.0100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osbourne A., Calway T., Broman M., McSharry S., Earley J., Kim G.H. Downregulation of connexin43 by microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J. Mol. Cell. Cardiol. 2014;74:53–63. doi: 10.1016/j.yjmcc.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gärtner C., Ziegelhöffer B., Kostelka M., Stepan H., Mohr F.W., Dhein S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol. Res. 2012;65:347–357. doi: 10.1016/j.phrs.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Anava S., Saad Y., Ayali A. The role of gap junction proteins in the development of neural network functional topology. Insect Mol. Biol. 2013;22:457–472. doi: 10.1111/imb.12036. [DOI] [PubMed] [Google Scholar]
- 74.Li M.W., Wang J., Zhao Y.O., Fikrig E. Innexin AGAP001476 is critical for mediating anti-Plasmodium responses in Anopheles mosquitoes. J. Biol. Chem. 2014;289:24885–24897. doi: 10.1074/jbc.M114.554519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calkins T.L., Piermarini P.M. Pharmacological and Genetic Evidence for Gap Junctions as Potential New Insecticide Targets in the Yellow Fever Mosquito, Aedes aegypti. PLoS ONE. 2015:1–15. doi: 10.1371/journal.pone.0137084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calkins T.L., Piermarini P.M. A Blood Meal Enhances Innexin mRNA Expression in the Midgut, Malpighian Tubules, and Ovaries of the Yellow Fever Mosquito Aedes aegypti. Insects. 2017;8:122. doi: 10.3390/insects8040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulz R., Görge P.M., Görbe A., Ferdinandy P., Lampe P.D., Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riquelme M.A., Kar R., Gu S., Jiang J.X. Neuropharmacology Antibodies targeting extracelular domain of connexins for studies of hemichannels. Neuropharmacology. 2013;75:525–532. doi: 10.1016/j.neuropharm.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bastide B., Briand J.P., Gros D. Effect of Antipeptide Antibodies Directed against Three Domains of Connexin43 on the Gap Junctional Permeability of Cultured Heart Cells. J. Membr. Biol. 1996;253:243–253. doi: 10.1007/s002329900048. [DOI] [PubMed] [Google Scholar]
- 80.Hertzberg E.L., Sa J.C., Corpina R.A., Roy C., Kessler J.A. Use of Antibodies in the Analysis of Connexin 43 Turnover and Phosphorylation. Acad. Press. 2000;20:129–139. doi: 10.1006/meth.1999.0931. [DOI] [PubMed] [Google Scholar]
- 81.Sosinsky G.E., Solan J.L., Gaietta G.M., Ngan L., Lee G.J., Mackey M.R., Lampe P.D. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antobodies. Biochem. J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clair C., Combettes L., Pierre F., Sansonetti P., Tran G., Nhieu V. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp. Cell Res. 2008;4 doi: 10.1016/j.yexcr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 83.Bohrmann J., Zimmermann J. Gap junctions in the ovary of Drosophila melanogaster: Localization of innexins 1, 2, 3 and 4 and evidence for intercellular communication via innexin-2 containing channels. BMC Dev. Biol. 2008;8:1–12. doi: 10.1186/1471-213X-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Güiza J., Barría I., Sáez J.C., Vega J.L. Innexins: Expression, Regulation, and Functions. Front. Physiol. 2018;9:1414. doi: 10.3389/fphys.2018.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evers M.M., Toonen L.J.A., Roon-mom W.M.C.V. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015;87:90–103. doi: 10.1016/j.addr.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Schoch K.M., Miller T.M. Review Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron. 2017;94:1056–1070. doi: 10.1016/j.neuron.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rinaldi C., Wood M.J.A. Antisense oligonucleotides: The next disorders. Nat. Rev. Neurol. 2017 doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 88.Green C.R., Law L., Lin J.S., Becker D.L. Spatiotemporal Depletion of connexins Using Antisense Oligonucleotides. Methods Mol. Biol. 2001;154:175–185. doi: 10.1385/1-59259-043-8:175. [DOI] [PubMed] [Google Scholar]
- 89.Danesh-Meyer H.V., Huang R., Nicholson L.F.B., Green C.R. Connexin43 antisense oligodeoxynucleotide treatment down-regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J. Clin. Neurosci. 2008;15:1253–1263. doi: 10.1016/j.jocn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Grupcheva C.N., Laux W.T., Rupenthal I.D., Mcghee J., Mcghee C.N.J., Green C.R. Improved Corneal Wound Healing through Modulation Connexin43-Specific Antisense Oligodeoxynucleotides. Investig. Ophthalmol. Vis. Sci. 2012:1130–1138. doi: 10.1167/iovs.11-8711. [DOI] [PubMed] [Google Scholar]
- 91.Dahl G., Werner R., Levine E., Rabadan-diehl C. Mutational analysis of gap junction formation. Biophys. J. 1992;62:172–182. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dahl G., Nonner W., Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys. J. 1994;67:1816–1822. doi: 10.1016/S0006-3495(94)80663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwak B.R., Jongsma H.J. Selective inhibition of gap junction channel activity by synthetic peptides. J. Physiol. 1999;516:679–685. doi: 10.1111/j.1469-7793.1999.0679u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin P.E.M., Wall C., Griffith T.M. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br. J. Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Z. Big data mining powers fungal research: Recent advances in fission yeast systems biology approaches. Curr. Genet. 2016 doi: 10.1007/s00294-016-0657-4. [DOI] [PubMed] [Google Scholar]
- 96.De Castro L. Fundamentals of natural computing: An overview. Phys. Life Rev. 2006;4:1–36. doi: 10.1016/j.plrev.2006.10.002. [DOI] [Google Scholar]
- 97.Mondal A., Moreno A.P. Heteromultimeric gap-junction channel permeance: Directional fluxes simulated using a Brownian dynamics model. Biophys. J. 2010;98:94a–95a. doi: 10.1016/j.bpj.2009.12.532. [DOI] [Google Scholar]
- 98.Kwon T., Harris A.L., Rossi A., Bargiello T.A. Molecular dynamics simulations of the Cx26 hemichannel: Evaluation of structural models with Brownian dynamics. J. Gen. Physiol. 2011;138:475–493. doi: 10.1085/jgp.201110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mondal A., Appadurai D.A., Nazem W., Sachse F.B., Moreno A.P. Computational Simulations of Asymmetric Fluxes of Large Molecules Through Gap Junction Channel Pores. J. Theor. Biol. 2016 doi: 10.1016/j.jtbi.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 100.Bargiello T.A., Oh S., Tang Q., Bargiello N.K., Dowd T.L., Kwon T. Gating of Connexin Channels by transjunctional-voltage: Conformations and models of open and closed states. Biochim. Biophys. Acta Biomembr. 2018;1860:22–39. doi: 10.1016/j.bbamem.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Janjua K., Garyantes T., Baron B. Identification of Gap Junction Blockers Using Automated Fluorescence Microscopy Imaging. J. Biomol. Screen. 2003;8:489–499. doi: 10.1177/1087057103257309. [DOI] [PubMed] [Google Scholar]
- 102.Casaleggio A., Hines M.L., Migliore M. Computational Model of Erratic Arrhythmias in a Cardiac Cell Network: The Role of Gap Junctions. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kononenko I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001;23:89–109. doi: 10.1016/S0933-3657(01)00077-X. [DOI] [PubMed] [Google Scholar]
- 104.Fooshee D., Mood A., Gutman E., Tavakoli M., Urban G., Liu F., Huynh N., van Vranken D., Baldi P. Deep learning for chemical reaction prediction. Mol. Syst. Des. Eng. 2018 doi: 10.1039/C7ME00107J. [DOI] [Google Scholar]
- 105.Wang J., Ding H., Bidgoli F.A., Zhou B., Iribarren C., Molloi S., Baldi P. Detecting Cardiovascular Disease from Mammograms with Deep Learning. IEEE Trans. Med Imaging. 2017;36:1172–1181. doi: 10.1109/TMI.2017.2655486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baldi P. Deep Learning in Biomedical Data Science. Annu. Rev. 2018:181–205. doi: 10.1146/annurev-biodatasci-080917-013343. [DOI] [Google Scholar]
- 107.Cai Y.D., Zhou G.P., Chou K.C. Support Vector Machines for Predicting Membrane Protein Types by Using Functional Domain Composition. Biophys. J. 2003;84:3257–3263. doi: 10.1016/S0006-3495(03)70050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guermeur Y., Pollastri G., Zelus D. Combining protein secondary structure prediction models with ensemble methods of optimal complexity. Neurocomputing. 2004;56:305–327. doi: 10.1016/j.neucom.2003.10.004. [DOI] [Google Scholar]
- 109.Lee E.Y., Fulan B.M., Wong G.C.L., Ferguson A.L. Mapping membrane activity in undiscovered peptide sequence space using machine learning. Proc. Natl. Acad. Sci. USA. 2016;113 doi: 10.1073/pnas.1609893113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karamzadeh R., Karimi-jafari M.H., Zarchi A.S. Machine Learning and Network Analysis of Molecular Dynamics Trajectories Reveal Two Chains of Red/Ox-specific Residue Interactions in Human Protein Disulfide Isomerase. Nat. Sci. Rep. 2017:1–11. doi: 10.1038/s41598-017-03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhardwaj N., Langlois R.E., Zhao G., Lu H. Kernel-based machine learning protocol for predicting DNA-binding proteins. Nucleic Acids Res. 2005;33:6486–6493. doi: 10.1093/nar/gki949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ballester P.J., Mitchell J.B.O. A machine learning approach to predicting protein—ligand binding affinity with applications to molecular docking. Bioinformatics. 2010;26:1169–1175. doi: 10.1093/bioinformatics/btq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.King R.D., Sternbergt M.J.E. Machine Learning Approach for the Prediction of Protein Secondary Structure. J. Mol. Biol. 1990;441:441–457. doi: 10.1016/S0022-2836(05)80333-X. [DOI] [PubMed] [Google Scholar]
- 114.Nielsen H., Brunak S., Heijne G.V. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 1999;12:3–9. doi: 10.1093/protein/12.1.3. [DOI] [PubMed] [Google Scholar]
- 115.Gourdie R.G., Green C.R., Severs N.J. Gap junction distribution in adult mammalian myocardium revealed by an anti-peptide antibody and laser scanning confocal microscopy. Pt 1J. Cell. Sci. 1991;99:41–55. doi: 10.1242/jcs.99.1.41. [DOI] [PubMed] [Google Scholar]
- 116.Rawanduzy A., Hansen A., Hansen T.W., Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J. Neurosurg. 1997;87:916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- 117.Takeuchi H., Mizoguchi H., Doi Y., Jin S., Noda M., Liang J. Blockade of Gap Junction Hemichannel Suppresses Disease Progression in Mouse Models of Amyotrophic Lateral Sclerosis and Alzheimer’s Disease. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor J., King R.D., Altmann T., Fiehn O. Application of metabolomics to plant genotype discrimination using statistics and machine learning. Bioinformatics. 2002;18:241–248. doi: 10.1093/bioinformatics/18.suppl_2.S241. [DOI] [PubMed] [Google Scholar]
- 119.Korolev D., Balakin K.V., Nikolsky Y., Kirillov E., Ivanenkov Y.A., Savchuk N.P., Ivashchenko A.A., Nikolskaya T. Modeling of Human Cytochrome P450-Mediated Drug Metabolism Using Unsupervised Machine Learning Approach. J. Med. Chem. 2003;46:3631–3643. doi: 10.1021/jm030102a. [DOI] [PubMed] [Google Scholar]
- 120.Ziliang Y.D.C., Lin Q., Xin K.Y.F., Bing M., Lu G.D.Z.W.C. Prediction of compounds’ biological function (metabolic pathways) based on functional group composition. Mol. Divers. 2008:131–137. doi: 10.1007/s11030-008-9085-9. [DOI] [PubMed] [Google Scholar]
- 121.Plaimas K., Mallm J.P., Oswald M., Svara F., Sourjik V., Eils R., König R. Machine learning based analysis on metabolic networks supports high-throughput knockout screens. BMC Syst. Biol. 2008;11:1–11. doi: 10.1186/1752-0509-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dale J.M., Popescu L., Karp P.D. Machine learning methods for metabolic pathway prediction. BMC Bioinform. 2010;11 doi: 10.1186/1471-2105-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martínez-Romero M., Vázquez-Naya J.M., Rabuñal J.R., Pita-Fernández S., Macenlle R., Castro-Alvariño J., López-Roses L., Ulla J.L., Martínez-Calvo A.V., Vázquez S., et al. Artificial Intelligence Techniques for Colorectal Cancer Drug Metabolism: Ontologies and Complex Networks. Curr. Drug Metab. 2010;11:347–368. doi: 10.2174/138920010791514289. [DOI] [PubMed] [Google Scholar]
- 124.Lima A.N., Philot E.A., Goulart G.H., Paulo L., Scott B., Maltarollo V.G. Expert Opinion on Drug Discovery Use of machine learning approaches for novel drug discovery. Expert Opin. Drug Discov. 2016;11:225–239. doi: 10.1517/17460441.2016.1146250. [DOI] [PubMed] [Google Scholar]
- 125.Cai Y.D., Liu X.J., Xu X.B., Chou K.C. Support Vector Machines for Predicting HIV Protease Cleavage Sites in Protein. J. Comput. Chem. 2002;23:267–274. doi: 10.1002/jcc.10017. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y.H., Li Y., Yang S.L., Yang L. Classification of Substrates and Inhibitors of P-Glycoprotein Using Unsupervised Machine Learning Approach. J. Chem. Inf. Model. 2005;45:750–757. doi: 10.1021/ci050041k. [DOI] [PubMed] [Google Scholar]
- 127.Kinnings S.L., Liu N., Tonge P.J., Jackson R.M., Xie L., Bourne P.E. A Machine Learning-Based Method to Improve Docking Scoring Functions and Its Application to Drug Repurposing. J. Chem. Inf. Model. 2011:408–419. doi: 10.1021/ci100369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Valdebenito S., Lou E., Baldoni J., Okafo G. The Novel Roles of Connexin Channels and Tunneling Nanotubes in Cancer Pathogenesis. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19051270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vargas A.A., Cisterna B.A., Saavedra-Leiva F., Urrutia C., Cea L.A., Vielma A.H., Gutierrez-Maldonado S.E., Martin A.J.M., Pareja-Barrueto C., Escalona Y., et al. On Biophysical Properties and Sensitivity to Gap Junction Blockers of Connexin 39 Hemichannels Expressed in HeLa Cells. Front. Physiol. 2017;8:1–15. doi: 10.3389/fphys.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bauer R., Löer B., Ostrowski K., Martini J., Weimbs A., Lechner H., Hoch M. Intercellular Communication: The Drosophila Innexin Multiprotein Family of Gap Junction Proteins. Chem. Biol. 2005;12:515–526. doi: 10.1016/j.chembiol.2005.02.013. [DOI] [PubMed] [Google Scholar]