Abstract
Background: Exercise preconditioning (EP+) is a useful and important procedure for the prevention of stroke. We aimed to ascertain whether EP+ protects against ischemic brain injury by preserving heat shock protein (HSP) 72-containing neurons in ischemic brain tissues.
Methods: Adult male Sprague-Dawley rats (n=240) were used to assess the contribution of HSP72-containing neurons to the neuroprotective effects of EP+ on ischemic brain injury caused by transient middle cerebral artery occlusion.
Results: Significant (P<0.05) increases in the percentages of both old HSP72-containing neurons (NeuN+HSP72 double positive cells) (18~20% vs. 40~50%) and newly formed HSP72-containing neurons (BrdU+NeuN+HSP72 triple positive cells); (2~3% vs. 16~20%) after 3 weeks of exercise coincided with significant (P<0.05) reductions in brain ischemia volume (250 mm3 vs. 100 mm3), brain edema (78% vs. 74% brain water content), blood-brain barrier disruption (1.5 μg/g vs. 0.7 μg/g tissue Evans Blue dye extravasation) and neurological motor deficits (neurological severity scores of 12 vs. 6 and maximal angles of 60° vs. 20°) in brain ischemia rats. Reductions in the percentages of both old (from 40~50% to 10~12%) and newly formed (from 18~20% to 5~7%) HSP72-containing neurons by gene silencing with an intracerebral injection of pSUPER small interfering RNA showed a significant (P<0.05) reversal in the neuroprotective outcomes. Our data provide an inverse correlation between the EP+-mediated increases in both old and newly formed HSP72-containing neurons and the extent of cerebral ischemic injury.
Conclusions: The percentages of both old and newly formed HSP72-containing neurons are inversely correlated with the outcomes of ischemic brain injury. Additionally, preischemic treadmill exercise improves the outcomes of ischemic brain injury by preserving both the old and newly formed HSP72-containing neurons in rats.
Keywords: Exercise, heat shock protein 72, neuroprotection, ischemic brain injury
Introduction
The American Heart Association/American Stroke Association has promoted that the primary prevention of stroke is particularly important 1. Exercise preconditioning (EP+) is widely believed to be a safe and effective preventive measure for people who undergo a stroke 2. Clinical data have also shown that physical inactivity before stroke predicts a higher risk of being dependent both before and after stroke 3, 4. Although preischemic EP+ can attenuate neurobehavioral deficits after ischemic brain injury 5, the exact neuroprotective mechanisms of preischemic EP+ against cerebral ischemic injury remain unclear.
In the normal rat brain, there is little to no expression of inducible heat shock protein (HSP) 72 6. However, following occlusion of the middle cerebral artery, HSP72 induction in the ischemic core, or penumbra, occurs primarily in neurons, but HSP72 can also be expressed in astrocytes, microglia and endothelial cells 6, 7. On the other hand, EP+ increases neuronal and astroglial levels of HSP72 in the gray matter of spinal cord tissue in normal rats 8. Transfection and viral overexpression of HSP72 in neurons and glia protects against in vitro ischemia 6 and in vivo ischemia 8, 9. Additionally, knocking out HSP72 worsens the outcomes and transgenic overexpression improves the outcomes of cerebral ischemia 10. Pharmacological induction of HSP72 protects against cerebral ischemia 11. Although it is well known that EP+ reduces neuronal apoptosis in stroke rats by upregulating HSP72 in the brain 12, it is unknown whether EP+ increases neuronal expression of HSP72 in normal rat brain tissues and protects against ischemic brain injury in rats by preserving old and newly formed HSP72-containing neurons.
In the present study, we first used immunofluorescence staining methods to elucidate whether old and/or newly formed HSP72-containing neurons can be upregulated by preischemic EP+ in rats with or without middle cerebral artery occlusion (MCAO). Second, we describe the contributions of old and/or newly formed HSP72-containing neurons to EP+-mediated attenuation of neurological injury (including brain infarct, neurological motor deficits, blood-brain barrier (BBB) disruption, and brain edema) in a ischemic brain injury model in rats 13. To inhibit HSP72 expression in the brain tissue, the ischemic brains were intraoperatively microinjected with pSUPER plasmid expressing HSP72 small interfering RNA (siRNA-HSP72) 12, 14.
Materials and methods
Animals and stroke model
Two hundred forty adult male Sprague-Dawley rats (weight, 248±15 g) were housed under controlled environmental conditions with an ambient temperature of 24±2°C, a relative humidity of 65% and a 12-h light/dark cycle, with free access to food and water. Three weeks after treadmill EP+, ischemic brain injury was induced by MCAO in rats by intraluminal filaments, using the relatively noninvasive technique detailed previously 15. The protocol was approved by the Institutional Review Board for Animal Care and Use (Assurance Number: 100120755).
Recombinant pSUPER plasmid expressing siRNA-HSP72 constructed using the pSUPER vector (Oligo Engine, Seattle, WA, USA), which contains the polymerase-III H1-RNA gene promoter and can direct the synthesis of siRNA-like transcripts. The target sequence for HSP72 (Gen Bank Accession No. NM-0319712) was chemically synthesized (Tri-1 Biotech, Taipei, Taiwan) as complementary oligonucleotides. The synthetic oligonucleotides of siRNA-HSP72 5'-GATCCCCGGAGATCATCGCCAACGACTAAGAGAGTTGGCGATGATCTCCTTTTTGGAAA-3' and 3'-GGGCCTCTAGTAGCGGTTGCTGAAGTTCTCTCAGCAACCGCTACTAGAGGAAAAACCTTTTCGAA-5' were annealed and cloned downstream of the H1 promoter to construct a recombinant pSUPER HSP72 plasmid. The cloned HSP72 target sequence was sequence-confirmed using a DNA sequencer (ABI Prism 377, Applied Biosystems, Foster City, CA, USA). During the MCAO surgery, an acute dose of siRNA-HSP72 (12.5 μg/rat in 25 μl of pSUPER RNAi delivery media [siRNA-vector]) was microinjected into the frontal cortex at a flow rate of 0.5 μl/min using a microinfusion pump (CMA 100, Carnegie Medicine AB, Stockholm, Sweden) according to the coordinates of the atlas of Paxinos and Watson 16. A single 28-gauge stainless steel injection cannula was lowered into the right frontal cortex (coordinates: 12 mm anterior to bregma, 4.6 mm lateral to midline and 3.0 mm ventral to the skull surface).
Exercise preconditioning (EP+) protocol
Animals were trained on a treadmill (model Exer-3/6, Columbus Instruments, Columbus, OH, USA) 5 days a week for 3 weeks. They were acclimatized to run for 15 min at 20 m/min at 0° for 3 days initially, and then they were running for 30 min at 20 m/min, 30 min at 30 m/min and 60 min at 30 m/min after 1, 2 and 3 weeks of training, respectively. Nonexercise preconditioning controls (EP-) were placed daily on a stationary treadmill and were given electrical stimulation in a manner identical to that used for the EP+ group.
Exercise groups and procedures
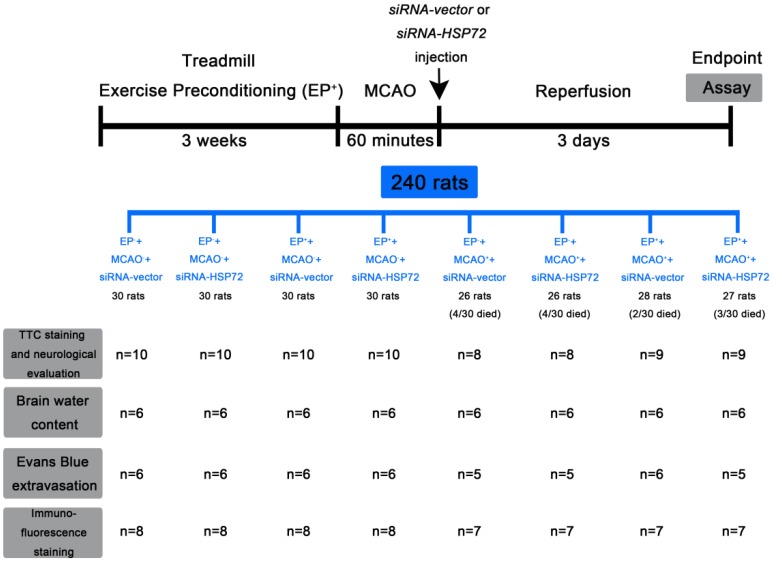
The animals were randomly assigned to one of eight groups: (i) EP-+MCAO- rats that received an intracerebral injection of siRNA-vector (EP-+MCAO-+siRNA-vector), (ii) EP-+MCAO-+siRNA-HSP72, (iii) EP++MCAO-+siRNA-vector, (iv) EP++MCAO-+siRNA-HSP72, (v) EP-+MCAO++siRNA-vector, (vi) EP-+MCAO++siRNA-HSP72, (vii) EP++MCAO++siRNA-vector, and (viii) EP++MCAO++siRNA-HSP72. The experimental procedures in this study are shown in Figure 1. Of all the rats in this study, 13 rats were excluded because of death prior to sacrifice, including 0 in the EP-+MCAO-+siRNA-vector group (i); 0 in the EP-+MCAO-+siRNA-HSP72 group (ii); 0 in the EP++MCAO-+siRNA-vector group (iii); 0 in the EP++MCAO-+siRNA-HSP72 group (iv); 4 in the EP-+MCAO++siRNA-vector group (v); 4 in the EP-+MCAO++siRNA-HSP72 group (vi); 2 in the EP++MCAO++siRNA-vector group (vii); 3 in the EP++MCAO++siRNA-HSP72 group (viii).
Figure 1.
Experimental procedure outline. Treadmill exercise preconditioning (EP+) was performed in Sprague-Dawley rats 3 weeks before middle cerebral artery occlusion (MCAO). The filament was removed after 60 min of MCAO to allow reperfusion. At the onset of reperfusion, separated groups of rats were given intracortical injections of 12.5 μg of siRNA-HSP72 or siRNA-vector. Three days after MCAO, neurological function tests, brain water content, Evans blue dye extravasation, and immunohistological examinations were performed.
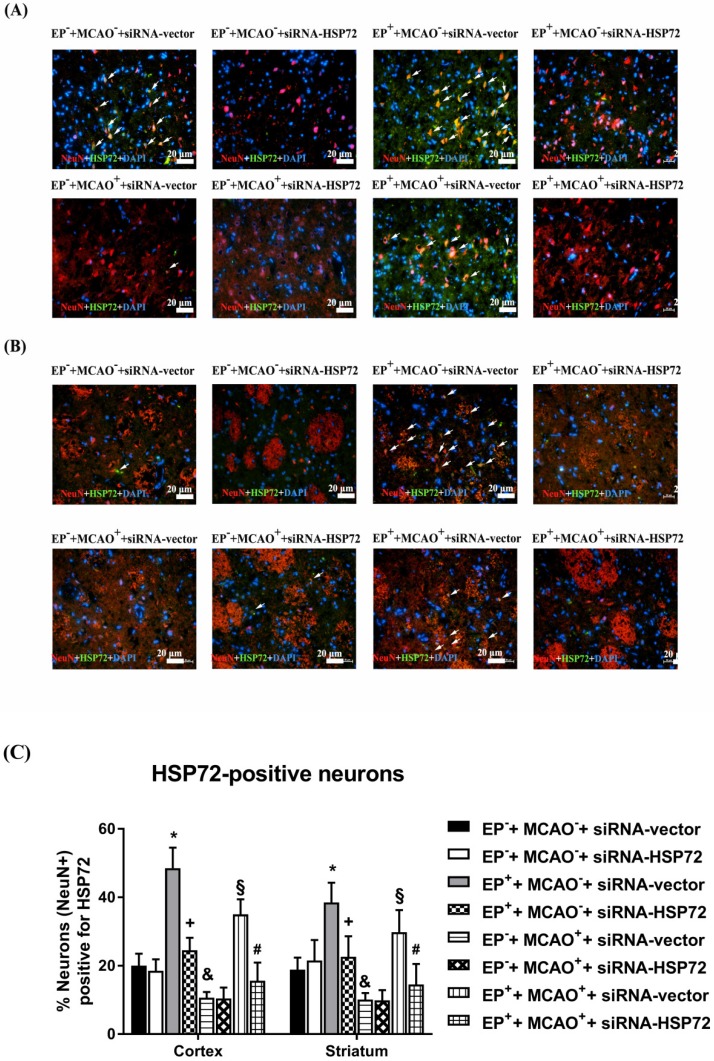
In experiment 1, the percentages of cortical and striatal old HSP72-containing neurons (i.e., HSP72+NeuN+DAPI-positive cells) were determined 3 days after the MCAO or sham operation in all groups (Figure 2).
Figure 2.
Immunofluorescence detection of heat shock protein (HSP)-72-containing (NeuN+HSP72+DAPI-positive) neurons in the frontal cortex (A) and striatum (B) 3 days after middle cerebral artery (MCAO). For group abbreviations, see the Methods section. Upper panels: the immunofluorescence images are representative of results from different groups of animals. Lower panels: values represent the mean ± SD of ten rats per group. *P<0.05, EP++MCAO-+siRNA-vector vs. EP-+MCAO-+siRNA-vector; +P<0.05, EP++MCAO-+siRNA-HSP72 vs. EP++MCAO-+siRNA-vector; &P<0.05, EP-+MCAO++siRNA-vector vs. EP-+MCAO-+siRNA-vector; §P<0.05, EP++MCAO++siRNA-vector vs. EP-+MCAO++siRNA-vector; and #P<0.05, EP++MCAO++siRNA-HSP72 vs. EP++MCAO++siRNA-vector. Arrows denote HSP72-containing neurons.
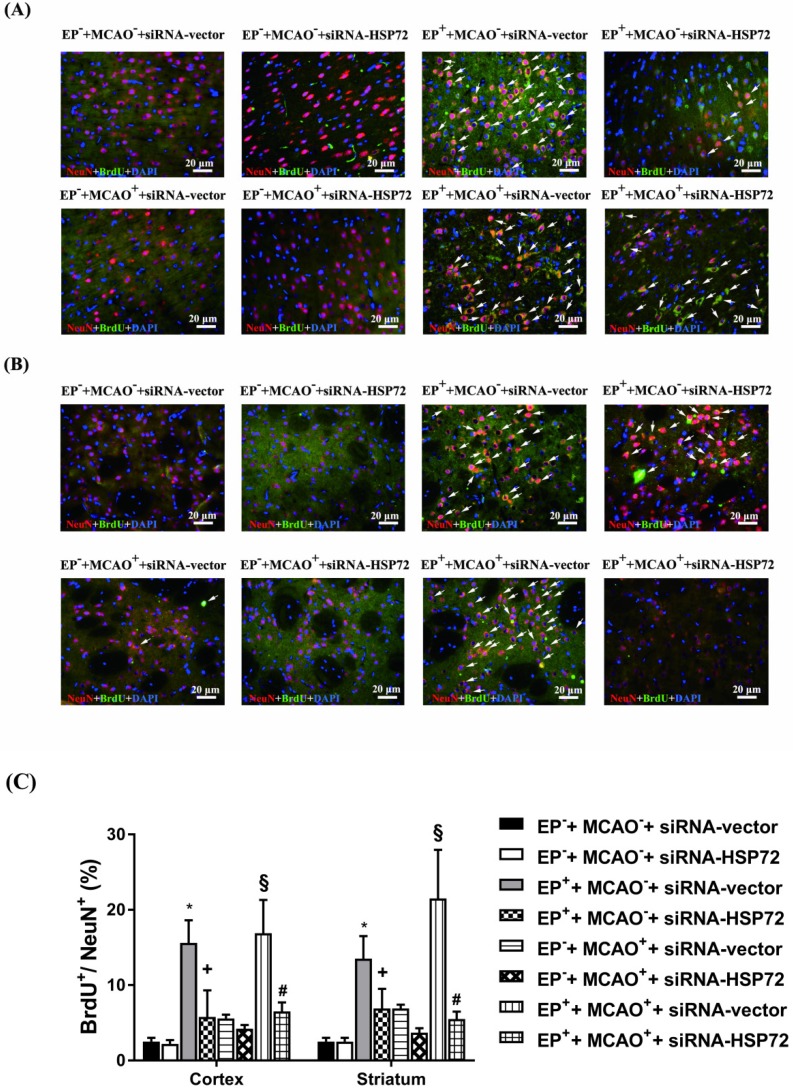
In experiment 2, the percentages of newly formed neurons containing HSP72 (i.e., HSP72+NeuN+BrdU+DAPI-positive cells) in cortical and striatal tissue of the ipsilateral brain were determined 3 days after MCAO or sham operation in all groups (Figure 3). We examined the same regions of the right frontal cortex and the striatum in both the ischemic brains and nonischemic brains.
Figure 3.
Immunofluorescence detection of newly formed HSP72-containing neurons (BrdU+NeuN+HSP72+DAPI-positive cells) in the frontal cortex (A) and striatum (B) 3 days after MCAO. For group abbreviations, see the Methods section. Upper panels: the immunofluorescence images are representative of results from different groups of animals. Lower panels: values represent the mean ± SD of ten rats per group. *P<0.05, EP++MCAO-+siRNA-vector vs. EP-+MCAO-+siRNA-vector; +P<0.05, EP++MCAO-+siRNA-HSP72 vs. EP++MCAO-+siRNA-vector; §P<0.05, EP++MCAO++siRNA-vector vs. EP-+MCAO++siRNA-vector; and #P<0.05, EP++MCAO++siRNA-HSP72 vs. EP++MCAO++siRNA-vector. Arrows denote newly formed HSP72-containing neurons.
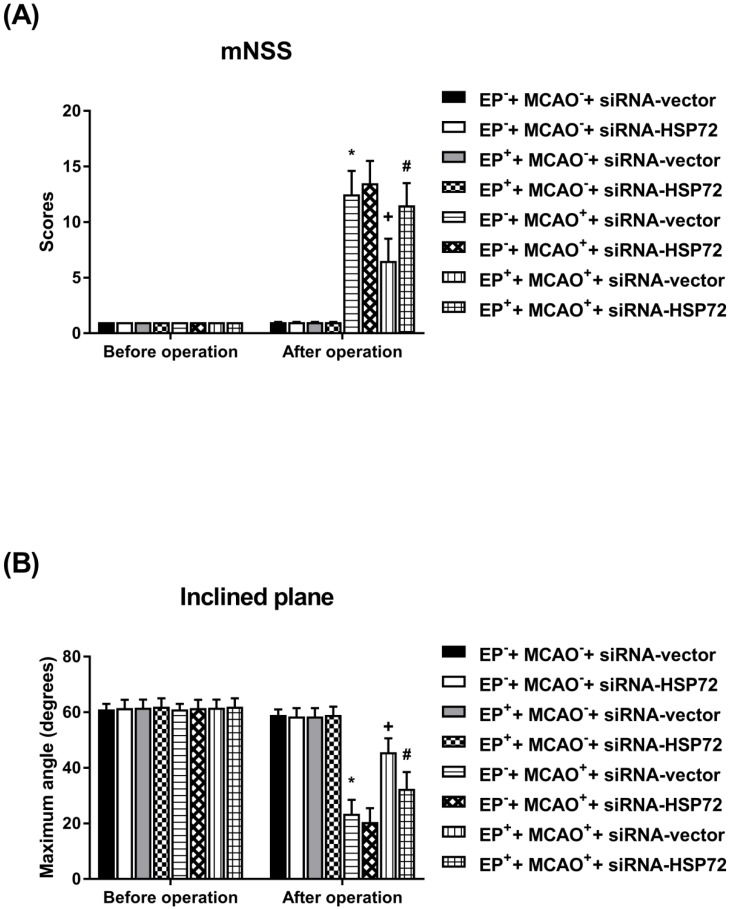
In experiment 3, the neurobehavioral functions were determined 1 day before the injury and 3 days after injury in all groups (Figure 4).
Figure 4.
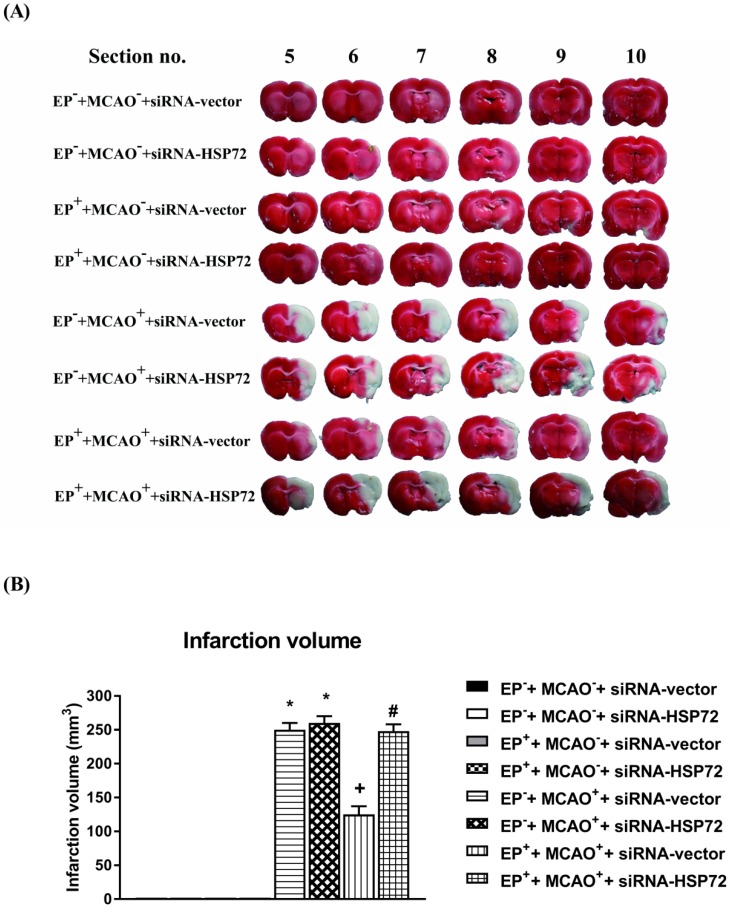
Effects of exercise on cerebral infarct volume in different groups of rats 3 days post-MCAO. Please see the Methods section for group abbreviations. Upper panels: the infarct volume (mm3), as revealed by negative 2,3,5-triphenyl tetrazolium chloride staining, indicating dehydrogenase-deficient tissue (pale area), was measured in each slice and summed using computerized planimetry for different groups of rats. Lower panels: values represent the mean ± SD of eight to ten rats per group.*P<0.01, EP-+MCAO++siRNA-vector vs. EP-+MCAO-+siRNA-vector; +P<0.05, EP++MCAO++siRNA-vector vs. EP-+MCAO++siRNA-vector; and #P<0.05, EP++MCAO++siRNA-HSP72 vs. EP++MCAO++siRNA-vector.
In experiment 4, the cerebral infarct volumes were determined 3 days after the MCAO or sham operation in all groups (Figure 5).
Figure 5.
Effects of exercise on both neurological severity scores (A) and maximal angles (B) in different groups of rats 3 days post-MCAO. Data represent the mean ± SD (n=8-10 per group). *P<0.01, EP-+MCAO++siRNA-vector vs. EP-+MCAO-+siRNA-vector or EP-+MCAO++siRNA-HSP72 vs. EP-+MCAO-+siRNA-vector; +P<0.05, EP++MCAO++siRNA-vector vs. EP-+MCAO++siRNA-vector; and #P<0.05, EP++MCAO++siRNA-HSP72 vs. EP++MCAO++siRNA-vector.
In experiment 5, the BBB permeability and Evans Blue dye extravasation were determined 3 days after the MCAO or sham operation in all groups (Figure 6).
Figure 6.
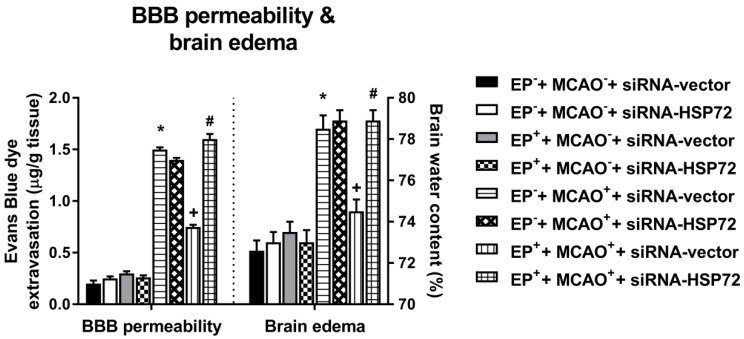
Effects of exercise on both blood-brain barrier (BBB) permeability (i.e., Evans Blue dye extravasation) and brain edema (i.e., brain water content) in different groups of rats 3 days post-MCAO. Data represent the mean ± SD (n=5-6 per group). *P<0.01, EP-+MCAO++siRNA-vector vs. EP-+MCAO-+siRNA-vector; +P<0.05, EP++MCAO++siRNA-vector vs. EP-+MCAO++siRNA-vector; #P<0.05, EP++MCAO++siRNA-HSP72 vs. EP++MCAO++siRNA-vector.
Neurobehavioral function test
Acute neurological motor deficits were assessed in all groups the day before and 3 days after MCAO or sham injury using both a neurological severity score test 17 and an inclined system with a microcontroller 18. The former reveals a composite of the motor, sensory, and reflex test scores, whereas the latter measures limb strength. The modified Neurological Severity Score (mNSS), which comprises tests of motor, sensory, beam balance, reflex absence and abnormal movement functions, was used to evaluate the neurological function of the rats before and after the operation. Neurological function was graded on a scale of 0-18: 1-6, mild injury; 7-12, moderate injury; 13-18, severe injury. The inclined plane was used to measure limb strength. The inclined plane used in this study was constructed of a 60 cm × 60 cm adjustable acrylic panel. A motor and a ball screw were used to control the inclined angle of the acrylic plane from 0° (horizontal) to 70° 18. The angle was increased or decreased in 5° increments to determine the maximal angle at which an animal could maintain balance on the plane. The data for each day were the mean values of the left- and right-side limb maximal angles.
Assessment of cerebral infarct
The rats were deeply anesthetized, and brains were rapidly removed, frozen in liquid nitrogen and then sectioned for immunohistochemistry. Brain sections were stained with 2,3,5-triphenyl-tetrazolium chloride (TTC) as detailed previously 19. The infarct volume (mm3) was measured in each slice and summed using computerized planimetry 20. We also adopted the method of Lin et al. 21 to correct the distortion of infarct volume caused by brain edema.
Bromodeoxyuridine labeling
Bromodeoxyuridine (BrdU), a thymidine analog that is incorporated into the DNA of dividing cells during the S-phase, was used for mitotic labeling (Roche Diagnostics, Indianapolis, IN, USA; 50 mg/kg) as described previously 22. BrdU was administered intraperitoneally immediately at the beginning of surgery and once daily for 3 consecutive days. Three days after MCAO, brains were removed for BrdU labeling. The BrdU immunostaining procedure with a specific antibody against BrdU (1:400; Roche Diagnostics) and the quantification of BrdU-immunoreactive cells were described previously 22.
Immunofluorescence staining
Three days after MCAO, rat brains were prepared as described above. The brains were embedded in paraffin and serially sectioned (10 μm) from the frontal cortex to the commencement of the hippocampus by a research assistant who was blinded to the study protocol. Every 10th section was then collected for immunofluorescence staining. For each animal, 6 serial coronal sections with 100-μm intervals were collected from the cortex. Sections were incubated in 2 mol/L HCl for 30 min, rinsed in 0.1 mol/L boric acid (pH 8.5) for 3 min at room temperature, and then incubated with primary antibodies in phosphate-buffered saline (PBS) containing 0.5% normal bovine serum at 4°C overnight; secondary antibodies were incubated for 1 h at room temperature. The sections were cover slipped with mounting medium (Fluorescent Mounting Medium; Dako). The labeled cells were calculated in 5 coronal sections from each rat and are expressed as the mean number of cells per section. For negative control sections, all the procedures were performed without the primary antibody. The primary and secondary antibodies for multiple staining are listed in Table 1.
Table 1.
Immunofluorescent staining in a focal ischemic injury rat model.
| Antibody | Antigen | Host | Company | Catalog# | Dilution |
|---|---|---|---|---|---|
| Primary Antibody | |||||
| NeuN | Neuron | Mouse | Millipore | MAB377 | 1:400 |
| HSP72 | HSP72 | Rabbit | Abgent | ASM10001 | 1:200 |
| DAPI | Nucleic acid | Invitrogen | D1306 | 1:20000 | |
| BrdU | Bromodeoxyuridine | Rat | Abcam | Ab6326 | 1:200 |
| Secondary Antibody (conjugation) | |||||
| Mouse IgG (Alexa Fluor 568) | Mouse IgG | Goat | Invitrogen | A11031 | 1:200 |
| Rabbit IgG (Alexa Fluor 488) | Rabbit IgG | Goat | Invitrogen | A11034 | 1:200 |
Brain water content and Evans blue dye extravasation assays
The water content of the ipsilateral brains was evaluated via the wet weight/dry weight ratio method, as previously reported 23. Evans blue dye extravasation assays were performed in accordance with the methods of Lin et al. 24. Three hours before sacrifice, animals received an intravenous dose of Evans Blue dye (4%, 0.25 ml/rat). The quantity of extravasated Evans Blue dye was detected by a spectrophotometer at 610 nm and quantified according to a standard curve. The results are presented as μg of Evans Blue dye per g of tissue.
Statistical analysis
GraphPad Prism (version 7.01 for Windows; GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. Data are expressed as the means±standard deviation (SD). Statistical analysis was performed using two-way analysis of variance (ANOVA) with Tukey's and Bonferroni post hoc testing to analyze the percentage of infarct area and the behavioral performance, respectively. Histological measures were analyzed using one-way ANOVA with Bonferroni post hoc tests. A value of P<0.05 was considered statistically significant.
Results
Cerebral percentages of old HSP72-containing neurons were higher in EP+ rats
As shown in Figure 2, the percentages of old HSP72-containing neurons (i.e., NeuN+HSP72-positive cells) in ipsilateral brain regions, including the frontal cortex and the striatum (Figure 1a, b), were ~20% in the EP-+MCAO-+siRNA-vector rats and 10% in the EP-+MCAO++siRNA-vector rats. EP+ significantly increased the percentage of old HSP72-containing neurons to 40-50% for the EP++MCAO++siRNA-vector rats and 30-35% for the EP++MCAO-+siRNA-vector rats (Figure 2). The increased percentage of old HSP72-containing neurons in the ipsilateral brains following EP+ was significantly reduced by intracerebral injection of pSUPER siRNA-HSP72 (down to 20-25%). Compared to the EP-+MCAO++siRNA-vector rats, the EP++MCAO++siRNA-vector rats had a significantly higher percentage of old HSP72-containing neurons (~35% vs. 10%). However, the EP++MCAO++siRNA-HSP72 rats had a significantly lower percentage of old HSP72-containing neurons (5%) than did the EP++MCAO++siRNA-vector rats.
Figure 3 shows that the percentages of newly formed HSP72-containing neurons (i.e., BrdU+NeuN+HSP72-positive cells) in ipsilateral brain regions, including the frontal cortex and the striatum, in both the EP-+MCAO-+siRNA-vector and EP-+MCAO-+siRNA-HSP72 rats were within 2-3%. EP+ significantly increased the percentage of newly formed HSP72-containing neurons to 13-15%, as shown in the EP++MCAO-+siRNA-vector rats (Figure 3). Additionally, the EP+-induced, newly formed HSP72-containing neurons in the ipsilateral frontal cortex and striatum were significantly reduced by an intracerebral injection of pSUPER siRNA-HSP72 (down to 4-5%), as shown in the EP++MCAO-+siRNA-HSP72 rats (Figure 3). Compared to the EP-+MCAO++siRNA-vector rats, the EP++MCAO++siRNA-vector rats had a significantly higher percentage of newly formed HSP72-containing neurons (16-20% vs. 4-5%) (Figure 3). Additionally, the increased percentages of newly formed HSP72-containing neurons in both the ipsilateral frontal cortex and striatum were significantly reversed by pSUPER siRNA-HSP72 but not by pSUPER siRNA-vector (~18% vs. ~4%) (Figure 2) in the MCAO+ rats.
Neurological motor deficits after MCAO were lower in EP+ rats
All EP-+MCAO-+siRNA-vector, EP-+MCAO-+siRNA-HSP72, EP++MCAO-+siRNA-vector and EP++MCAO-+siRNA-HSP72 rats displayed normal neurological motor function as evidenced by “0” mNSS and 60° maximal angles (Figure 4). Both the EP-+MCAO++siRNA-vector and EP-+MCAO++siRNA-HSP72 groups of rats had significantly (P<0.01) higher neurological severity scores (12.5±2.1 vs. 2.0±0.2) and lower maximal angles (18±1° vs. 60±3°) than did the EP- control rats. However, the EP++MCAO++siRNA-vector rats had significantly (P<0.05) lower neurological severity scores (6.2±0.4 vs. 12.5±2.1) and higher maximal angles (18±2° vs. 6.2±0.4°) than did the EP+ control rats. Additionally, the beneficial effects of EP+ in promoting neurological motor recovery following MCAO were significantly (P<0.05) reversed by siRNA-HSP72 as shown in the EP++MCAO++siRNA-HSP72 rats (P<0.05) (Figure 4).
Cerebral infarction and edema after MCAO were attenuated in EP+ rats
All EP-+MCAO-+siRNA-vector, EP-+MCAO-+siRNA-HSP72, EP++MCAO-+siRNA-vector and EP++MCAO-+siRNA-HSP72 rats displayed insignificant differences among the groups regarding cerebral infarction (Figure 5), Evans Blue dye extravasation and water content (Figure 6). Both the EP-+MCAO++siRNA-vector and EP-+MCAO++siRNA-HSP72 groups had significantly (P<0.01) greater infarct volume (250±12 mm3 vs. 0 mm3), increased Evans Blue dye extravasation (1.42±0.07 μg/g vs. 0.25±0.06 μg/g) and higher water content (78.5±1.1% vs. 73.2±0.8%) than did the EP- control rats. However, the EP++MCAO++siRNA-vector rats had significantly (P<0.05) lower brain infarction (125±11 mm3), decreased Evans Blue dye extravasation (0.68±0.04 μg/g), and lower brain water content (74.6±10%) than did the EP-+MCAO++siRNA-vector rats. However, the beneficial effects of EP+ in attenuating brain infarction, Evans Blue dye extravasation and brain water content following MCAO were significantly (P<0.05) reversed by siRNA-HSP72 (Figure 5 and Figure 6) (brain infarct volume, 247±12 mm3; Evans Blue dye extravasation, 0.68±0.04 μg/g; and brain water content, 78.9±1.0%).
Discussion
The most striking findings of the present study are that EP+ provides significant neuroprotection against ischemic brain injury by preserving both old HSP72-containing neurons and newly formed HSP72-containing neurons. Our present results are consistent with many previous findings. For example, both in vitro cerebral ischemic injury and in vivo spinal cord ischemic injury can be ameliorated by transfection and viral overexpression of HSP72 in neurons and glia. Mice and rats overexpressing HSP72 are protected against cerebral infarction 25-27. Knockout of HSP72 worsens ischemic brain injury in rodents with focal brain ischemia 28. Geldanamycin induces HSP72 in the brain and protects against focal ischemic brain injury in rats 29, 30.
Previous studies have demonstrated that EP+ induced a 70% higher expression of HSP72 in the ischemic brain and diminished neuronal injury in rats with MCAO 6. Antibody inhibition of EP+-induced mild HSP72 expression in the ischemic brain completely blocked EP+-induced neuroprotection. This result indicates that the increased HSP72 levels prior to ischemia and reperfusion brain injury may be one of the key issues. In the present study, in sham-operated rats (i.e., MCAO- rats), EP+ increased the percentage of both old HSP72-containing neurons (from 19% to 45%) and newly formed HSP72-containing neurons (from 2.5% to 16%) (Figure 3a, 3b). Intracerebral injection of pSUPER siRNA-HSP72 significantly reduced the percentage of both old and newly formed HSP72-containing neurons to 25% and 10%, respectively. At the same time, MCAO+ significantly attenuated the percentage of old and newly formed HSP72-containing neurons to 10% and 5%, respectively. The reduction of HSP72-containing neurons in MCAO rats was blocked by EP+ (30% and 18%, respectively, for old and newly formed HSP72-containing neurons). The beneficial effects of EP+ in preserving the old HSP72-containing neurons and increasing the newly formed HSP72-containing neurons in ipsilateral brain tissues were attenuated by intracerebral injection of siRNA-HSP72 to 15% and 4.5%, respectively. These results suggest that HSP72 antibody or siRNA-HSP72 gene silencing had an effect by neutralizing HSP72 or reducing the accumulation of HSP72-containing neurons produced by EP+ in the brain, leading to interference of an anti-neural injury mechanism.
EP+ provides significant protection against acute cerebral ischemic injury (present results and 31). EP+ induces brain ischemic tolerance through the promotion of angiogenesis, mediation of inflammatory responses, and inhibition of neuronal apoptosis. EP+-mediated overexpression of cerebral HSP72 improves outcomes of severe ischemic injury via increasing brain derived neurotrophic factor (BDNF) production and/or decreasing neuroinflammation and neuronal apoptosis (via elevated Bcl-2/Bax ratio, preventing formation of the apoptosome, and inhibition of caspase-3) 12, 32-34. Cerebral overexpression of HSP72 exerts both anti-inflammatory and antiapoptotic effects and facilitates neuroprotection. In mice with comparable cerebral ischemic injury, overexpression of HSP72 reduced the density of glial fibrillary acidic protein (GFAP)-positive cells and decreased astrocyte morphological complexity 35. In addition, HSP72-mediated EP+ may attenuate BBB disruption and cerebral ischemic injury in rats via inhibiting the matrix metalloproteinase-9 (MMP-9) 36/nuclear factor NF-κB 6 pathway. Furthermore, HSP72-mediated preconditioning exercise might reduce cerebral ischemic injury and neuronal apoptosis through endogenous 14-3-3γ 31.
Conclusion
In conclusion, a significant (P<0.05) increase in the percentages of both old and newly formed HSP72-containing neurons after 3 weeks of exercise coincided with a significant (P<0.05) reduction in brain infarct volume, brain edema, BBB disruption, and neurological motor deficits in cerebral ischemic injury rats. Reductions in the percentages of both the old and newly formed HSP72-containing neurons by pSUPER siRNA showed a significant (P<0.05) reversal in the neuroprotective effects of EP+. Our data provide an inverse correlation between the EP+-mediated upregulation of old and newly formed HSP72-containing neurons and the extent of cerebral ischemic injury. Our data demonstrate that preischemic treadmill exercise improves cerebral ischemic injury by preserving the percentages of both old HSP72-containing neurons and newly formed HSP72-containing neurons.
Acknowledgments
This study is partially supported by the Ministry of Science and Technology (Taiwan) under grant no. MOST 107-2314-B-384-007-MY3, grant no. MOST 108-2314-B-715-001, and the Chi Mei Medical Center, Tainan, Taiwan under grant no. CMFHT10401.
Author contributions
Study design: Fong-Chin Su, Willy Chou
Performed the experiments & data collection: Yu-Ling Wang, Cheng-Hsien Lin, Chi-Chun Chen
Statistical analysis: Ching-Ping Chang, Kao-Chang Lin
Data interpretation: Yu-Ling Wang, Willy Chou
Manuscript preparation: Fong-Chin Su, Willy Chou
Literature search: Yu-Ling Wang, Ching-Ping Chang, Kao-Chang Lin
Abbreviations
- EP+
exercise preconditioning
- HSP72
heat shock protein 72
- siRNA
small interfering RNA
- BrdU
bromodeoxyuridine
- NeuN
neuronal nuclei
- pSUPER
suppression of endogenous RNA
- MCAO
middle cerebral artery occlusion
- TTC
2,3,5-triphenyl-tetrazolium chloride
- PBS
phosphate-buffered saline
- DAPI
4',6-diamidino-2-phenylindole
References
- 1.Goldstein LB, Bushnell CD, Adams RJ. et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Clagett GP, Easton JD. et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 1999;30:1991–4. doi: 10.1161/01.str.30.9.1991. [DOI] [PubMed] [Google Scholar]
- 3.Reinholdsson M, Palstam A, Sunnerhagen KS. Prestroke physical activity could influence acute stroke severity (part of PAPSIGOT) Neurology. 2018;91:e1461–e67. doi: 10.1212/WNL.0000000000006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rist PM, Capistrant BD, Mayeda ER. et al. Physical activity, but not body mass index, predicts less disability before and after stroke. Neurology. 2017;88:1718–26. doi: 10.1212/WNL.0000000000003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao JH, Meng XL, Zhang J. et al. Oxygen glucose deprivation post-conditioning protects cortical neurons against oxygen-glucose deprivation injury: role of HSP70 and inhibition of apoptosis. J Huazhong Univ Sci Technolog Med Sci. 2014;34:18–22. doi: 10.1007/s11596-014-1225-0. [DOI] [PubMed] [Google Scholar]
- 6.Sharp FR, Zhan X, Liu D-Z. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Translational stroke research. 2013;4:685–92. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp FR, Lu A, Tang Y. et al. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–32. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Chang CK, Chou W, Lin HJ. et al. Exercise preconditioning protects against spinal cord injury in rats by upregulating neuronal and astroglial heat shock protein 72. Int J Mol Sci. 2014;15:19018–36. doi: 10.3390/ijms151019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan X, Ander BP, Liao IH. et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41:538–43. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giffard RG, Han R-Q, Emery JF. et al. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109:339–48. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanan A, Deshpande S, Jamadarkhana PG. et al. Delayed intervention in experimental stroke with TRC051384-a small molecule HSP70 inducer. Neuropharmacology. 2011;60:991–9. doi: 10.1016/j.neuropharm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chio CC, Lin HJ, Tian YF. et al. Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-kappaB/IL-6/synapsin I axis in traumatic brain injury rats. J Neuroinflammation. 2017;14:90. doi: 10.1186/s12974-017-0867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CM, Chang CK, Chang CP. et al. Protecting against ischaemic stroke in rats by heat shock protein 20-mediated exercise. Eur J Clin Invest. 2015;45:1297–305. doi: 10.1111/eci.12551. [DOI] [PubMed] [Google Scholar]
- 14.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 15.Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–24. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. New York Elsevier Academic Press; 2013. [Google Scholar]
- 17.Chen SF, Hsu CW, Huang WH. et al. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol. 2008;155:1279–96. doi: 10.1038/bjp.2008.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang MW, Young MS, Lin MT. An inclined plane system with microcontroller to determine limb motor function of laboratory animals. J Neurosci Methods. 2008;168:186–94. doi: 10.1016/j.jneumeth.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Bederson JB, Pitts LH, Germano SM. et al. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 20.Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol. 1997;147:353–60. doi: 10.1006/exnr.1997.6635. [DOI] [PubMed] [Google Scholar]
- 21.Lin TN, He YY, Wu G. et al. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 22.Zhang RL, Zhang ZG, Zhang L. et al. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 23.Huang B, Krafft PR, Ma Q. et al. Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis. 2012;46:204–14. doi: 10.1016/j.nbd.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HJ, Hsu CC, Chio CC. et al. Gamma-Secretase Inhibitors Attenuate Neurotrauma and Neurogenic Acute Lung Injury in Rats by Rescuing the Accumulation of Hypertrophic Microglia. Cell Physiol Biochem. 2017;44:1726–40. doi: 10.1159/000485778. [DOI] [PubMed] [Google Scholar]
- 25.van der Weerd L, Lythgoe MF, Badin RA. et al. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia-an MRI study. Exp Neurol. 2005;195:257–66. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Rajdev S, Hara K, Kokubo Y. et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–91. [PubMed] [Google Scholar]
- 27.Hoehn B, Ringer TM, Xu L. et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–9. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Kwon HM, Kim YJ. et al. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35:2195–9. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- 29.Kwon HM, Kim Y, Yang SI. et al. Geldanamycin protects rat brain through overexpression of HSP70 and reducing brain edema after cerebral focal ischemia. Neurol Res. 2008;30:740–5. doi: 10.1179/174313208X289615. [DOI] [PubMed] [Google Scholar]
- 30.Lu A, Ran R, Parmentier-Batteur S. et al. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–64. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 31.Otsuka S, Sakakima H, Terashi T, Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3gamma after focal brain ischemia in rats. Brain Struct Funct; 2018. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka S, Sakakima H, Sumizono M. et al. The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav Brain Res. 2016;303:9–18. doi: 10.1016/j.bbr.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 33.Hayes K, Sprague S, Guo M. et al. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–96. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo JH, Kwon IS, Kang EB. et al. Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer's disease. Journal of exercise nutrition & biochemistry. 2013;17:151–60. doi: 10.5717/jenb.2013.17.4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barreto GE, White RE, Xu L. et al. Effects of heat shock protein 72 (Hsp72) on evolution of astrocyte activation following stroke in the mouse. Exp Neurol. 2012;238:284–96. doi: 10.1016/j.expneurol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhry K, Rogers R, Guo M. et al. Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett. 2010;474:109–14. doi: 10.1016/j.neulet.2010.03.020. [DOI] [PubMed] [Google Scholar]