Abstract
Purpose: The aim of this study was to verify the potential risk factors of ventilator-associated pneumonia (VAP) in elderly Chinese patients receiving mechanical ventilation (MV). The secondary aim of this study was to present logistical regression prediction models of VAP occurrence in elderly Chinese patients receiving MV.
Methods: Patients (aged 80 years or above) receiving MV for ≥48 h were enrolled from the Chinese People’s Liberation Army (PLA) General Hospital from January 2011 to December 2015. A chi-squared test and Mann–Whitney U-test were used to compare the data between participants with VAP and without VAP. Univariate logistic regression models were performed to explore the relationship between risk factors and VAP.
Results: A total of 901 patients were included in the study, of which 156 were diagnosed as VAP (17.3%). The incidence density of VAP was 4.25/1,000 ventilator days. Logistic regression analysis showed that the independent risk factors for elderly patients with VAP were COPD (OR =1.526, P0.05), intensive care unit (ICU) admission (OR=1.947, P0.01), the MV methods (P0.023), the number of antibiotics administered (OR=4.947, P0.01), the number of central venous catheters (OR=1.809, P0.05), the duration of indwelling urinary catheter (OR=1.805, P0.01) and the use of corticosteroids prior to MV (OR=1.618, P0.05). Logistic regression prediction model of VAP occurrence in the Chinese elderly patients with mechanical ventilation:
Conclusion: VAP occurrence is associated with a variety of controllable factors including the MV methods and the number of antibiotics administered. A model was established to predict VAP occurrence so that high-risk patients could be identified as early as possible.
Keywords: aged, 80 and over, pneumonia, ventilator-associated, risk factors, prediction model
Introduction
Ventilator-associated pneumonia (VAP) is defined as pneumonia that occurs 48–72 h (or later) following endotracheal intubation. VAP is characterized by the presence of new or progressive infiltrates, systemic infection (fever, altered white blood cell counts), changes in sputum characteristics, and the detection of a causative agent.1 VAP is the most frequent cause of nosocomial infections amongst patients requiring mechanical ventilation (MV).2,3 It has been reported that the incidence of VAP is 9–27% with a mortality of 25–50%.4–6 VAP can lead to the deterioration of a patient’s condition, endanger the patient’s life, and increase the health-care burden.6–8 As the average age of the Chinese population is increasing, the number of elderly patients requiring MV is rising. Elderly patients have a compromised immune system and are more prone to opportunistic infections, increasing the likelihood of VAP. The factors affecting the occurrence of VAP are complex and diverse, making its management difficult. Specific risk factors include tracheal catheterization, MV, the duration of intensive care unit (ICU) stay, nursing measures, increased colonization of upper gastrointestinal pathogens and the aspiration of contaminated secretions.9,10 The risk factors for VAP in elderly patients have not been assessed in detail. Clinical practices possess no effective theoretical based evidence for the prevention of VAP in elderly patients. This study retrospectively analyzed the incidence of VAP and related risk factors in elderly patients receiving MV to provide suggestions for the prevention of VAP and elderly patient care.
Methods
Participants
Elderly patients who underwent MV from the Chinese People’s Liberation Army (PLA) General Hospital from January 2011 to December 2015 were included. A total of 901 patients were enrolled, including 755 males and 146 females, with an average age of (86.734.818) years. Inclusion criteria for the study were: 1) ventilator use ≥48 h; 2) no VAP infection prior to MV; 3) ≥80 years old. Exclusion criteria were: 1) pneumonia diagnosed before MV; (2) death, ceased treatment, discharged or transferred within 48 h; (3) patients with incomplete clinical data.
Data collection
Retrospective survey methods were used to collect data on elderly patients who underwent MV from the Chinese People’s Liberation Army (PLA) General Hospital from January 2011 to December 2015. The investigation was conducted with the assistance of staff from the Hospital Infection Management Section. The investigating members consisted of three nursing staff and one hospital infection management staff, all of whom received uniform training. For patients with MV, routine VAP prevention protocols were available in our hospital. All procedures involving human participants were performed in accordance with the basic principles of the Declaration of Helsinki. Ethical approval was obtained from the Beijing Municipal Commission of Science and Technology Program. Informed consent was waived due to the retrospective design. The electronic database at the Chinese People’s Liberation Army General Hospital includes discharge records for all patients treated in the hospital. All patient data were anonymized and maintained with confidentiality.
Study variables
Study variables included basic patient characteristics (gender, disorder of consciousness, smoking, alcohol consumption, nutritional status, surgery, ICU admission, underlying diseases, hospitalization length of stay, ICU stay, origin of patients, duration and number of catheters), MV related data (MV methods, duration of MV and number of reintubations), and medication-related data (antibiotics use after surgery, duration of antibiotics use after surgery, number of antibiotics administered, duration of antibiotics administered, combined application of antibiotics, number of combined antibiotics administered, duration of the combined antibiotics, use of acid suppressant agent, use of sedation, and use of corticosteroids).
Diagnosis of VAP
Chest radiograph or CT showing new or progressive infiltration, consolidation, or ground glass, and at least two of the following criteria: 1) fever (>38.3°C) or hypothermic (<36°C); 2) leukocytosis (>11×109/L) or leukopenia (<4×109/L); and 3) purulent tracheal secretions confirmed by microscopic examination, and a quantitative bacterial cultures of 106 CFU/mL from an endotracheal specimens or 104 CFU/mL from bronchoalveolar lavage fluid. Pulmonary edema, acute respiratory distress syndrome (ARDS), tuberculosis, pulmonary embolism, and other diseases were discounted.11 VAP rate was defined as the number of VAPs/1,000 ventilator days.12 It can be of two types: 1) early-onset VAP, which is defined as VAP that occurs within the first 4 days of ventilation; and 2) late-onset VAP, which is defined as VAP that occurs more than 4 days after initiation of mechanical ventilation.13
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software. The Kolmogorov–Smirnov test was used to determine the normality of distributions. Continuous variables were presented as median, max, and min, while categorical variables were presented as percentages. Mann–Whitney U-test or chi-squared test was used to compare the data between participants with VAP and participants without VAP. Univariate logistic regression models were used to analyze the risk factors of VAP. The hypothesis test significance level was 0.05. OR>1 was a risk factor and OR<1 was a protective factor. When P≤0.05 (bilateral), differences were considered statistically significant.
Quality control
According to the criteria for the inclusion and exclusion of cases, completed unified data collection forms were used to screen and collect cases that met the study criteria. Double data entries and logic verification were used to ensure data accuracy. Upon completion, all data were checked, missing data were completed, and duplicated or erroneous data were removed.
Results
VAP incidence
From January 2011 to December 2015, a total of 901 elderly patients receiving MV who met the criteria for inclusion were assessed. Of these patients, 156 had VAP (17.3%). The incidence of VAP in elderly patients receiving MV significantly decreased from 23.4% to 8.4% over the five-year study period (χ2=23.634, P<0.001). VAP incidence was calculated as follows: (number of cases with VAP/total number of patients who received MV×100)= VAP rate per 100 patients. See Table 1. The VAP incidence density was calculated as follows: (number of cases with VAP/number of ventilator days)×1,000= VAP rate per 1,000 ventilator days.14 The incidence density of VAP was 4.25/1,000 ventilator days (156/36,720×1,000=4.25 per 1,000 ventilator days). Out of the 156 cases, 47 (30.13%) were categorized under early-onset group and 109 (69.87%) under the late-onset group.
Table 1.
Incidence of VAP in elderly patients receiving MV
| Year | Number (N) | VAP, n (%) | NVAP, n (%) | χ2 | p |
|---|---|---|---|---|---|
| 2011 | 154 | 36(23.4) | 118 (76.6) | ||
| 2012 | 255 | 40(15.7) | 215 (84.3) | ||
| 2013 | 288 | 44(15.3) | 244 (84.7) | ||
| 2014 | 312 | 32(10.3) | 280 (89.7) | ||
| 2015 | 285 | 24(8.4) | 261 (91.6) | ||
| Total | 901 | 156(17.3) | 745 (82.7) | 23.634 | 0.000 |
Abbreviations: MV, mechanical ventilation; NVAP, non-ventilator-associated pneumonia ; VAP, ventilator-associated pneumonia.
Patient characteristics
Analysis of the basic characteristics of the elderly patients who received MV showed significant differences in the effects of surgery (χ2=5.018, P<0.05) and ICU admission (χ2=8.445, P<0.05). COPD influenced the occurrence of VAP (χ2=6.264, P<0.05). VAP occurred in 22.6% of COPD patients, which was higher than patients with other diseases. Compared with the non-VAP group, the hospitalization length of stay in the VAP group was prolonged, and the difference was statistically significant (Z=−4.677, P<0.01). ICU length of stay also significantly differed between the VAP group and the non-VAP group (Z=−4.938, P<0.01). According to patient origin, we sub-divided the patients into internal medicine, surgery, emergency department, ICU and others. Statistical analysis showed a large number of internal medicine patients had VAP (12.9%) and the incidence of VAP was influenced by patient origin (χ2=13.519, P<0.05). The analysis of central venous catheter and indwelling urinary catheter in elderly patients receiving MV showed that the duration of central venous catheter >90 days had a significant influence on VAP (P<0.01). Patients with indwelling urinary catheter >90 days had a higher incidence of VAP compared to patient’s ≤90 days. The number of central venous catheters also significantly influenced VAP (χ2=18.350, P<0.001) (Table 2).
Table 2.
Characteristics between elderly patients receiving MV with VAP and without VAP
| Patient characteristics | VAP(N=156) | NVAP(N=745) | χ2/Z | p |
|---|---|---|---|---|
| Gender | 1.591 | 0.207 | ||
| Male | 136(18.0) | 619(82.0) | ||
| Female | 20(13.7) | 126(86.3) | ||
| Disorder of consciousness | 3.806 | 0.051 | ||
| Yes | 58(21.0) | 218(79.0) | ||
| No | 98(15.7) | 527(84.3) | ||
| Smoking | 0.090 | 0.764 | ||
| Yes | 64(17.8) | 296(82.2) | ||
| No | 92(17.0) | 449(83.0) | ||
| Alcohol consumption | 1.028 | 0.311 | ||
| Yes | 45(15.5) | 246(84.5) | ||
| No | 111(18.2) | 499(81.8) | ||
| Nutritional status | 1.301 | 0.522 | ||
| Poor | 26(17.0) | 127(83.0) | ||
| Moderate | 86(18.6) | 376(81.4) | ||
| Good | 44(15.4) | 242(84.6) | ||
| Surgery | 5.018 | 0.025 | ||
| Yes | 73(20.9) | 277(79.1) | ||
| No | 83(15.1) | 468(84.9) | ||
| ICU admission | 9.594 | 0.002 | ||
| Yes | 101(21.0) | 381(79.0) | ||
| No | 55(13.1) | 364(86.9) | ||
| Underlying diseases | ||||
| Hypertension | 83(19.1) | 352(80.9) | 1.833 | 0.176 |
| Diabetes | 27(14.4) | 161(85.6) | 1.446 | 0.229 |
| COPD | 45(22.6) | 154(77.4) | 5.009 | 0.025 |
| Respiratory failure | 32(13.3) | 209(86.7) | 3.744 | 0.053 |
| Heart dysfunction | 44(14.9) | 252(85.1) | 1.847 | 0.174 |
| Brain infarction | 47(19.9) | 189(80.1) | 1.511 | 0.219 |
| Malignancy | 41(16.7) | 204(83.3) | 0.014 | 0.907 |
| Renal insufficiency | 29(15.7) | 156(84.3) | 0.305 | 0.581 |
| Origin of patients | ||||
| Internal medicine | 59(12.9) | 397(87.1) | 13.519 | 0.009 |
| Surgery | 10(18.9) | 43(81.1) | ||
| Emergency department | 1(11.1) | 8(88.9) | ||
| ICU | 85(22.5) | 293(77.5) | ||
| Others | 1(20.0) | 4(80.0) | ||
| Hospitalization length of stay | 120(9,2,535) | 70(4,1,607) | −4.677 | <0.01 |
| ICU length of stay | 25(0,1,263) | 4(0,1,548) | −4.938 | <0.01 |
| Duration of catheters(days) | ||||
| Central venous catheter | ||||
| 0–90 | 90(15.3) | 498(84.7) | 4.767 | 0.029 |
| >90 | 66(21.1) | 247(78.9) | ||
| Indwelling urinary catheter | ||||
| 0–90 | 92(13.6) | 582(86.4) | 25.090 | <0.001 |
| >90 | 64(28.2) | 163(71.8) | ||
| Number of catheters | ||||
| Central venous catheter | ||||
| 0–2 | 118(15.2) | 660(84.8) | 18.350 | <0.001 |
| >2 | 38(30.9) | 85(69.1) | ||
| Indwelling urinary catheter | ||||
| 0–2 | 112(14.7) | 651(85.3) | 0.839 | 0.360 |
| >2 | 44(19.3) | 184(80.7) |
Note: Data are shown as number (percentage) or median (min and max).
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation; NVAP, non-ventilator-associated pneumonia; VAP, ventilator-associated pneumonia.
Effects of MV on VAP occurrence
From 2011 to 2015, the number of elderly patients with tracheal intubation was the highest (596) and the rate of VAP was 13.4%. However, the incidence of VAP in patients with tracheotomy was 28.4%, followed by 23.7% in patients who underwent tracheotomy after tracheal intubation. Chi-squared tests of the patient’s MV methods revealed its influence on VAP (χ2=19.616, P<0.001). When the duration of ventilation between groups was compared, significant differences were observed (Z=−5.983, P<0.01). The number of MVs received also significantly influenced VAP (χ2=7.633, P<0.01). The incidence of VAP in patients suffering the number of reintubations >2 (28.4%) was higher than the number of reintubations ≤2 (16.2%) (Table 3).
Table 3.
Comparison of MV characteristics between VAP group and non-VAP group
| Ventilation characteristics | VAP(N=156) | NVAP(N=745) | χ2/Z | p |
|---|---|---|---|---|
| MV methods | ||||
| Tracheal intubation | 80(13.4) | 516(86.6) | 19.615 | <0.001 |
| Tracheostomy | 21(28.8) | 52(71.2) | ||
| Tracheotomy after tracheal intubation | 55(23.7) | 177(76.3) | ||
| Duration of MV | 71.5(4,1,588) | 34(2,1,584) | −5.983 | <0.01 |
| Number of reintubations | ||||
| 0–2 | 133(16.2) | 687(83.8) | 7.633 | 0.006 |
| >2 | 23(28.4) | 58(71.6) |
Note: Data are shown as number (percentage) or median (min and max).
Abbreviations: MV, mechanical ventilation; NVAP, non-ventilator-associated pneumonia; VAP, ventilator-associated pneumonia.
Medication use
The analysis of medication use in elderly patients receiving MV in hospital showed that the use of antibiotics after surgery (χ2=4.652, P<0.05), the duration of antibiotics use post-surgery (χ2=6.868, P<0.05), the number of antibiotics (χ2=18.645, P<0.05), the duration of antibiotic administered (χ2=6.101, P<0.05), the combined application of antibiotics (χ2=5.098, P<0.05), the number of combined antibiotics (χ2=9.508, P<0.05), the duration of combined antibiotics (χ2=16.732, P<0.05) and the use of corticosteroids prior to MV (χ2=5.483, P<0.05) all significantly influenced VAP (Table 4).
Table 4.
Effects of medication use on the occurrence of VAP
| Medication use | VAP(N=156) | NVAP(N=745) | χ2 | p |
|---|---|---|---|---|
| Antibiotics use after surgery | 4.652 | 0.031 | ||
| Yes | 72(20.7) | 275(79.3) | ||
| No | 84(15.2) | 470(84.8) | ||
| Duration of antibiotics use after surgery (days) | 6.868 | 0.009 | ||
| ≤14 | 93(15.1) | 524(84.9) | ||
| >14 | 63(22.2) | 221(77.8) | ||
| Number of antibiotics administered | 18.645 | <0.001 | ||
| ≤3 | 4(3.4) | 115(96.7) | ||
| >3 | 152(19.4) | 630(80.6) | ||
| Duration of antibiotics administered (days) | 6.101 | 0.014 | ||
| ≤14 | 6(5.1) | 112(94.9) | ||
| >14 | 150(18.3) | 670(81.7) | ||
| Combined application of antibiotics | 5.098 | 0.024 | ||
| Yes | 151(18.1) | 682(81.9) | ||
| No | 5(7.4) | 63(92.6) | ||
| Number of combined antibiotics administered | 9.508 | 0.002 | ||
| ≤3 | 22(10.3) | 191(89.7) | ||
| >3 | 134(19.5) | 554(80.5) | ||
| Duration of combined antibiotics administered (days) | 16.732 | <0.001 | ||
| ≤14 | 20(8.6) | 213(91.4) | ||
| >14 | 136(20.4) | 532(79.6) | ||
| Before mechanical ventilation | ||||
| Use of acid suppressant agent | 107(19.2) | 451(80.8) | 3.548 | 0.060 |
| Use of sedation | 48(19.5) | 198(80.5) | 1.142 | 0.285 |
| Use of corticosteroids | 58(21.9) | 207(78.1) | 5.483 | 0.019 |
| After mechanical ventilation | ||||
| Use of acid suppressant agent | 135(17.1) | 655(82.9) | 0.228 | 0.633 |
| Use of sedation | 88(16.4) | 449(83.6) | 0.797 | 0.372 |
| Use of corticosteroids | 65(18.3) | 290(81.7) | 0.406 | 0.524 |
Note: Data are number (percentage).
Abbreviations: NVAP, non-ventilator-associated pneumonia; VAP, ventilator-associated pneumonia.
Logistical analysis of factors related to VAP infection
Retrospective analysis of elderly patients with MV was performed from 2011 to 2015. Two-class logistic analysis was performed to identify whether VAP was a dependent variable and to perform single-factor analysis of the risk factors for VAP (P<0.05). Using the forward conditional method for stepwise regression, the significance level α of the selected variables was determined as 0.05. Dummy variables were set for multi-category variables such as MV methods and the origin of patients. The results showed that the COPD (X1) (OR=1.526, P0.05), the ICU admission (X2) (OR=1.947, P0.01), the MV methods (P0.023) (tracheal intubation (X3), tracheostomy (X4), tracheotomy after tracheal intubation (X5)), the number of central venous catheter (X6) (OR=1.809, P0.05), the duration of indwelling urinary catheter (X7) (OR=1.805, P0.01), the number of antibiotics administered (X8) (OR=4.947, P0.01) and the use of corticosteroids prior to MV (X9) (OR=1.618, P0.05) were all single risk factors of VAP (Table 5).
Table 5.
Logistic regression analysis of VAP risk factors in elderly patients with MV
| Risk factors | B | SE | Wald | p | OR | 95%CI |
|---|---|---|---|---|---|---|
| COPD | 0.423 | 0.211 | 4.015 | 0.045 | 1.526 | 1.009–2.308 |
| ICU admission | 0.666 | 0.199 | 11.239 | 0.001 | 1.947 | 1.319–2.875 |
| Mechanical ventilation methods | ||||||
| Tracheal intubation | 7.584 | 0.023 | ||||
| Tracheostomy | −0.501 | 0.222 | 5.123 | 0.024 | 0.606 | 0.392–0.935 |
| Tracheotomy after tracheal intubation | 0.122 | 0.321 | 0.145 | 0.703 | 1.130 | 0.602–2.122 |
| Number of central venous catheter | 0.593 | 0.234 | 6.424 | 0.011 | 1.809 | 1.144–2.860 |
| Duration of indwelling urinary catheters | 0.590 | 0.206 | 8.226 | 0.004 | 1.805 | 1.206–2.701 |
| Number of antibiotics administered | 1.599 | 0.525 | 9.263 | 0.002 | 4.947 | 1.767–13.852 |
| Before mechanical ventilation | ||||||
| Use of corticosteroids | 0.481 | 0.195 | 6.075 | 0.014 | 1.618 | 1.104–2.372 |
| Constant | −6.468 | 1.100 | 34.557 | <0.001 | 0.002 |
Abbreviations: MV, mechanical ventilation; ICU, intensive care unit; VAP, ventilator-associated pneumonia.
Establish logistic regression prediction model
Logistic regression prediction model
Logistic regression prediction model of VAP infection in elderly patients with mechanical ventilation:
The model is used for the preliminary prediction of VAP, predicting whether VAP will occur in patients undergoing mechanical ventilation. The closer the P-value is to 1, the more likely VAP will occur in patients undergoing mechanical ventilation. The closer the P-value is to 0, the less likely the patient will have VAP.
Prediction effect evaluation of logistic regression models
The likelihood ratio test, Hosmer–Lemeshow goodness-of-fit test and receiver-operating characteristic (ROC) curve were used to evaluate the prediction effect of the model.
Overall validity of the model
The likelihood ratio test showed that χ2=315.332, df=1, P<0.001, indicating that the prediction model has statistical significance. The Wald test demonstrated that χ2=34.557, df=1, P<0.001, that is, the coefficients of the regression equation were statistically significant.
Goodness-of-fit of logistic regression equations
Hosmer–Lemeshow goodness-of-fit test showed that the goodness of fit model was favorable (χ2=4.613, df=7, P=0.707).
Discriminant ability of logistic regression equations
The ROC curve was drawn with “1-specificity” as the abscissa and “sensitivity” as the ordinate. When 0.5≤ the area under the curve (AUC) <0.7, it was considered that the discriminant value of the model was acceptable. When 0.7≤AUC<0.9, it was considered that the discriminant value of the model was favorable. When 0.9≤AUC, the value of the model was considered outstanding.
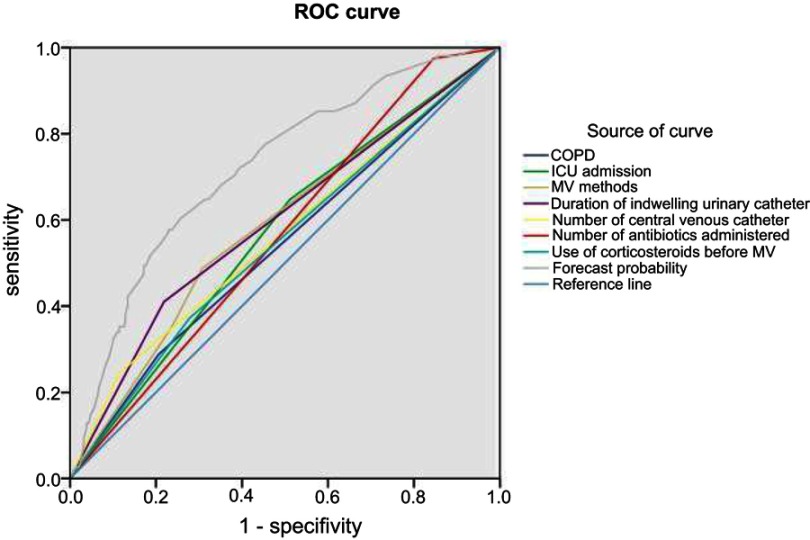
The results of this study show that the AUC of the prediction probability of the new variable was 0.722, indicating that the discriminant effect of the model was very good. The AUC of the comprehensive index was higher than that of other indices (P<0.05), indicating that the comprehensive index more accurately identified VAP, and performed better than that of the single index for the identification of VAP (Figure 1 and Table 6).
Figure 1.
Logistic regression prediction model ROC curve.
Abbreviation: ROC, receiver-operating characteristic.
Table 6.
Area under curve
| Test result variable | Area chart | The standard errora | Asymptotically significant levelb | 95% CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| COPD | 0.541 | 0.026 | 0.108 | 0.490 | 0.592 |
| ICU admission | 0.568 | 0.025 | 0.007 | 0.519 | 0.617 |
| Duration of mechanical ventilation | 0.586 | 0.025 | 0.001 | 0.536 | 0.636 |
| Number of central venous catheter | 0.565 | 0.027 | 0.001 | 0.513 | 0.617 |
| Duration of indwelling urinary catheter | 0.596 | 0.026 | 0.000 | 0.545 | 0.647 |
| Number of antibiotics use | 0.564 | 0.023 | 0.011 | 0.519 | 0.610 |
| Use of corticosteroids before MV | 0.547 | 0.026 | 0.065 | 0.496 | 0.598 |
| Forecast probability | 0.722 | 0.022 | 0.000 | 0.679 | 0.765 |
Notes: aAssume nonparametric. bOriginal hypothesis: real area =0.5
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation.
Discussion
VAP is a common complication during MV and a leading cause of death in MV patients.15,16 The clinical symptoms of VAP are complex and early diagnosis is difficult. Once VAP occurs, patients suffer difficulties withdrawing from MV, have prolonged hospitalization, increased hospitalization expenses and an increased danger to life.6–8 Of the 901 elderly MV patients observed in this study, 156 met the diagnostic criteria for VAP (incidence=17.3%). The incidence density of VAP in our hospital was 4.25‰, which was mainly classed as late onset. This was less than previously reported values that ranged from 18% to 32%.17–19 The incidence of VAP varied because of the target population and methods of diagnosis. In this study, the target population was elderly Chinese patients. Analyzing the risk factors for the occurrence of VAP provides a theoretical basis for effective preventive measures. Logistic regression revealed that the risk factors of VAP in elderly patients were COPD, ICU admission, the MV methods, the number of central venous catheters, the duration of an indwelling urinary catheter, the number of antibiotics administered and the use of corticosteroids prior to MV.
MV methods are VAP risk factors
The methods of MV influenced the occurrence of VAP. The incidence of VAP was 13.4% for tracheal intubation, 28.4% for tracheostomy, and 23.7% for tracheostomy after tracheal intubation. The incidence of VAP in patients with tracheotomy was lower than that of patients who underwent tracheostomy after tracheal intubation. This is contrary to previous clinical data. Patients with MV should therefore undergo tracheotomy as quickly as possible to reduce the risk of secondary catheterization and the incidence of VAP. Wang20 and Griffiths et al21 found that early tracheotomy failed to reduce the incidence of VAP and did not shorten the duration of MV or the duration of ICU stay. This is because tracheotomy damages the normal physiological and anatomical function of the trachea. The respiratory tract directly contacts the external environment and the protective effects of upper respiratory tract filtration and humidification are weakened. This leads to a loss of cough and reflex function of the trachea, leading to pathogenic microorganisms colonizing in the tracheal tube. Colonized pathogens form biofilms, increasing the likelihood of lower respiratory tract infections.22,23 In this study, the incidence of VAP in patients suffering >2 (28.4%) reintubations was higher than those with ≤2 (16.2%) reintubations. These differences, however, were not statistically significant. This differed from previous studies that identified reintubation as an important predictor of VAP development.24–26 This may have been due to the number of re-intubations we compared, as opposed to the number of re-intubations that occurred.
ICU admission and COPD are VAP risk factors
Patients admitted to ICU are between 5 and 10 times more likely to acquire a nosocomial infection than patients in other hospital areas.27 VAP is the most common hospital-associated infection among adult patients in ICUs, with frequencies of 15–45%. The reported rate of VAP in North American and European ICU settings is 1–53 cases per 1,000 ventilator days, affecting up to 30% of patients receiving MV.28–30 A 2005 study across 14 ICUs revealed a rate of 28%.31 The incidence of VAP varied according to the type of ICU (medical, surgical, coronary). This was higher amongst patients with burns, or in those within neurosurgical or trauma units (17–20%).32 These findings did not agree with Torres et al, who found that the type of ICU population did not influence VAP occurrence.33 In this study, ICU admission was the major risk factor for the occurrence of VAP. We also found that patients who developed VAP had longer ICU stays than those who did not, which was consistent with other reports.34 ICU patients have more chronic co-existing diseases and more severe acute physiological dysfunction and suffer more invasive procedures, so they are in a state of relative immunosuppression.3 In addition, due to the widespread use of antibiotics and improper isolation measures, cross-infection is also increasing, and the possibility of infection is increasing. Therefore, reducing the incidence of ventilator-associated pneumonia and exploring the related factors causing ventilator-associated pneumonia in ICU have been the main research focus in this field.
COPD is a well-recognized risk factor for community-acquired pneumonia.35,36 A history of COPD has also been identified as a risk factor for VAP development.37 The duration of MV and ICU stay were both longer in COPD patients with VAP.38 The development of VAP in COPD patients resulted in an increase of 17% in mortality rates compared to COPD patients that did not develop VAP.39 In this study, COPD was the major risk factor of VAP occurrence (χ2=6.264, P<0.05). VAP occurred in 22.6% of COPD patients, which was higher than in patients with other diseases. This may be due to the patient’s advanced age, high colonization of the lower airways, the inhibition of mucociliary function due to cigarette smoking, the inability to generate an effective cough response due to airway obstruction, and the suppressive effects of corticosteroids on lung host defenses.40 When patients with COPD develop VAP, they present an increased risk of infection of specific pathogenic bacterial species.41 COPD leads to physiological changes which predispose patients to infections, particularly from Gram-negative bacilli.42
Impact of medication on the occurrence of VAP
The number of antibiotics administered by patients was a risk factor for VAP. Logistic regression showed that the incidence of VAP in patients using the number of antibiotics >3 was 4.947 times higher than those receiving ≤3, the differences of which were statistically significant. A large number of antibiotics can alter the parasitism of normal microorganisms, leading to infection by opportunistic pathogens or the emergence of drug-resistant bacterial strains, increasing the incidence of VAP.43–45 Prior antibiotic therapy has been recognized as a risk factor for VAP in adults.46,47 However, it is unclear whether the number of antibiotics prolongs hospitalization or intubation, both of which are risk factors for VAP. This relationship requires further exploration and clarification. A controversial issue is the selection of antibacterials. The judicious use of appropriate antibiotics may reduce patient colonization and subsequent infections with multidrug-resistant bacteria. Data from global studies suggest that multi-resistant bacteria are increasing, but these data may not be applicable to local hospitals. Therefore, based on our knowledge of bacterial flora in our hospital, the selection of adequate therapeutic regimens will decrease both morbidity and mortality.
Reports on the effects of corticosteroids on VAP have been variable. Both experimental48 and clinical49 data suggest that corticosteroid use decreases the occurrence and severity of nosocomial pneumonia in patients treated in the ICU. In the case of ARDS patients, corticosteroids reduce the incidence of suspected VAP.50,51 A multicenter trial that included 150 intubated patients admitted to the ICU due to severe trauma showed that the use of intravenous hydrocortisone over a period of seven consecutive days resulted in a decreased risk of hospital-acquired pneumonia and an increased duration of MV-free days.51 Mortality rates, the length of the ICU stay and the length of MV were all significantly lower in VAP patients receiving corticosteroids.52 Alternative results have however been reported. For patients with traumatic brain injury, the use of corticosteroids failed to reduce the incidence of VAP.53 In other studies, the use of low-dose steroids to prevent VAP was not favored.54 However, we found that the use of corticosteroids prior to intubation in patients with MV increases the occurrence of VAP. The use of corticosteroids prior to MV was the major risk factor of VAP. The reason might be that the use of corticosteroids leads to myelosuppression, liver and kidney dysfunction, decreased immune function, and infection. In addition, corticosteroids can increase the incidence of ulcers and damage the integrity of the gastrointestinal mucosal tissue structure, leading to the adsorption and transplantation of gastrointestinal pathogens, an important risk factor of VAP. Thus, corticosteroids should be administered reasonably to elderly patients when required.
Invasive procedures influence the occurrence of VAP
The number of central venous catheters and the duration of indwelling urinary catheters were identified as risk factors for VAP. Logistic regression showed that the incidence of VAP in the number of central venous catheter >2 of patients was 1.809 times higher than those receiving ≤2, the differences of which were statistically significant. The incidence of VAP in the duration of indwelling urinary catheters >90 days of patients was 1.805 times higher than those receiving ≤90 days. Indwelling catheters bypass the host’s natural defense mechanisms with high frequency, providing a method through which microbes invade important organs in the body. In the process of maintaining these catheters, medical personnel require frequent patient contact, leaving the patients vulnerable to the colonization and infection of hospital pathogens. In addition, maintenance devices act as reservoirs for pathogens, leading to the horizontal spread of pathogens between patients.55 More regular catheters of prolonged duration is more likely to damage a patient’s resistance and provide opportunities for pathogenic bacteria to enter the body, increasing the risk of infection. Therefore, invasive procedures in patients should be minimized to reduce the incidence of infection.
Establishment of VAP prediction model in elderly patients with MV
There are many factors affecting the occurrence of VAP. A model was constructed to predict the probability of VAP occurrence in patients with mechanical ventilation in Chinese elderly, to find high-risk patients infected with VAP as early as possible, to strengthen prevention measures in time, and to effectively reduce the occurrence of VAP. In this study, logistic regression was used to establish the prediction model of VAP infection in elderly patients with mechanical ventilation for the preliminary identification of VAP. By the logistic regression prediction model it is found that the number of antibiotics administered and mechanical ventilation methods have important effects on the occurrence of VAP, especially tracheotomy on the impact of VAP. Therefore, this study provides evidence for the clinical research to focus on the mechanical ventilation methods and the number of antibiotics administered to prevent VAP.
This study had several potential limitations. First, this was a cross-sectional study. Only correlations rather than causal relationships were established due to the study design. Secondly, this was a single-center study and all participants were from the same tertiary hospital in China. As such, the results cannot be generalized to other demographic groups. Further multicenter, prospective cohort studies that enroll participants with different demographic characteristics are now required.
Conclusion
The incidence of VAP in elderly patients with MV was 17.3%. The incidence density of VAP was 4.25/1,000 ventilator days. The risk factors of VAP mainly include the MV methods and the number of antibiotics administered. Based on the risk factors of VAP in the elderly Chinese patients, a prediction model was established to facilitate the early detection of high-risk patients. Further multicentered, prospective cohort studies are needed.
Acknowledgment
This study was sponsored by Beijing Municipal Commission of Science and Technology Program (Z171100000417032) and Beijing Natural Science Foundation Program (7192197).
Ethical approval
The ethical approval was obtained from Beijing Municipal Commission of Science and Technology Program.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 2.Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi: 10.1186/cc13712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. JAMA. 1995;274(8):639–644. [PubMed] [Google Scholar]
- 4.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.8.2106104 [DOI] [PubMed] [Google Scholar]
- 5.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the healthcare infection control practices advisory committee. MMWR Recomm Rep. 2004;53(RR–3):1–36. [PubMed] [Google Scholar]
- 6.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115 [DOI] [PubMed] [Google Scholar]
- 7.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31(5):1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06 [DOI] [PubMed] [Google Scholar]
- 8.Baid H. Patient safety: identifying and managing complications of mechanical ventilation. Crit Care Nurs Clin North Am. 2016;28(4):451–462. doi: 10.1016/j.cnc.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Jovanovic B, Milan Z, Markovic-Denic L, et al. Risk factors for ventilator-associated pneumonia in patients with severe traumatic brain injury in a Serbian trauma centre. Int J Infect Dis. 2015;38:46–51. doi: 10.1016/j.ijid.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Blot S, Koulenti D, Dimopoulos G, et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med. 2014;42(3):601–609. doi: 10.1097/01.ccm.0000435665.07446.50 [DOI] [PubMed] [Google Scholar]
- 11.File TM Jr. Recommendations for treatment of hospital-acquired and ventilator-associated pneumonia: review of recent international guidelines. Clin Infect Dis. 2010;51(Suppl 1):S42–7. doi: 10.1086/653048 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues DO, Cezário RC, Filho PP. Ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa vs. other microorganisms at an adult clinical-surgical intensive care unit in a Brazilian University Hospital: risk factors and outcomes. Int J Med Sci. 2009;1:432–437. [Google Scholar]
- 13.Morehead RS, Pinto SJ. Ventilator-associated pneumonia. Arch Intern Med. 2000;160(13):1926–1936. doi: 10.1001/archinte.160.13.1926 [DOI] [PubMed] [Google Scholar]
- 14.Khattab AA, El-Lahony DM, Fathy W. Ventilator associated pneumonia in a neonatal intensive care unit. J Am Sci. 2013;9(11):251–258. [Google Scholar]
- 15.Makris D, Luna C, Nseir S. Ten ineffective interventions to prevent ventilator- associated pneumonia. Intensive Care Med. 2018;44(1):83–86. doi: 10.1007/s00134-017-4978-7 [DOI] [PubMed] [Google Scholar]
- 16.Corrado RE, Lee D, Lucero DE, et al. Burden of adult community-acquired, healthcare-associated, hospital-acquired, and ventilator-associated pneumonia-New York City, 2010-2014. Chest. 2017;S0012369217307791. doi:10.1016/j.chest.2017.04.162 [DOI] [PubMed] [Google Scholar]
- 17.Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. Effectiveness of an educational program to reduce ventilator-associated pneumonia in a tertiary care center in Thailand: a 4-year study. Clin Infect Dis. 2007;45(6):704–711. doi: 10.1086/520987 [DOI] [PubMed] [Google Scholar]
- 18.Kulvatunyou N, Boonbarwornrattanakul A, Soonthornkit Y, Kocharsanee C, Lertsithichai P. Incidence of ventilator-associated pneumonia (VAP) after the institution of an educational program on VAP prevention. J Med Assoc Thai. 2007;90(1):89–95. [PubMed] [Google Scholar]
- 19.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120(2):555–561. doi: 10.1378/chest.120.2.555 [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Wu Y, Bo L, et al. The timing of tracheotomy in critically ill patients undergoing mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;140(6):1456–1465. doi: 10.1378/chest.11-0401 [DOI] [PubMed] [Google Scholar]
- 21.Griffiths J, Barber VS, Morgan L, et al. systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(5):1243–1246. doi: 10.1136/bmj.38467.485671.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sands KM, Wilson MJ, Lewis MA, et al. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J Crit Care. 2017;37:30–37. doi:10.1016/j.jcrc.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 23.Machado MC, Webster TJ. Decreased Pseudomonas aeruginosa biofilm formation on nanomodified endotracheal tubes: a dynamic lung mode. Int J Nanomedicine. 2016;11:3825–3831. doi:10.2147/IJN.S108253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaviroj S, Cherdrungsi R, Chaiwat O. Incidence and risk factors for ventilator-associated pneumonia in the surgical intensive care unit, Siriraj Hospital. J Med Assoc Thai. 2014;97(Suppl 1):S61–8. [PubMed] [Google Scholar]
- 25.Kollef MH, Von Harz B, Prentice D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest. 1997;112(3):765–773. doi: 10.1378/chest.112.3.765 [DOI] [PubMed] [Google Scholar]
- 26.Torres A, Gatell JM, Aznar E, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152(1):137–141. doi: 10.1164/ajrccm.152.1.7599836 [DOI] [PubMed] [Google Scholar]
- 27.Widmer AF. Infection control and presentation strategies in the ICU. Intensive Care Med. 1994;20(Suppl.4):7–11. doi: 10.1007/BF01713976 [DOI] [PubMed] [Google Scholar]
- 28.Rea-Neto A, Youssef NC, Tuche F, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12(2):R56. doi: 10.1186/cc6996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charles MP, Easow JM, Joseph NM, et al. Incidence and risk factors of ventilator associated pneumonia in a tertiary care hospital. Australas Med J. 2013;6(4):178–182. doi: 10.4066/AMJ.2013.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipovy B, Rihova H, Gregorova N, et al. Epidemiology of ventilator-associated tracheobronchitis and ventilator-associated pneumonia in patients with inhalation injury at the burn centre in Brno (Czech Republic). Ann Burns Fire Disasters. 2011;24(3):120–125. [PMC free article] [PubMed] [Google Scholar]
- 31.Boots RJ, Lipman J, Bellomo R, et al. Disease risk and mortality prediction in intensive care patients with pneumonia. Australian and New Zealand practice in intensive care (ANZPIC II). Anaesth Intensive Care. 2005;33(1):101–111. doi: 10.1177/0310057X0503300116 [DOI] [PubMed] [Google Scholar]
- 32.System N. National Nosocomial infections surveillance (NNIS) system report, data summary from January 1990-May 1999, issued June 1999. A report from the NNIS system. Am J Infect Control. 1999;27(6):520–532. doi: 10.1016/S0196-6553(99)70031-3 [DOI] [PubMed] [Google Scholar]
- 33.Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142(3):523–528. doi: 10.1164/ajrccm/142.3.587 [DOI] [PubMed] [Google Scholar]
- 34.Sofianou DC, Constandinidis TC, Yannacou M, Anastasiou H, Sofianos E. Analysis of risk factors for ventilator-associated pneumonia in a multidisciplinary intensive care unit. Eur J Clin Microbiol Infect Dis. 2000;19(6):460–463. doi: 10.1007/s100960000236 [DOI] [PubMed] [Google Scholar]
- 35.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 36.Restrepo MI, Sibila O, Anzueto A. Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2018;81(3):187–197. doi: 10.4046/trd.2018.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundar KM, Nielsen D, Sperry P. Comparison of ventilator-associated pneumonia (VAP) rates between different ICUs: implications of a zero VAP rate. J Crit Care. 2012;27(1):26–32. doi: 10.1016/j.jcrc.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 38.Hadda V, Dubey G, Nallan R, et al. Impact of ventilator associated pneumonia on outcome in patients with chronic obstructive pulmonary disease exacerbation. Lung India. 2014;31(1):4–8. doi: 10.4103/0970-2113.125886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koulenti D, Blot S, Dulhunty JM, et al. COPD patients with ventilator-associated pneumonia: implications for management. Eur J Clin Microbiol Infect Dis. 2015;34(12):2403–2411. doi: 10.1007/s10096-015-2424-8 [DOI] [PubMed] [Google Scholar]
- 40.Charles MP, Kali A, Easow JM, et al. Ventilator-associated pneumonia. Australas Med J. 2014;7(8):334–344. doi: 10.4066/AMJ.2014.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rello J, Torres A, Ricart M, et al. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1545–1549. [DOI] [PubMed] [Google Scholar]
- 42.Talon D, Mulin B, Rouget C, et al. Risks and routes for ventilator-associated pneumonia with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157(3 Pt 1):978–984. [DOI] [PubMed] [Google Scholar]
- 43.Charles EL, Nicolas B, Alain C, et al. Delivering antibiotics to the lungs of patients with ventilation-associated pneumonia: an update. Expert Rev Anti Infect Ther. 2013;11(5):511–521. doi: 10.1586/eri.13.36 [DOI] [PubMed] [Google Scholar]
- 44.Kadri SS, O’Grady NP. Review: short and long courses of antibiotics do not differ for mortality in ventilator-associated pneumonia. Ann Intern Med. 2014;160(10):JC3–JC3. [DOI] [PubMed] [Google Scholar]
- 45.Almuneef M, Memish ZA, Balkhy HH, Alalem H, Abutaleb A. Ventilator-associated pneumonia in a pediatric intensive care unit in Saudi Arabia: a 30-month prospective surveillance. Infect Control Hosp Epidemiol. 2004;25(9):753–758. doi: 10.1086/502472 [DOI] [PubMed] [Google Scholar]
- 46.Bauer TT, Ferrer R, Angrill J, Schultze-Werninghaus G, Torres A. Ventilator-associated pneumonia: incidence, risk factors, and microbiology. Semin Respir Infect. 2000;15(4):272–279. doi: 10.1053/srin.2000.20938 [DOI] [PubMed] [Google Scholar]
- 47.Bonten MJ, Weinstein RA. Infection control in intensive care units and prevention of ventilator-associated pneumonia. Semin Respir Infect. 2000;15(4):327–335. doi: 10.1053/srin.2000.20936 [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann I, Briegel J, Schliephake F, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008;34(2):344–349. doi: 10.1007/s00134-007-0865-y [DOI] [PubMed] [Google Scholar]
- 49.Steinberg KP, Hudson LD, Goodman RB, et al. National heart, lung, and blood institute acute respiratory distress syndrome (ARDS) clinical trials network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693 [DOI] [PubMed] [Google Scholar]
- 50.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693 [DOI] [PubMed] [Google Scholar]
- 51.Roquilly A, Mahe PJ, Seguin P, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA. 2011;305(12):1201–1209. doi: 10.1001/jama.2011.360 [DOI] [PubMed] [Google Scholar]
- 52.Nseir S, Di Pompeo C, Soubrier S, et al. Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest. 2005;128(3):1650–1656. doi: 10.1378/chest.128.3.1650 [DOI] [PubMed] [Google Scholar]
- 53.Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–1328. doi: 10.1016/S0140-6736(04)17188-2 [DOI] [PubMed] [Google Scholar]
- 54.Chaari A, Habib ME, Ghdhoun H, et al. Does low-dose hydrocortisone therapy prevent ventilator-associated pneumonia in trauma patients? Am J Ther. 2013;22(1):22–28. doi: 10.1097/MJT.0b013e3182691af0 [DOI] [PubMed] [Google Scholar]
- 55.Kaye KS, Marchaim D, Smialowicz C, Bentley L. Suction regulators: a potential vector for hospital-acquired pathogens. Infect Control Hosp Epidemiol. 2010;31(7):772–774. doi: 10.1086/653820 [DOI] [PubMed] [Google Scholar]