Abstract
Pregnancy parallels an elaborate assortment of dynamic changes allowing intimate approximation of genetically discordant maternal and fetal tissues. Although the cellular and molecular details for how this works remains largely undefined, important clues arise from evaluating how prior pregnancy influences the outcome of future pregnancy. Risk of complications is consistently increased with complication in prior pregnancy. Reciprocally, prior successful pregnancy protects against complications in future pregnancy. Here we summarize immunological perturbations associated with fetal loss, with particular focus on how these harmful and protective adaptations may persist in mothers. Immunological aberrancy as a root cause of pregnancy complications is also considered given their shared overlapping risk factors and the sustained requirement for averting maternal-fetal conflict throughout pregnancy. Understanding pregnancy induced immunological changes can not only expose new therapeutic strategies for improving pregnancy outcomes, but also new facets on how immune tolerance works applicable in other physiological and pathological contexts.
Keywords: Spontaneous abortion, microchimerism, stillbirth, preeclampsia, prematurity, regulatory T cells
INTRODUCTION
Discrimination between “self” compared with “non-self”, activation of adaptive immune components upon exposure to genetically foreign non-self antigens, and the ability to “remember” these prior antigenic encounters are each interrelated hallmark features of the mammalian immune system. Almost every facet of how we evaluate immunological responses, generate new hypotheses to investigate the inter-workings of the immune system in health and disease, and the design immune-based therapeutic strategies is based on these foundational immunological pillars. For example, vaccines work because they stimulate in individuals the expansion and long-term persistence of adaptive immune components with specificity to genetically foreign microbe-expressed antigens. In turn, these memory adaptive immune components capable of more robust and efficient activation with subsequent microbial antigen encounter confer protection against future infection. Reciprocally, these same adaptive immune components that protect against microbial infection are also the major obstacles in transplantation where donor tissues are attacked with similar vigor until all genetically foreign cells are rejected.
Importantly however, there remain major shortcomings regarding how immunology works under this framework when applied to reproduction and pregnancy. To the extent that pregnancy health and reproductive success are dominant factors driving trait selection – refining how our immune systems optimally work, these shortcomings should not be viewed as curious exceptions to traditional immunological rules. Instead, pregnancy and reproduction likely hold important immunological secrets, that if more comprehensively understood, has enormous applicability on how immune tolerance is achieved in many other physiological and pathological contexts. With these fundamental gaps in knowledge unresolved, it should be no surprise that pregnancy complications remain the leading cause of under-age five childhood mortality, and that therapies for unfortunately common human disorders such as infertility, spontaneous abortion, stillbirth, preeclampsia and prematurity remain non-existent or at best rudimentary.
With regards to “self” versus “non-self” antigen distinction, accumulating evidence already shows many examples where immunological tolerance is naturally expanded to encompass a variety of genetically foreign “extended-self” antigens. Along with the expanded tolerance of mothers to fetal-expressed paternal antigens, another prominent example of tolerance to extended self antigens include those expressed by commensal microbes that also reside in close approximation within our tissues (1). Given the primitive ancestral origins whereby symbiosis between commensal microbes and larger multicellular organisms is predicted to have evolved, that predates viviparity and genetic heterogeneity amongst individuals that gives rise to the conundrum of maternal-fetal immunological conflict (2), it is not surprising to find many commonalities in how tolerance to commensal microbes and tolerance to fetal expressed antigens is achieved during pregnancy – both require expanded peripheral immune tolerance to genetically foreign non-self antigens.
Here, we evaluate reproduction and pregnancy from the other related immunological hallmark feature of “memory”. In particular, available human epidemiological data comparing the risk of complications for women during a first pregnancy compared with subsequent pregnancies, and the partner specificity of these effects is highlighted. Results from complementary animal models tracking maternal adaptive immune components during pregnancy, their disposition after parturition, and their response to stimulation in future pregnancies is also summarized, together with what we view as important priorities and questions for future research.
Pregnancy induced immunological shifts.
The relationship between mother and fetus during pregnancy remains an immunological marvel and enigma. The fetus is semi-allogeneic, containing equal genetic contributions from the mother and father. Under the classical immunological tenets of “self” versus “non-self” antigenic distinction (3, 4), fetal tissues should prime robust activation of maternal adaptive immune components that leads to the rejection of all antigenically foreign fetal cells. Why does this not occur during pregnancy?
Sir Peter Medawar posited three theoretical explanations on this immunological paradox (5). The first considered anatomical separation of the fetus from the mother, or the placenta as a physical barrier separating genetically discordant maternal and fetal tissues. While there are indeed unique immunological facets at the maternal-fetal interface that could create a functional barrier including entrapment of decidual antigen presenting cells (6), local exclusion of effector T cells through chemokine gene silencing (7), and reduced complement deposition (8), this explanation does not account for how nutrients and waste products are exchanged, and the increasing appreciation for the highly conserved bi-directional systemic transfer of intact cells between mother and fetal offspring during pregnancy (9, 10).
Another consideration focused on antigenic immaturity of the fetus, that allow fetal cells to evade recognition and rejection by maternal immune components. This is best illustrated by reduced trophoblast cell expression of classical human leukocyte antigens (HLA) that defines the unique immunological identity of individuals (11). Instead, trophoblast cells selectively express other non-polymorphic HLA subsets (e.g. HLA-G), that simultaneously allows evasion of “missing self” recognition and rejection by natural killer cells (12, 13). However, with the development of antibodies with increased specificity to HLA antigens, individual trophoblast cell subsets have since been shown to express classical HLA-A, HLA-B or HLA-C antigens, albeit at somewhat reduced levels (14-16). Likewise, normal fertility and pregnancy outcomes have been shown for HLA-G null individuals and non-human primate species with predicted non-functional HLA-G variants (17, 18). Together, these results highlight the existence of additional, and likely more dominant mechanisms, whereby fetal tolerance is universally maintained. This could involve the third of Medawar’s postulates considering the immunological inertness of the mother, and in particular, pregnancy induced shifts in responsiveness of systemic and local maternal immune components with fetal specificity.
Classical examples demonstrating dampened systemic responsiveness of mothers come from animal pregnancy showing selectively reduced numbers of peripheral maternal T cells with fetal MHC allo-specificity, and selective acceptance of tumor allografts bearing genetically foreign fetal-expressed MHC haplotypes during pregnancy (19). Using more refined immunological tools to track maternal T cells with surrogate fetal specificity, maternal CD8+ T cells were shown to readily proliferate in an antigen-specific fashion in response to fetal antigen stimulation, but these proliferating cells quickly undergo clonal deletion without priming for cytolytic effector function (20). Interestingly, pregnancy induced tolerance is likely not restricted only to genetically foreign fetal-expressed paternal antigens, but can extend to self-antigens driving autoimmune disorders as well. For example, sharp reductions in disease severity or remission in symptoms are observed during pregnancy for women with various autoimmune disorders that affect non-reproductive tissues such as multiple sclerosis, rheumatoid arthritis, autoimmune hepatitis and thyroiditis (21-24). Similarly, in mouse models of autoimmunity, inflammatory arthritis and ascending paralysis caused by activated T cells with specificity to defined neuronal antigens are each alleviated during pregnancy (25-27). However, these findings do not imply pregnancy-induced global immunological suppression since there are clearly other instances where maternal immunity remains robust and effective. These include the protective response to intrapartum maternal immunization against influenza A or tetanus infection, that is not only effective in improving the health of mothers, but also immunity in offspring through passively transferred antibodies (28, 29). Thus, the response of mothers during pregnancy is dampened against some antigens (fetal expressed or genetically encoded self antigens), but remains intact against others such as microbe specific antigens. Here, it may be important to highlight that while the response to genetically foreign fetal-expressed antigens have appropriately been the focus on studies investigating maternal-fetal immunological conflict, fetal cells also likely express nearly all maternal self antigens that are the targets for autoimmunity. Thus, an interesting commonality between the remission of autoimmunity and expanded fetal tolerance that simultaneously occurs during pregnancy is the enhanced tolerance that occurs to fetal-expressed antigens, regardless of whether they are genetically foreign to the mother or maternally self-encoded.
Treg cells and expanded fetal tolerance.
One of the main functions of immune system is to discriminate between self and non-self, and exclusively maintain self-tolerance. These tolerogenic mechanisms actively suppress the activation of immune components with self-specificity to protect against autoimmunity (3, 4). By extension, suppressed activation of immune components with commensal microbe extended-self specificity protects against auto-inflammatory disorders in mucosal barrier tissues (1). Peripheral tolerance to self and extended-self antigens are primarily maintained by a dedicated immune suppressive subset of CD4+ T cells, called regulatory T (Treg) cells (30, 31). Treg cells usually constitute 5-15% of peripheral CD4+ T cells in both humans and mice. The appreciation of Treg cells a separate functional CD4+ T cell subset were spearheaded by finding expression of the IL-2 receptor, CD25, marks cells with unique suppressive capacity (32), and later identification of forkhead box P3 (FOXP3) as the master transcriptional regulator and lineage defining marker for Treg cells (33). Humans with FOXP3 mutations develop multi-organ systemic autoimmunity characterized as the immune dysregulation polyendocrinopathy X-linked (IPEX) syndrome (34). A similar phenotype occurs in mice with either spontaneous null FOXP3 mutations, targeted foxp3 gene deletion or after selective depletion of FOXP3+ cells (35, 36).
Most Treg cells are self-reactive and acquire FOXP3 expression during thymic maturation and suppress other self-reactive immune components that escape central tolerance (30, 31, 37). However, FOXP3 expression can also be induced amongst peripheral non-Treg effector CD4+ T cells. Induced FOXP3 expression and conversion into Treg cells occurs for peripheral CD4+ T cells after high-affinity cognate TCR stimulation in the presence of TGF-β (38), plus a potentially wide variety of other tolerogenic cytokines or tissue specific environmental cues. This capacity for induced conversion of peripheral CD4+ T cells with specificity for a near infinite array of genetically foreign antigens into Treg cells creates an adaptable arsenal of immune suppressive cells capable of responding to fluctuating environmental conditions, such as pregnancy, when expanded immunological tolerance is required. It is now clear from the analysis of human pregnancy and complementary animal studies that maternal FOXP3 Treg cells expand locally at the maternal fetal interface and systemically during pregnancy, and that the sustained expansion of these cells is required for maintaining fetal tolerance [Reviewed in (39-42)]. However, additional investigation is needed to establish the origin of these cells, how they work, and their requirements for fetal-specificity.
Decidual Treg cells can be derived from either the induced differentiation of systemically recruited fetal-specific CD4+ T cells or proliferation of thymus derived Treg cells. Before implantation, Treg cells accumulate in the uterine lymph nodes in response to hormonal and paternal antigen stimulation (43, 44). Treg cells expand and traffic to the pregnant uterus by human chorionic gonadotropin-induced chemoattractant mechanisms and establish residency in the decidua (45). Human chorionic gonadotropin also augments the suppressive potency of Treg cells and primes a tolerogenic phenotype amongst antigen presenting cells (46). Paternal antigens present in the seminal fluid further drive the expansion of Treg cells from naive CD4+ T cells (47). Saito et al proposed that dendritic cells detect paternal antigens from the seminal plasma after coitus and present them to locally recruited CD4+ T cells (48). In turn, hormone conditioned antigen presenting cells prime Treg cell differentiation and tolerogenic, as opposed to activated effector T cell phenotypes (6, 49). Thymic stromal lymphopoietin produced by trophoblast cells further augments the capacity of dendritic cells to induce FOXP3 and CD25 expression among decidual human CD4+ T cells (50). Together, these interactions promote the selective accumulation of Treg cells at the maternal fetal interface beginning from the earliest stages of pregnancy.
Despite an increasing wide variety of Treg cell associated or secreted molecules having been shown to mediate the suppressive function of these cells [Reviewed in (51, 52)], how they mediate context specific immune suppression remains largely undefined. Decidual Treg cells secrete inhibitory cytokines, such as transforming growth factor (TGF)-β, IL-10 and IL-35 to suppress the activation of non-Treg effector cells and inhibit their proliferation and production of proinflammatory cytokines [Reviewed in (53)]. Expanded decidual Treg cells in human pregnancy express high levels of cytotoxic T lymphocyte antigen-4 (CTLA-4) (54). In turn, CTLA-4 functionally sequesters the expression of CD80/CD86 costimulatory molecules, and induces expression of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO) in decidual dendritic cells (54, 55). IDO mediated tryptophan catabolism efficiently inhibits the proliferation and activation of non-Treg effector cells, whereas IDO inhibition during pregnancy results in rapid T cell induced wastage of allogeneic concepti in mice (56). Galectin-1 and programmed death ligand-1 (PDL-1) have each also independently been shown to promotes apoptosis of potentially harmful activated maternal T cells during pregnancy (57, 58). Expanded fetal tolerance may also be aided by non-Treg T cell subsets since decidual CD8+ T cells have recently been shown to adopt cell intrinsic dysfunctional phenotypes (59). Thus, considering the overall necessity of expanded maternal tolerance in sustaining healthy pregnancy (39, 40), prioritizing and establishing how maternal Treg cells work, and work together with other leukocyte subsets, during the progression of pregnancy represent important areas for future investigation.
Partner-specific immunological tolerance prior to pregnancy.
Semen plays a critical role in conditioning female reproductive tissues for expanded tolerance. Although sperm is MHC negative, paternal antigens expressed by epithelial cells and cellular particles in seminal fluid represents the first exposure of female immune components to paternal allo-antigens. Seminal fluid contains steroid hormones, including estrogen, testosterone and prostaglandins (60), all potent modulators of myeloid antigen presenting cells and lymphocyte development. TGF-β in seminal fluid stimulates production of granulocyte macrophage colony stimulating factor that synergizes in promoting immunological tolerance (61,62). Seminal fluid also induces expression of the chemokine CCL19, that promotes uterine recruitment of Treg cells (44). In humans, seminal fluid further promotes the recruitment of T cells with a memory phenotype into the cervix within 12 hours after coitus (63); however, whether these memory T cells then differentiate into Treg cells or help recruit circulating Treg cells remains undefined.
Preeclampsia is an idiopathic disorder clinically characterized by high maternal blood pressure and proteinuria. Severe cases are associated with hemolysis, low platelet count, plus liver and kidney dysfunction. Left untreated, it can progress to eclampsia, defined by the presence of maternal seizures. Circulating levels of proinflammatory cytokines including TNF-α and IL-6 are each significantly increased in women with preeclampsia compared with gestational age matched healthy pregnancy (64, 65). Although preeclampsia has been widely attributed to abnormal trophoblastic invasion and placentation (66), that in turn drives endothelial cell dysfunction, oxidative stress and ensuing vasoconstriction that results in end-organ damage, multiple independent lines of evidence suggest maternal-fetal immunological conflict likely play important causative roles (67, 68). In the early stages of pregnancy, perturbations in tolerance to invasive fetal trophoblast cells may lead to inadequately remodeled spiral arteries and shallow implantation. In the second half of pregnancy when preeclampsia occurs, the only definitive treatment is delivery of the fetus and all products of conception.
In humans, there are provocative data highlighting the positive association between duration of sexual cohabitation (prolonged semen exposure prior to conception) and protection against preeclampsia, pregnancy-induced hypertension and fetal growth restriction (69-71). There is also interesting partner specificity to these protective benefits [Reviewed in (72)]. Amongst nulliparous women with history of prior spontaneous abortion, the risk of preeclampsia is reduced by nearly 50% in subsequent pregnancies with the same partner, but these protective effects are eliminated amongst women who conceived with a new partner (73). Preeclampsia is more common in women who conceived following the use of contraceptives (e.g. condoms, diaphragms, spermicides) which prevents exposure to seminal fluid (74). Oral sex, with tolerogenic exposure to fetal expressed paternal antigens in non-reproductive mucosal tissues, is also protective against preeclampsia that occurs in a partner-specific fashion (75). The incidence of preeclampsia is significantly reduced in pregnancies initiated by artificial intrauterine insemination with partner sperm compared with sperm from non-partner donors (76), and a recent meta-analysis of seven randomized controlled trials show significant improvement in clinical pregnancy rate amongst women with natural exposure to seminal plasma around the time of embryo transfer (77).
Dendritic cells in the cervix respond to paternal antigens in seminal fluid, including HLA in seminal plasma, and induce paternal MHC class I specific tolerance along with the expansion of paternal-antigen specific Treg cells [Reviewed in (78, 79)]. This, in turn, promotes tolerance to paternal antigens expressed by sperm and suppresses anti-sperm immunity, which could otherwise cause infertility. Repeated exposure to seminal fluid drives enhanced accumulation of maternal Treg cells with paternal HLA specificity that facilitates optimal implantation and development of embryos conceived by the same male partner. Reciprocally, reduced Treg cell frequencies in the decidual CD4+ T cell pool and peripheral blood for women with recurrent fetal loss suggest that absent or reduced pre-exposure to paternal antigens negatively impacts pregnancy outcomes (80, 81). Thus, the mechanism linking seminal fluid with protection against pregnancy complications is likely to involve early recruitment of protective immune cells to female reproductive tissues even prior to conception, particularly expansion of Treg cells with partner-specificity, that promotes optimal trophoblast cell implantation and placentation when pregnancy eventually occurs.
Further evidence of partner specificity and the cumulative benefit of semen exposure on protective partner-specific immunological tolerance is derived from animal pregnancy models. Seminal fluid expands the pool of Treg cells in the para-aortic lymph nodes draining the uterus, and promotes accumulation of Treg cells in the uterus of mice even prior to embryo implantation (44). In turn, paternal HLA contained in semen primes in mice expansion of maternal Treg cells with paternal HLA specificity (47). Treg cells are essential for the maintenance of allogeneic pregnancy since reconstituting T cell deficient mice with non-Treg effector cells alone selectively causes resorption of allogeneic, but not syngeneic fetal offspring (82). Fetal wastage occurring after depletion of Treg cells during mouse allogeneic pregnancies using either anti-CD25 antibodies or transgenic mice that co-express with FOXP3 the human high affinity receptor for diphtheria toxin further re-enforce the necessity for these cells in healthy pregnancy (83-85).
Paternal antigen in the acellular fraction of the ejaculate presented in the context of immune-modulating signals, such as TGF-β, likely drive expansion of Treg cells in an antigen-specific manner after mating (61). Maternal antigen presenting cells cross-present antigens from the ejaculate in the para-aortic lymph nodes (47), facilitated in part by seminal fluid contained TGF-β, that initiates functional tolerance of maternal T cells even prior to embryo implantation (19, 44). Strain-specific tolerance of tumor cells conferred by mating with intact, but not vasectomized males, even in the absence of ensuing pregnancy further supports the notion that exposure to seminal fluid is sufficient for activating expansion of maternal Treg cells (62). Studies tracking donor cells from T cell receptor transgenic mice with fixed specificity to model antigens (e.g. ovalbumin) during pregnancies sired by male mice that ubiquitously express ovalbumin in all cells, further demonstrate that exposure to seminal fluid primes maternal T cell awareness of fetal-expressed antigens beginning prior to embryo implantation (47). Ovalbumin reactive T cells with surrogate paternal specificity adoptively transferred into female mice proliferate and become activated within 3 days post-coitum in the para-aortic lymph nodes, despite embryo implantation not occurring until the 4th day after mating in this species (47). Together, these observations of male partner specificity and the protective benefits of seminal fluid exposure on susceptibility to complications in future pregnancy suggest that immunological tolerance of a partner's antigenic repertoire is programmed and boosted by each round of insemination before embryo implantation, regardless of whether pregnancy occurs.
Immune pathogenesis of recurrent fetal loss and other pregnancy complications.
Circulating Treg cell frequencies in women undergo profound changes during the menstrual cycle, with expansion in the late follicular phase followed by a dramatic decrease in the luteal phase (86). Female reproductive hormones, including estrogen (87, 88) and progesterone (89), likely play dominant roles driving these dynamic Treg cells shifts. Interestingly, circulating Treg cells during mensuration are functionally diminished in women with recurrent spontaneous abortion (86), whereas endometrial FOXP3 mRNA levels are reduced in women with infertility (90). Sasaki and colleagues first described a significantly reduced frequency (by ~66%) of CD25bright CD4+ Treg cells in the decidual tissue of spontaneous abortion compared induced abortions cases (80), and this association between reduced accumulation of decidual maternal Treg cells and spontaneous abortion was later confirmed by analysis of FOXP3 expression (81). Women with history of recurrent fetal loss with decreased frequency of Treg cells in the first trimester have reduced fertility compared with those with higher Treg cell levels (91). Reciprocally, immunomodulatory therapies for infertility further support the need for expanded maternal Treg cells since lymphocyte immunization that boosts the number of maternal Treg cells results in successful delivery of a live infant in 80-90% of patients (92).
Along with reduced Treg cell numbers, qualitative reductions in their suppressive potency are also likely involved in pregnancy complications. Decreased efficiency whereby CD25+ FOXP3+ CD4+ Treg cells suppress the proliferation of responder T cells after stimulation with third-party allogeneic cells are found in women with recurrent fetal loss (93). Sperm specific Treg cells in women with recurrent fetal loss express less Ubc13, a ubiquitin-conjugating enzyme essential for maintaining the suppressive function of Treg cells (94). Expression of intracellular TGF-β and IL-10 by maternal Treg cells is also reduced in pregnant women with recurrent fetal loss (93). Maternal serum levels of IL-35, a cytokine implicated in some aspects of immune suppression by Treg cells (51), are increased during healthy pregnancies, but reduced in women with recurrent spontaneous abortion (95). Expression of galectin-1, which promotes apoptosis of activated T cells, is reduced amongst Treg cells from women with recurrent fetal loss compared with healthy pregnant women (96).
This apparent necessity for functionally expanded accumulation of maternal Treg cells is not limited to infertility and spontaneous abortion, but has since been shown for a wide variety of complications in human pregnancy including those that primarily occur in the second half of pregnancy such as preeclampsia, prematurity and stillbirth [reviewed in (40)]. For example, the frequency of conventional Treg cells and activated CD4+ CD25high FOXP3high cells were lower in pregnant women with preeclampsia compared to non-pregnant controls (97). Circulating maternal Treg cells are significantly diminished in women with preeclampsia (98-100), and reduced maternal Treg cell levels are inversely correlated with increased numbers of activated non-Treg effector T cells in the decidua of women with preeclampsia (101). Treg cells likely suppress the activation of non-Treg effector Th1 and Th17 CD4+ T cells with paternal specificity since accumulation of IFN-γ+ and IL-17+ CD4+ T cells with sperm specificity is reciprocally associated with dampened expansion of sperm-specific Treg cells in the peripheral blood of women with recurrent miscarriage (94). Likewise, the number of IL-17+ T cells and ratio of IL-17+ T cells/Treg cells are significantly increased in the peripheral blood of women with recurrent spontaneous abortion or fetal loss (102, 103).
Despite these very compelling observations linking healthy pregnancy and expanded accumulation of maternal Treg cells, together with the reciprocal association between blunted maternal Treg cell expansion or their dampened suppressive potency in pregnancy complications, these human analyses without experimental manipulation cannot definitively establish the cause and effect relationship between these biological parameters. In this regard, the analysis of animal pregnancy, especially allogeneic pregnancies that recapitulate the natural heterogeneity between maternal and fetal-expressed paternal antigens, have been invaluable for not only validating the protective necessity for maternal Treg cells, but also exposing critical mechanistic insights on how these cells work to sustain healthy pregnancy. Circulating maternal Tregs cells expand significantly above background levels within two days after pregnancy, and reach peak ~2-fold expanded levels by midgestation (82, 84). Reciprocally in abortion prone mating between defined strains of inbred mice (e.g. DBA/2J [H2d] ♂ × CBA/J [H2k] ♀), the expansion of maternal Treg cells is dampened to levels comparable non-pregnant controls (104). Fetal wastage is caused by blunted maternal Treg cell expansion in this context since adoptive transfer of CD25+ CD4+ Treg cells from mice with healthy pregnancies prevents fetal rejection (105), whereas expansion of FOXP3+ Treg cells, either directly by low dose of IL-2 or indirectly by Fms-related tyrosine kinase 3 ligand, led to normal pregnancy rates in abortion-prone mouse pregnancies (106). Interestingly, the degree of antigenic mis-match between maternal and fetal expressed paternal antigens also drives the magnitude of maternal Treg cell expansion. For example, pregnancy induced accumulation of maternal Treg cells becomes sharply reduced or eliminated during syngeneic matings amongst genetically homogenous mice where the only potential source of antigenic mismatch are those encoded by the Y chromosome, compared with allogeneic pregnancies using strains of mice with discordant MHC haplotype alleles (84, 107).
Importantly, sustained expansion of maternal Treg cells is essential for enforcing fetal tolerance because even partial transient depletion of these cells to pre-pregnancy levels causes fetal wastage that is associated with sharply increased activation and expansion of non-Treg effector CD4+ and CD8+ T cells with fetal specificity (84). Likewise, infection induced reductions in the suppressive potency of maternal Treg cells also cause placental inflammation and unleash the activation of harmful fetal-specific effector T cells (108, 109). With placental inflammation, the epigenetically silenced expression of Th1 chemokines (e.g. CXCL9, CXCL10, CXCL11) by decidual stromal cells is overcome by local recruitment of chemokine producing acute inflammatory neutrophil and macrophage cells (7, 108). In turn, fetal wastage is mediated by decidual infiltration and accumulation of activated maternal CD8+ T cells with fetal specificity since blocking their decidual infiltration using CXCR3 deficient mice or the administration of CXCR3 neutralizing antibodies each efficiently protects against fetal wastage (108). Likewise, preventing fetal recognition by maternal CD8+ T cells using TCR transgenic mice with a fixed monoclonal repertoire of these cells, but polyclonal repertoire of other adaptive immune cells, protects against fetal wastage (108). Thus, fetal wastage in mice is primarily mediated by maternal CD8+ T cells with fetal specificity that become unleashed for activation when Treg cells are depleted or their suppressive potency is diminished.
Fetal tolerance maintained by maternal Treg cells is further reinforced by the presence of evolutionarily conserved genetic elements that drive induced FOXP3 expression in humans and other eutherian placental mammals, which are conspicuously absent in marsupials and egg-laying mammalian species (2, 110). This includes the Foxp3 intronic enhancer element, conserved non-coding sequence 1 (CNS1), which is required for peripheral induced differentiation of naive CD4+ T cells into FOXP3+ Treg cells (111). Interestingly, allogeneic pregnancies in mice with targeted defects in Cns1, thus selectively lacking peripheral induced Treg cells show increased rates of fetal resorption with more pronounced decidual inflammation and abnormal spiral artery remodeling similar to the pathological features of preeclampsia in human pregnancy (2). Together with the aforementioned discussion on expansion of maternal FOXP3+ Tregs with fetal specificity prior to and during pregnancy, these results highlight the importance of maternal Treg cells with fetal specificity in sustaining maternal-fetal tolerance. On the other hand, peripheral induction does not imply exclusive specificity to fetal-expressed paternal antigens since pre-eliminating FOXP3+ cells amongst a polyclonal repertoire of donor CD4+ T cells transferred into virgin female mice prior to allogeneic pregnancy only suppressed expansion of fetal-specific Treg cell by ~50% (112). Similarly, others have shown optimal pregnancy induced expansion of maternal Treg cells with other surrogate fetal specificities (e.g. hemagglutinin) occurs optimally when recombinant strains of male and female mice that each express these antigens are used for mating (106). This potential overlap in protective functional roles between peripherally induced maternal Treg cells and pregnancy induced expansion of thymus derived Treg cells responsive to fetal antigen stimulation may explain the more modest levels of fetal loss that occurs when induced Treg cells are eliminated based on Cns1 deficiency or preconceptual stable differentiation of maternal CD4+ T cells with fetal specificity into the Th1 effector lineage, compared with the depletion of bulk Treg cells based on FOXP3 expression (2, 84, 113).
Pregnancy complications: discrete entities or a pathological continuum?
Despite rapidly approaching a population of 8 billion individuals, human reproduction is arguably still a relatively inefficient process. Extraordinarily high rates of implantation failure, spontaneous abortion and stillbirth occur, and 30 - 60% of human pregnancies do not achieve offspring viability (114, 115). Among pregnancies that survive to the later stages, 10% are affected by preeclampsia-eclampsia (116), another 10% suffer from prematurity (117) making offspring susceptible to ensuing sequelae such infection, blindness, neuro-developmental disorders, chronic lung disease and mortality. Barker’s theory posits that babies born from pregnancies affected by these syndromes also have high risk for diabetes, chronic inflammatory disorders and cardiovascular disease later in life (118). Therefore, complications in pregnancy, and their long-term sequelae in surviving offspring, remain important unresolved causes of economic, social and emotional human suffering.
While these complications in human pregnancy each have strict defining parameters based on primarily on in utero offspring viability, gestational age and maternal clinical parameters, they also share many key overlapping features, risk factors, and frequently occur in the same pregnancy. For example, an estimated 15% of premature births are caused by preeclampsia-eclampsia, whereas mild maternal hypertension alone is associated with an ~25% incidence of preterm birth (119). Maternal diabetes, cigarette smoking, hypertension and infection during pregnancy are each also independent dominant risk factors for spontaneous abortion, stillbirth, preeclampsia and prematurity (120-122). Importantly, women with a history of these complications are also at high risk for recurrence of these complications in future pregnancies. The risk of preeclampsia is consistently increased for women with preeclampsia in a prior pregnancy compared with nulliparous women undergoing a first pregnancy (123-126). A prospective analysis of over 700,000 first time mothers from the Swedish birth register showed the 4.1% risk of preeclampsia in a first pregnancy was increased to 14.7% during a second pregnancy, and 31.9% in women with preeclampsia in two previous pregnancies (127). Women with history of stillbirth have significantly higher (up to 5.7-fold) risk of stillbirth in subsequent pregnancies (128-130). The risk of preterm birth is also consistently increased in women with preterm birth in prior pregnancy (131-133), and best illustrated by greater than 5-fold increased risk of preterm birth amongst women with prior preterm birth in a retrospective analysis of over 1 million deliveries from the Danish Family Relations database (134).
Interestingly, having prior pregnancy affected by these complications not only predispose women to the same complication, but also increases susceptibility to other complications in future pregnancies (135). For example, the risk of fetal demise is increased 1.5-fold in women with history of preeclampsia in prior pregnancy (136) and the risk of preterm birth is increased ~ 3-fold in women with a history of stillbirth in prior pregnancy (137, 138). Likewise, the risk preeclampsia is significantly increased in women with a history of preterm birth without preeclampsia in the first pregnancy (126), and prematurity in a first pregnancy increased the risk of stillbirth in a second pregnancy (139). In the aforementioned prospective analysis of over 700,000 mothers in the Swedish birth register, prematurity (birth before 34 gestational weeks) occurred with ~15 and 30-fold increased frequency amongst women with preeclampsia in one and two prior pregnancies, respectively (127). With these stark associations that span and bypass the boundaries of how individual pregnancy complications are traditionally defined, it may be instructive to consider commonalities in their pathophysiology, and unifying mechanistic and pathological root causes.
A striking commonality highlighted in the aforementioned discussion is the association between reduced numerical and/or functional suppressive potency by maternal Treg cells and susceptibility to pregnancy complications such spontaneous abortion, stillbirth, preeclampsia and prematurity that each may stem from maternal-fetal immunological conflict (Figure 1). Given the continuous need for expanded tolerance to fetal-expressed paternal antigens that span the earliest stages of pregnancy through parturition, fractured fetal tolerance at any time during pregnancy would be expected to trigger complications associated with pathological activation of maternal immune components, immune-mediated fetal injury and/or premature fetal rejection. When during pregnancy disruptions in fetal tolerance occur, together with the tempo and severity whereby known (e.g. prenatal infection) or idiopathic instigators that disrupt fetal tolerance likely cause differences in the clinical presentation and range of maternal symptomatology that eventually terminate with delivery of the fetus and all macroscopic products of conception.
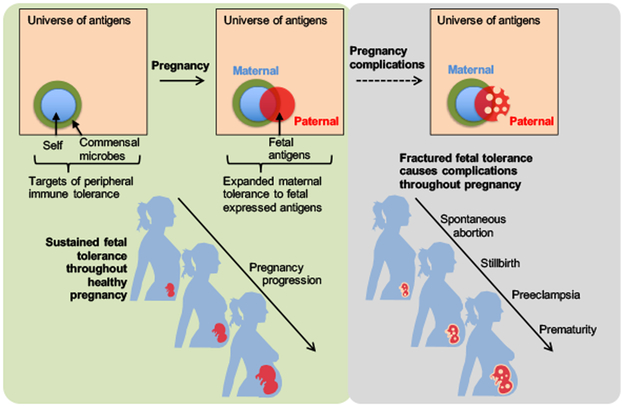
Figure 1. Expanded maternal tolerance to fetal expressed antigens is essential for averting complications throughout pregnancy.
Peripheral immune tolerance expands to encompass fetal expressed paternal antigens during uncomplicated healthy pregnancy. Fractured fetal tolerance at any time during pregnancy can cause gestational time specific complications such as spontaneous abortion, stillbirth, preeclampsia and prematurity.
It can be further hypothesized that if aberrantly activated maternal T cells cause pregnancy complications, then persistence of these cells in women may predispose them to complications associated with disrupted fetal tolerance in future pregnancies with the same partner (Figure 2). On the other hand, if protective maternal Treg cells primed by prior pregnancy persist in women after parturition, then enforced fetal tolerance and reduced susceptibility to complications caused by disruptions in fetal tolerance is expected. Answers to these questions as to whether protective or harmful maternal immune components are retained in mothers after pregnancy, and how they potentially work together in influencing the outcomes of future pregnancy require more comprehensive and dedicated analysis of complication incidence in humans during a first pregnancy compared with future pregnancies, and the partner specificity for these potential shifts in susceptibility to pregnancy complications. However, considering the inherent wide variability of humans and their unpredictability in mating and partner selection, together with the aforementioned immunological impacts of intercourse without pregnancy and differences in penetrance of other non-immune genetic elements that influence birth timing (140), animal pregnancy models that limit these experimental variables are likely to be more practical in testing these hypotheses.
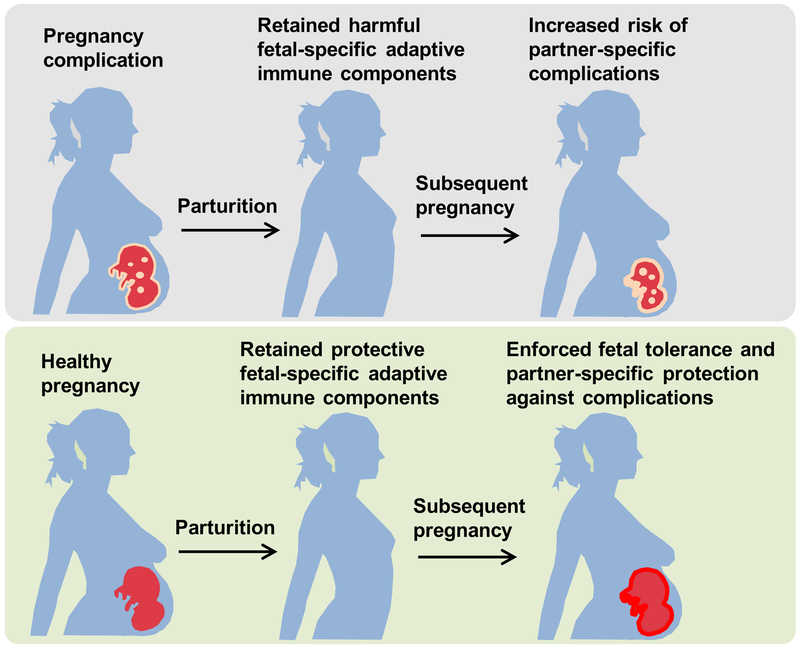
Figure 2. Fetal specific adaptive immune components retained in mothers after pregnancy influence susceptibility to complications in future pregnancies.
Harmful adaptive immune components with fetal specificity primed with pregnancy complications are retained in mothers after parturition, and cause increased partner-specific risk of complications in subsequent pregnancies (top). Protective fetal specific adaptive immune components retained in mothers after healthy pregnancy enforce fetal tolerance and confer partner-specific protection against complications in subsequent pregnancies (bottom).
Fate of maternal immune components with fetal specificity after parturition.
Given maternal immunological awareness of genetically foreign fetal-expressed antigens, and pregnancy-induced expansion of maternal adaptive immune components with these specificities, important questions for consideration include their disposition after parturition and their potential impacts on the outcomes of future pregnancy. Human pregnancy is known to efficiently sensitize mothers with antibodies to genetically foreign fetal-expressed paternal antigens. Using a more sensitive luminex based platform for analysis, nearly 50% of mothers without a prior history of transfusion or transplantation were found to contained antibodies with paternal HLA allo-specificity (141). Several other studies have found a positive correlation between increased HLA sensitization in women and the number of prior pregnancies (142, 143). Importantly, how these antibodies impact the outcomes of future pregnancies remains incompletely defined, and likely depends on the target antigen, their pattern of expression in fetal tissues, the antibody isotype and affinity, and when during gestation these antibodies are generated. For example, sensitization of rhesus (Rh) factor negative women during pregnancy with an Rh+ partner (and Rh+ concepti) primes anti-Rh antibodies in mothers that persist after pregnancy (144). In future pregnancies bearing Rh+ concepti, these vertically transferred antibodies cause destruction of fetal red blood cells, and in severe cases fatal hemolytic disease of the newborn. Paradoxically however, the presence of anti-paternal cytotoxic antibodies is associated with sharply reduced susceptibility to spontaneous abortion since anti-paternal antibodies are found in 32% of women with normal term pregnancies, whereas anti-paternal antibodies were present in only 10% of women with pregnancies that terminated in spontaneous abortion when sampled after parturition (142).
In mice, maternal CD8+ T cells with pre-existing fetal specificity were recently shown to persist after parturition, and adopt a dysfunctional phenotype characterized by expression of the T cell exhaustion marker, programmed death (PD)-1, and diminished production of the canonical effector cytokine IFN-γ (145). These cells fail to undergo secondary expansion with fetal antigen re-stimulation, and their presence did not negatively (or positively) impact the outcome of secondary pregnancy. Interestingly however, pregnancy-induced maternal sensitization in this context did cause accelerated rejection of skin allografts that express the same repertoire of genetically foreign allo-antigens encountered in prior pregnancy (145). In humans, pregnancy induced allo-sensitization also negatively impacts donor selection resulting in an estimated 30% reduced rate of kidney allo-transplantation in women (146). Thus, the long-term impacts of pregnancy induced immunological shifts are not limited only to the outcomes of future pregnancies, but can extend to other physiological contexts when the need for expanded peripheral tolerance arises. Therefore, one important area for future investigation is dissecting the key context specific differences between pregnancy and allograft tolerance that with secondary antigen stimulation either reinforces tolerance or enhanced sensitization.
Persistence of maternal Treg cells with pre-existing fetal specificity was found in the spleen and peripheral lymph nodes of mice in the first 100 days after parturition (112). With fetal antigen restimulation in secondary pregnancy, these cells re-expand with sharply more accelerated kinetics and tempo reminiscent of true memory cells primed in other contexts (147). In turn, this more expanded pool of fetal-specific Treg cells in secondary compared with primary pregnancy was associated with sharply increased resiliency against fetal wastage caused by partial depletion of bulk maternal FOXP3+ cells, presumably because maternal Treg cells with fetal specificity more efficiently enforce fetal tolerance overriding the need for expanded Treg cells of other specificities (112). Preeclampsia is more common during first pregnancies, and a healthy prior pregnancy protects against preeclampsia in subsequent pregnancies. For example, a prospective analysis of over 700,000 primiparous mothers showed the overall 4.1% rate of preeclampsia in a first pregnancy was reduced to 1.7% in subsequent pregnancies (127). These results may provide critical mechanistic insights as to how prior successful pregnancy protects against preeclampsia and other complications which are associated with fractured fetal tolerance in subsequent pregnancies (123-127), and the partner specificity of these protective benefits (73, 148-152).
However, these findings also raise new questions with regards to the durability of memory maternal Treg cells with pre-existing fetal specificity and whether they require ongoing antigenic “reminders” similar to that described for non-Treg effector CD4+ T cell memory subsets (153, 154). An interesting explanation may entail trans-placentally transferred fetal cells that persist in mothers at very rare “microchimeric” levels (9, 10). In this case, pregnancy induced expansion of peripheral immune tolerance in mothers does not cease with parturition, but persists indefinitely with the long-term retention of these genetically foreign fetal microchimeric cells. Provocative new questions based on this reasoning is the potential interactions between each wave of genetically foreign fetal microchimeric cells that seed maternal tissues in each successive pregnancy – whether new cells functionally displace old ones, are agnostic, or synergize their tolerogenic or sensitization properties.
Reciprocal fetal-maternal immunological tolerance.
The semi-allogeneic fetus expresses paternal derived traits that make it genetically foreign to the mother. However, since individual offspring only contain traits encoded by half of homologous chromosomes from their mothers through Mendelian inheritance, mothers are equally foreign (genetically and immunologically) to their offspring. Given the need for expanded tolerance of mothers to foreign paternal antigens expressed by the developing fetus, healthy pregnancies likely also require tolerance of the fetus to an equally vast array of non-inherited maternal antigens (NIMA). This consideration would be especially prominent for humans and other species where fetal adaptive immune components mature and are functional beginning in utero prior to term parturition.
T cells seed human fetal lymphoid tissue as early as gestational week 10, and fetal T cells isolated from gestational week 20 fetuses are capable of vigorous alloantigen-induced proliferation (155). Interestingly, this proliferative response of human fetal CD4+ and CD8+ T cells can extend to maternal alloantigens, but the presence of expanded fetal Treg cells likely suppresses this type of fetal-maternal allo-reactivity during in utero development (156). Fetal compared with adult T cells preferentially undergo differentiation into Treg cells in response to alloantigen stimulation. In turn, this expanded pool of fetal Treg cells is thought to avert fetal-maternal conflict by suppressing the activation of NIMA-specific effector T cells since their depletion selectively unleashes the proliferative response of fetal effector T cells responsive to stimulation by maternal antigen presenting cells, but not cells from unrelated individuals (156). These findings demonstrating fetal immune cells coopt a similar mechanism for achieving tolerance during unavoidable exposure to extended-self maternal antigens to protect against anti-maternal “rejection” during in utero development highlight the likely dual importance of Treg cells operating on both sides of the placenta for sustaining human pregnancy.
Interestingly, tolerance of human offspring to NIMA does not cease with parturition, and the postnatal persistence of NIMA-specific tolerance is highlighted by some remarkable immunological phenotypes. For example, before the availability of purified erythropoietin, transfusion dependent individuals that were broadly exposed to allogeneic HLA were found to selective lack antibodies against one or more NIMA HLA haplotypes compared to the response of these individuals to noninherited paternal HLA (157). Similarly, before the widespread use of more potent immune suppressive therapies in organ transplantation, long-term survival of kidney allografts was shown to be significantly improved in sibling donor recipient pairings if matched for noninherited maternal compared with the noninherited paternal HLA haplotype alleles (158). Reciprocally, the severity of graft-versus-host disease (GVHD) after bone marrow transplantation is reduced in recipients of NIMA-matched donor stem cells (159, 160).
Curiously, postnatal persistence of NIMA-specific tolerance is not unique to humans, but also found in animals. For example, long-term survival of cardiac H-2d allografts selectively occurs in recipient mice developmentally exposed to H-2d as a NIMA compared with the rapid rejection of these grafts in genetically identical recipient mice without such H-2d developmental exposure (161). Likewise, the incidence and severity of GVHD is sharply reduced in mice after engraftment of allogeneic stem cells from donor mice with developmental exposure to NIMAs that overlap with foreign antigens in recipient mice (162). However, these findings demonstrating that developmental exposure to NIMAs can dominantly shape the reactivity of adaptive immune components in offspring also highlight new gaps in knowledge with regards to how such postnatal tolerance is immunologically achieved and sustained.
The aforementioned selective lack of antibodies with NIMA-HLA specificity in transfusion dependent adult individuals would suggest some form of sustained antigen-specific B cell anergy (157). On the other hand, Treg cells with NIMA-specificity primed during in utero development that persist postnatally in individuals may also sustain NIMA-specific tolerance. The importance of Treg cells in maintaining postnatal persistence of NIMA-specific tolerance is supported by the observation that depletion of CD25+ Treg cells selectively unleashes the proliferation of effector T cells after stimulation by maternal antigen presenting cells in children up to 17 years of age (156). Reciprocally, adoptively transferred CD4+ CD25+ Treg cells from NIMA-exposed mice suppress the rejection of NIMA allografts in recipient mice (163). More recently by transforming model antigens into surrogate NIMA, the selective accumulation and long-term postnatal persistence of FOXP3+ Treg cells with NIMA-specificity was shown in NIMA-exposed rodent offspring (164). However, similar to the aforementioned discussion on durability for memory CD4+ T cells after infection or in mothers after parturition (153, 154, 165), prolonged postnatal retention of Treg cells in offspring with NIMA specificity raise new questions with regards to potential sources of postnatal maternal antigen exposure. Cells in breast milk are likely important since the survival of maternal kidney allografts is significantly improved in children that were breast-fed compared with non-breast fed recipients (166). Similarly, in animal cross-fostering studies, significantly reduced accumulation of Treg cells with NIMA specificity and loss of tolerance to NIMA matched donor allograft tissue occurs in NIMA recipients when exposure to maternal breast milk is eliminated (161, 164).
The porous placental interface allows bi-directional transfer of fetal cells into mothers, as well as maternal cells into offspring. Curiously, these genetically foreign cells also persist at very low microchimeric levels in both individuals after parturition (9, 10). In adult humans and mice, microchimeric maternal cells have been shown to be distributed across a range of tissues (167, 168), and this persistence parallels the systemic postnatal retention of NIMA-specific Treg cells (156, 164, 169). Reciprocally, a rapid decline in NIMA-specific FOXP3+ CD4+ Treg cells occurs following the depletion of maternal microchimeric cells which demonstrates ongoing postnatal NIMA exposure maintains the expanded accumulation of NIMA-specific memory Treg cells in offspring (164).
Perhaps a more fundamental issue raised by these findings of persistent NIMA specific tolerance is what teleological advantage may promote the conserved postnatal persistence of maternal microchimeric cells and this immunological phenotype in offspring. Importantly since NIMA-specific tolerance initially described in humans is readily found in mice where fetal adaptive immune components do not functionally mature until after birth (155), there are likely to be more dominant factors driving such conservation beyond the need for averting fetal rejection of mothers during in utero development. An interesting clue comes from the persistence of maternal microchimeric cells in female reproductive tissues (e.g. uterus, ovaries), but their absence in analogous male tissues (e.g. prostate and testes) (164). Considering the importance whereby genetic fitness promotes trait selection, and the need for expanded tolerance during pregnancy in each successive generation, one possibility is enhanced fetal tolerance during next-generation pregnancies recently shown by sharply increased resiliency against fetal wastage in mice during allogeneic pregnancies sired by males that express NIMA-matched MHC haplotypes compared with pregnancies sired by mismatched males (164). Depletion of microchimeric maternal cells prior to mating that eliminates the expanded accumulation of NIMA-specific Treg cells also overrides this resiliency against fetal wastage during allogeneic pregnancies sired by males that express NIMA-matched MHC haplotypes (164).
These cross-generational reproductive benefits shown in mice are consistent with classical observations of reduced susceptibility to Rh sensitization among Rh− women born to mothers that are Rh+ compared with Rh− negative mothers (144). Reduced Rh sensitization in mothers would be predicted to promote the survival of next generation Rh+ offspring through protection against neonatal Rh hemolytic disease. In other words, the antigenic composition of maternal grandmothers may dominantly shape the outcomes of next-generation pregnancies through vertically transferred microchimeric maternal cells that functionally expand tolerance in female offspring. Interestingly and despite significantly reduced Rh sensitization, the incidence of hemolytic disease of newborn remained similar among Rh-tolerant and Rh-intolerant mothers (144), suggesting NIMA-specific tolerance to this single alloantigen alone may not be sufficient to confer survival benefits to next-generation offspring or that other influences (e.g. medical interventions) dilute the protective benefits of this biologically engrained pathway. It may also be interesting to consider that the transfer of cells from mother to offspring may not be limited only to genetically encoded maternal cells. If transfer and persistence of these cells is indeed purposeful (and not accidental), then the possibility that offspring could be transferred cells retained in mothers from prior pregnancies (older siblings) and their own mothers (maternal grandmothers) are each intriguing consideration, with immunological implications that need to be evaluated (9, 10). Together, these provocative observations in human and animal pregnancy suggest that in addition to the transfer of genetic traits from one generation to the next through Mendelian inheritance, the vertical transfer of genetically foreign maternal cells and their postnatal retention in offspring can promote the maintenance of non-inherited hereditary traits that span multiple generations.
In the broader context, these additional clues gained from reproduction and pregnancy highlighting individuals as being constitutively chimeric – with a potentially wide assortment of genetically foreign cells that are each programmed to persist, can fundamentally change how we view immunological identity. Can autoimmunity instead represent cases where NIMA-specific tolerance is perturbed, and instead be caused by alloimmunity to genetically foreign maternal cells purposefully retained in the tissue of offspring? Reciprocally, if fetal microchimeric cells persist in mothers to enforce fetal tolerance during future pregnancy with genetically similar younger siblings, could autoimmunity represent alloimmunity of mothers to microchimeric fetal cells or immunological intolerance between multiple subsets of genetically foreign cells within the same individual? Despite some supporting evidence for these provocative hypotheses that include the sharp gender disparity of autoimmune disorders in women and enriched levels of microchimeric cells in diseased tissue of individuals with autoimmunity (9, 10), more focused investigation based on the experimental manipulation of these cells is needed. Nonetheless, these findings suggest the temporal events occurring during pregnancy can have long lasting implications not restricted only to that offspring during in utero development, but also for the outcomes of future and next-generation pregnancies, along with shaping susceptibility to health and disease for the life of mother and offspring.
Summary and perspectives.
Reproduction and pregnancy are fundamental to life and lie at the core of our existence as individuals and as a species. However, we still know so very little about how these basic biological processes work. Traditionally, many foundational concepts in immunology were based on observations and experimental perturbations during pregnancy and early life development. However, after recognizing some key differences in the anatomy of cells at the maternal-fetal interface and tempo of fetal-neonatal immune cell maturation between humans compared with animals, reproductive immunology and early life development has become more a “niche” field amongst more prominently expanding areas such as autoimmunity, transplantation, cancer, commensal tolerance and dysbiosis, vaccinology and antimicrobial host defense. This is unfortunate, and in many ways represents an important missed scientific opportunity.
As immunologists, it can be argued that pregnancy remains our best and most relevant model for investigating how immune tolerance naturally works. These analyses would pertain not only to improving the outcomes of pregnancy, but likely have wide applicability to many other immunological disciplines. For example, regarding the pathogenesis of autoimmunity, important clues are likely garnered by understanding how pregnancy efficiently causes clinical improvement and often disease remission (21-24, 170, 171). In transplantation, can understanding how the fetal allograft averts rejection be used for designing improved antigen-specific therapies for protecting donor allografts? A provocative recent review highlighted how immune tolerance to the first cells of the genetically foreign fetal conceptus is more analogous to how the immune system would respond to genetically aberrant malignant cells that eventually develops into cancerous tumors (172). Regarding how the immunological identity of individuals is defined, the ubiquitous presence of genetically discordant microchimeric cells, and potentially from a wide variety of hereditary relevant source individuals, further blurs the distinction between self and non-self.
We have conveniently assigned these antigens to the nebulous expanding bucket of “extended self” antigens as a basis for their discussion in the current immunological framework of self versus non-self antigen distinction. However, it may be worthwhile to consider how the purposeful presence of genetically foreign microchimeric cells inside us and commensal microbes at mucosal barriers fit with alternative ways to conceptually frame immunological identity, tolerance, and how the response to infection-immunization can be so divergent even in genetically identical individuals (173). One prominent alternative is the danger model that posits the initial event activating immunity is tissue damage and cellular stress, instead of non-self recognition (174, 175). In this case, genetically foreign fetal cells and tissues may not provoke as much “danger” compared with infection or other inflammatory insults. However, given the substantial tissue remodeling that occurs with trophoblast invasion of the uterine mucosa even in healthy pregnancy, it would be hard to image that some sort of danger is not activated. Given the dominant impacts reproductive and pregnancy health have in trait selection, it may be worthwhile to place observations based on reproduction as the centerpiece in refining old or creating more comprehensive new theories on how immunology works.
Pregnancy complications remain the largest single cause of infant and childhood mortality. Individuals are the most vulnerable when they are first born – when the denominator is live births. However, one could argue vulnerability is equally high in utero when offspring need to simultaneously navigate the development of vital organs, grow exponentially, ward off infection, and avoid “rejection” by maternal immune cells. As fathers (each of us have two healthy children) we marvel with amazement that everything went right. As pediatricians tasked with care of sick infants and children, and being constantly reminded of the sobering realities of neonatal mortality, we are grateful for this forum allowing us to highlight mostly what we still do not know and the need for additional research in these impactful, but often forgotten areas.
SUMMARY POINTS.
Common pregnancy complications such as infertility, spontaneous abortion, stillbirth, preeclampsia-eclampsia and prematurity share many overlapping risk factors, dominantly influence susceptibility to the same and other complications in future pregnancy, and therefore likely share the same or overlapping pathological roots.
Expanded immunological tolerance during pregnancy is associated with sustained expansion of maternal immune suppressive regulatory T cells. Diminished regulatory T cell expansion occurs in a wide variety of human pregnancy complications, whereas depletion of these cells in animals causes fetal wastage and pathological activation of maternal immune components with fetal specificity.
Maternal immune components with fetal specificity persist after parturition, raising the possibility that aberrantly activated components causing complications in prior pregnancy increase the risk of complications in future pregnancy. Reciprocally, postpartum persistence of protective maternal immune cells may reduce susceptibility to complications in future pregnancy.
Bi-directional transfer of microchimeric cells between mothers and offspring, and the persistence of these cells in both individuals suggest temporal events occurring during pregnancy have durable implications not restricted only to that offspring during in utero development, but also on the outcomes of future and next-generation pregnancies, and the immunological identity for the life of mother and offspring.
Given the dominant role pregnancy health and reproductive fitness play in trait selection, refining how our immune systems optimally work, more comprehensive analysis of pregnancy induced immunological changes can expose new therapeutic strategies for improving the outcomes of pregnancy, and fundamental new insights on how immune tolerance naturally works with applicability to many other physiological and pathological contexts.
ACKNOWLEDGEMENTS
We are indebted to Feiyang Wang for critical review of this manuscript. Given space constraints, we apologize for not being able to include many important primary references. The writing of this manuscript was made possible through funding by the US National Institutes of Health, Office of the Director (DP1AI131080 to S.S.W.); the US National Institute of Allergy and Infectious Disease (R01AI100934, R01AI120202, R21AI123089, R21AI128932 to S.S.W.); the US National Institute of Child Health and Human Development (K08HD084686 to H.S.D); the Parker B. Francis Fellowship (2015-28 to H.S.D) and the March of Dimes Foundation (FY15-254 to S.S.W.). S.S.W. is a Burroughs Wellcome Fund Investigator in the pathogenesis of infectious disease and Howard Hughes Medical Institute Faculty Scholar.
REFERENCES:
- 1.Maynard CL, Elson CO, Hatton RD, Weaver CT. 2012. Reciprocal interactions of the intestinal microbiota and immune system. Nature 489: 231–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul WE. 2010. Self/Nonself-Immune Recognition and Signaling: A new journal tackles a problem at the center of immunological science. Self Nonself 1: 2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janeway CA Jr. 1992. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13: 11–6 [DOI] [PubMed] [Google Scholar]
- 5.Medawar PB. 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol 7: 320–38 [Google Scholar]
- 6.Collins MK, Tay CS, Erlebacher A. 2009. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest 119: 2062–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. 2012. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336: 1317–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. 2000. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287: 498–501 [DOI] [PubMed] [Google Scholar]
- 9.Nelson JL. 2012. The otherness of self: microchimerism in health and disease. Trends Immunol 33: 421–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinder JM, Stelzer IA, Arck PC, Way SS. 2017. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol 17: 483–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt JS, Andrews GK, Wood GW. 1987. Normal trophoblasts resist induction of class I HLA. J Immunol 138: 2481–7 [PubMed] [Google Scholar]
- 12.Fisher SJ, Damsky CH. 1993. Human cytotrophoblast invasion. Semin Cell Biol 4: 183–8 [DOI] [PubMed] [Google Scholar]
- 13.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. 1997. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A 94: 11520–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaschitz A, Hutter H, Dohr G. 2001. HLA Class I protein expression in the human placenta. Early Pregnancy 5: 67–9 [PubMed] [Google Scholar]
- 15.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. 2009. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127: 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunderland CA, Redman CW, Stirrat GM. 1981. HLA A, B, C antigens are expressed on nonvillous trophoblast of the early human placenta. J Immunol 127: 2614–5 [PubMed] [Google Scholar]
- 17.Casro MJ, Morales P, Rojo-Amigo R, Martinez-Laso J, Allende L, Varela P, Garcia-Berciano M, Guillen-Perales J, Arnaiz-Villena A. 2000. Homozygous HLA-G*0105N healthy individuals indicate that membrane-anchored HLA-G1 molecule is not necessary for survival. Tissue Antigens 56: 232–9 [DOI] [PubMed] [Google Scholar]
- 18.Moreau P, Dausset J, Carosella ED, Rouas-Freiss N. 2002. Viewpoint on the functionality of the human leukocyte antigen-G null allele at the fetal-maternal interface. Biol Reprod 67: 1375–8 [DOI] [PubMed] [Google Scholar]
- 19.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. 1995. T cell awareness of paternal alloantigens during pregnancy. Science 270: 630–3 [DOI] [PubMed] [Google Scholar]
- 20.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. 2007. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest 117: 1399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. 1998. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 339: 285–91 [DOI] [PubMed] [Google Scholar]
- 22.Finkelsztejn A, Brooks JB, Paschoal FM Jr., Fragoso YD. 2011. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG 118: 790–7 [DOI] [PubMed] [Google Scholar]
- 23.Ostensen M, Villiger PM. 2007. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol 29: 185–91 [DOI] [PubMed] [Google Scholar]
- 24.Buchel E, Van Steenbergen W, Nevens F, Fevery J. 2002. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol 97: 3160–5 [DOI] [PubMed] [Google Scholar]
- 25.Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. 2002. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol 169: 1084–91 [DOI] [PubMed] [Google Scholar]
- 26.McClain MA, Gatson NN, Powell ND, Papenfuss TL, Gienapp IE, Song F, Shawler TM, Kithcart A, Whitacre CC. 2007. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol 179: 8146–52 [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Suano A, Kallikourdis M, Sarris M, Betz AG. 2012. Regulatory T cells protect from autoimmune arthritis during pregnancy. J Autoimmun 38: J103–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, Breiman RF, M BBSK. 2010. Influenza immunization in pregnancy--antibody responses in mothers and infants. N Engl J Med 362: 1644–6 [DOI] [PubMed] [Google Scholar]
- 29.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. 1993. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 168: 647–56 [DOI] [PubMed] [Google Scholar]
- 30.Littman DR, Rudensky AY. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–58 [DOI] [PubMed] [Google Scholar]
- 31.Wing K, Sakaguchi S. 2010. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11: 7–13 [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182: 18–32 [DOI] [PubMed] [Google Scholar]
- 33.Hori S, Nomura T, Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–61 [DOI] [PubMed] [Google Scholar]
- 34.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–1 [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–6 [DOI] [PubMed] [Google Scholar]
- 36.Kim JM, Rasmussen JP, Rudensky AY. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–7 [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101: 455–8 [DOI] [PubMed] [Google Scholar]
- 38.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. 2004. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant 4: 1614–27 [DOI] [PubMed] [Google Scholar]
- 39.Erlebacher A. 2013. Immunology of the maternal-fetal interface. Annu Rev Immunol 31: 387–411 [DOI] [PubMed] [Google Scholar]
- 40.Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, Xin L, Way SS. 2014. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol 192: 4949–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aluvihare VR, Betz AG. 2006. The role of regulatory T cells in alloantigen tolerance. Immunol Rev 212: 330–43 [DOI] [PubMed] [Google Scholar]
- 42.Guerin LR, Prins JR, Robertson SA. 2009. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 15: 517–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson SA, Sharkey DJ. 2001. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol 13: 243–54 [DOI] [PubMed] [Google Scholar]
- 44.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. 2011. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod 85: 397–408 [DOI] [PubMed] [Google Scholar]
- 45.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa SD, Zimmermann G, Nitschke C, Volk HD, Alexander H, Gunzer M, Zenclussen AC. 2009. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol 182: 5488–97 [DOI] [PubMed] [Google Scholar]
- 46.Schumacher A, Heinze K, Witte J, Poloski E, Linzke N, Woidacki K, Zenclussen AC. 2013. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol 190: 2650–8 [DOI] [PubMed] [Google Scholar]
- 47.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. 2009. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol 182: 8080–93 [DOI] [PubMed] [Google Scholar]
- 48.Saito S, Nakashima A, Shima T, Ito M. 2010. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 63: 601–10 [DOI] [PubMed] [Google Scholar]
- 49.McCloskey ML, Curotto de Lafaille MA, Carroll MC, Erlebacher A. 2011. Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J Exp Med 208: 135–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, Tao Y, Li YH, Zhang D, Zhu R, Fu Q, Li DJ. 2014. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 192: 1502–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vignali DA, Collison LW, Workman CJ. 2008. How regulatory T cells work. Nat Rev Immunol 8: 523–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sojka DK, Huang YH, Fowell DJ. 2008. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology 124: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Rocca C, Carbone F, Longobardi S, Matarese G. 2014. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162: 41–8 [DOI] [PubMed] [Google Scholar]
- 54.Heikkinen J, Mottonen M, Alanen A, Lassila O. 2004. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol 136: 373–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, Takikawa O, Saito S. 2005. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod 11: 865–70 [DOI] [PubMed] [Google Scholar]
- 56.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–3 [DOI] [PubMed] [Google Scholar]
- 57.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. 2007. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 13: 1450–7 [DOI] [PubMed] [Google Scholar]
- 58.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. 2007. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol 179: 5211–9 [DOI] [PubMed] [Google Scholar]
- 59.van der Zwan A, Bi K, Norwitz ER, Crespo AC, Claas FHJ, Strominger JL, Tilburgs T. 2018. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A 115: 385–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pohanka M, Hampl R, Sterzl I, Starka L. 2002. Steroid hormones in human semen with particular respect to dehydroepiandrosterone and its immunomodulatory metabolites. Endocr Regul 36: 79–86 [PubMed] [Google Scholar]
- 61.Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. 2002. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J Reprod Immunol 57: 109–28 [DOI] [PubMed] [Google Scholar]
- 62.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. 2009. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 80: 1036–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. 2012. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 188: 2445–54 [DOI] [PubMed] [Google Scholar]
- 64.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. 2007. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–5 [DOI] [PubMed] [Google Scholar]
- 65.Pinheiro MB, Martins-Filho OA, Mota AP, Alpoim PN, Godoi LC, Silveira AC, Teixeira-Carvalho A, Gomes KB, Dusse LM. 2013. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine 62: 165–73 [DOI] [PubMed] [Google Scholar]
- 66.Fisher SJ. 2015. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 213: S115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redman CW, Sargent IL. 2010. Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–43 [DOI] [PubMed] [Google Scholar]
- 68.Saito S, Sakai M, Sasaki Y, Nakashima A, Shiozaki A. 2007. Inadequate tolerance induction may induce pre-eclampsia. J Reprod Immunol 76: 30–9 [DOI] [PubMed] [Google Scholar]
- 69.Kho EM, McCowan LM, North RA, Roberts CT, Chan E, Black MA, Taylor RS, Dekker GA, Consortium S. 2009. Duration of sexual relationship and its effect on preeclampsia and small for gestational age perinatal outcome. J Reprod Immunol 82: 66–73 [DOI] [PubMed] [Google Scholar]
- 70.Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E. 1994. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet 344: 973–5 [DOI] [PubMed] [Google Scholar]
- 71.Einarsson JI, Sangi-Haghpeykar H, Gardner MO. 2003. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol 188: 1241–3 [DOI] [PubMed] [Google Scholar]
- 72.Dekker GA, Robillard PY, Hulsey TC. 1998. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. Obstet Gynecol Surv 53: 377–82 [DOI] [PubMed] [Google Scholar]
- 73.Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. 2003. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol 157: 1108–14 [DOI] [PubMed] [Google Scholar]
- 74.Klonoff-Cohen HS, Savitz DA, Cefalo RC, McCann MF. 1989. An epidemiologic study of contraception and preeclampsia. JAMA 262: 3143–7 [PubMed] [Google Scholar]
- 75.Koelman CA, Coumans AB, Nijman HW, Doxiadis II, Dekker GA, Claas FH. 2000. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol 46: 155–66 [DOI] [PubMed] [Google Scholar]
- 76.Kyrou D, Kolibianakis EM, Devroey P, Fatemi HM. 2010. Is the use of donor sperm associated with a higher incidence of preeclampsia in women who achieve pregnancy after intrauterine insemination? Fertil Steril 93: 1124–7 [DOI] [PubMed] [Google Scholar]
- 77.Crawford G, Ray A, Gudi A, Shah A, Homburg R. 2015. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta-analysis. Hum Reprod Update 21: 275–84 [DOI] [PubMed] [Google Scholar]
- 78.Saito S, Shima T, Nakashima A, Inada K, Yoshino O. 2016. Role of Paternal Antigen-Specific Treg Cells in Successful Implantation. Am J Reprod Immunol 75: 310–6 [DOI] [PubMed] [Google Scholar]
- 79.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. 2009. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J Reprod Immunol 83: 109–16 [DOI] [PubMed] [Google Scholar]
- 80.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10: 347–53 [DOI] [PubMed] [Google Scholar]
- 81.Inada K, Shima T, Nakashima A, Aoki K, Ito M, Saito S. 2013. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol 97: 104–11 [DOI] [PubMed] [Google Scholar]
- 82.Aluvihare VR, Kallikourdis M, Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–71 [DOI] [PubMed] [Google Scholar]
- 83.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. 2010. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 85: 121–9 [DOI] [PubMed] [Google Scholar]
- 84.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. 2011. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. 2006. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett 102: 106–9 [DOI] [PubMed] [Google Scholar]
- 86.Arruvito L, Sanz M, Banham AH, Fainboim L. 2007. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 178: 2572–8 [DOI] [PubMed] [Google Scholar]
- 87.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. 2004. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol 173: 2227–30 [DOI] [PubMed] [Google Scholar]
- 88.Prieto GA, Rosenstein Y. 2006. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 118: 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JH, Lydon JP, Kim CH. 2012. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol 42: 2683–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jasper MJ, Tremellen KP, Robertson SA. 2006. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod 12: 301–8 [DOI] [PubMed] [Google Scholar]
- 91.Winger EE, Reed JL. 2011. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am J Reprod Immunol 66: 320–8 [DOI] [PubMed] [Google Scholar]
- 92.Wu L, Luo LH, Zhang YX, Li Q, Xu B, Zhou GX, Luan HB, Liu YS. 2014. Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod Biol Endocrinol 12: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bao SH, Wang XP, De Lin Q, Wang WJ, Yin GJ, Qiu LH. 2011. Decidual CD4+CD25+CD127dim/- regulatory T cells in patients with unexplained recurrent spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol 155: 94–8 [DOI] [PubMed] [Google Scholar]
- 94.Liu C, Wang XZ, Sun XB. 2013. Assessment of sperm antigen specific T regulatory cells in women with recurrent miscarriage. Early Hum Dev 89: 95–100 [DOI] [PubMed] [Google Scholar]
- 95.Yue CY, Zhang B, Ying CM. 2015. Elevated Serum Level of IL-35 Associated with the Maintenance of Maternal-Fetal Immune Tolerance in Normal Pregnancy. PLoS One 10: e0128219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu M, Liu P, Cheng L. 2015. Galectin-1 reduction and changes in T regulatory cells may play crucial roles in patients with unexplained recurrent spontaneous abortion. Int J Clin Exp Pathol 8: 1973–8 [PMC free article] [PubMed] [Google Scholar]
- 97.Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B, Rigo J Jr., Molvarec A. 2012. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol 68: 175–80 [DOI] [PubMed] [Google Scholar]
- 98.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. 2009. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy 28: 300–11 [DOI] [PubMed] [Google Scholar]
- 99.Steinborn A, Haensch GM, Mahnke K, Schmitt E, Toermer A, Meuer S, Sohn C. 2008. Distinct subsets of regulatory T cells during pregnancy: is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin Immunol 129: 401–12 [DOI] [PubMed] [Google Scholar]
- 100.Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, Treszl A. 2008. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand 87: 1229–33 [DOI] [PubMed] [Google Scholar]
- 101.Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, Oleszczuk J. 2007. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol 58: 39–45 [DOI] [PubMed] [Google Scholar]
- 102.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. 2010. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 84: 164–70 [DOI] [PubMed] [Google Scholar]
- 103.Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A, Kwak-Kim J. 2011. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 26: 2964–71 [DOI] [PubMed] [Google Scholar]
- 104.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. 2005. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 166: 811–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon Bertoja A, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J, Volk HD. 2006. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol 36: 82–94 [DOI] [PubMed] [Google Scholar]
- 106.Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, Klatzmann D. 2013. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol 191: 2273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao JX, Zeng YY, Liu Y. 2007. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol 75: 71–81 [DOI] [PubMed] [Google Scholar]
- 108.Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, Jones HN, Way SS. 2015. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest 125: 1713–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rowe JH, Ertelt JM, Xin L, Way SS. 2012. Listeria monocytogenes cytoplasmic entry induces fetal wastage by disrupting maternal Foxp3+ regulatory T cell-sustained fetal tolerance. PLoS Pathog 8: e1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andersen KG, Nissen JK, Betz AG. 2012. Comparative Genomics Reveals Key Gain-of-Function Events in Foxp3 during Regulatory T Cell Evolution. Front Immunol 3: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rowe JH, Ertelt JM, Xin L, Way SS. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xin L, Ertelt JM, Rowe JH, Jiang TT, Kinder JM, Chaturvedi V, Elahi S, Way SS. 2014. Cutting edge: committed Th1 CD4+ T cell differentiation blocks pregnancy-induced Foxp3 expression with antigen-specific fetal loss. J Immunol 192: 2970–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. 1982. Early embryonic mortality in women. Fertil Steril 38: 447–53 [PubMed] [Google Scholar]
- 115.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. 1988. Incidence of early loss of pregnancy. N Engl J Med 319: 189–94 [DOI] [PubMed] [Google Scholar]
- 116.Ananth CV, Keyes KM, Wapner RJ. 2013. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347: f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. 2015. Births: Final Data for 2014. Natl Vital Stat Rep 64: 1–64 [PubMed] [Google Scholar]
- 118.Barker DJ, Osmond C. 1986. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1: 1077–81 [DOI] [PubMed] [Google Scholar]
- 119.Barton JR, Barton LA, Istwan NB, Desch CN, Rhea DJ, Stanziano GJ, Sibai BM. Elective delivery at 340/7 to 366/7 weeks' gestation and its impact on neonatal outcomes in women with stable mild gestational hypertension. American Journal of Obstetrics & Gynecology 204: 44.e1–.e5 [DOI] [PubMed] [Google Scholar]
- 120.Smulian JC, Ananth CV, Vintzileos AM, Scorza WE, Knuppel RA. 2002. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol 100: 1183–9 [DOI] [PubMed] [Google Scholar]
- 121.Pineles BL, Park E, Samet JM. 2014. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 179: 807–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cui H, Gong TT, Liu CX, Wu QJ. 2016. Associations between Passive Maternal Smoking during Pregnancy and Preterm Birth: Evidence from a Meta-Analysis of Observational Studies. PLoS One 11: e0147848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koike T, Minakami H, Izumi A, Watanabe T, Matsubara S, Sato I. 2002. Recurrence risk of preterm birth due to preeclampsia. Gynecol Obstet Invest 53: 22–7 [DOI] [PubMed] [Google Scholar]
- 124.Xiong X, Fraser WD, Demianczuk NN. 2002. History of abortion, preterm, term birth, and risk of preeclampsia: a population-based study. Am J Obstet Gynecol 187: 1013–8 [DOI] [PubMed] [Google Scholar]
- 125.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. 2000. Rates of and factors associated with recurrence of preterm delivery. JAMA 283: 1591–6 [DOI] [PubMed] [Google Scholar]
- 126.Rasmussen S, Ebbing C, Irgens LM. 2017. Predicting preeclampsia from a history of preterm birth. PLoS One 12: e0181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernandez-Diaz S, Toh S, Cnattingius S. 2009. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 338: b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melve KK, Skjaerven R, Rasmussen S, Irgens LM. 2010. Recurrence of stillbirth in sibships: Population-based cohort study. Am J Epidemiol 172: 1123–30 [DOI] [PubMed] [Google Scholar]
- 129.Lamont K, Scott NW, Jones GT, Bhattacharya S. 2015. Risk of recurrent stillbirth: systematic review and meta-analysis. BMJ 350: h3080. [DOI] [PubMed] [Google Scholar]
- 130.Smith GC, Shah I, White IR, Pell JP, Dobbie R. 2007. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: a retrospective cohort study, Scotland, 1992-2001. Am J Epidemiol 165: 194–202 [DOI] [PubMed] [Google Scholar]
- 131.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. 2006. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 195: 643–50 [DOI] [PubMed] [Google Scholar]
- 132.Esplin MS, O'Brien E, Fraser A, Kerber RA, Clark E, Simonsen SE, Holmgren C, Mineau GP, Varner MW. 2008. Estimating recurrence of spontaneous preterm delivery. Obstet Gynecol 112: 516–23 [DOI] [PubMed] [Google Scholar]
- 133.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, Caritis SN, Miodovnik M, Menard MK, Thurnau GR, Dombrowski MP, Roberts JM, McNellis D. 1999. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 181: 1216–21 [DOI] [PubMed] [Google Scholar]
- 134.Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Feenstra B, Melbye M. 2009. Maternal contributions to preterm delivery. Am J Epidemiol 170: 1358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R, A WS, Lavin T, Pereira G. 2018. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG 125: 183–92 [DOI] [PubMed] [Google Scholar]
- 136.Mbah AK, Alio AP, Marty PJ, Bruder K, Whiteman VE, Salihu HM. 2010. Pre-eclampsia in the first pregnancy and subsequent risk of stillbirth in black and white gravidas. Eur J Obstet Gynecol Reprod Biol 149: 165–9 [DOI] [PubMed] [Google Scholar]
- 137.Black M, Shetty A, Bhattacharya S. 2008. Obstetric outcomes subsequent to intrauterine death in the first pregnancy. BJOG 115: 269–74 [DOI] [PubMed] [Google Scholar]
- 138.Robson S, Chan A, Keane RJ, Luke CG. 2001. Subsequent birth outcomes after an unexplained stillbirth: preliminary population-based retrospective cohort study. Aust N Z J Obstet Gynaecol 41: 29–35 [DOI] [PubMed] [Google Scholar]
- 139.Gordon A, Raynes-Greenow C, McGeechan K, Morris J, Jeffery H. 2012. Stillbirth risk in a second pregnancy. Obstet Gynecol 119: 509–17 [DOI] [PubMed] [Google Scholar]
- 140.Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, Miller DE, Litterman N, Jiang PP, Russell L, Hinds DA, Hu Y, Weirauch MT, Chen X, Chavan AR, Wagner GP, Pavlicev M, Nnamani MC, Maziarz J, Karjalainen MK, Ramet M, Sengpiel V, Geller F, Boyd HA, Palotie A, Momany A, Bedell B, Ryckman KK, Huusko JM, Forney CR, Kottyan LC, Hallman M, Teramo K, Nohr EA, Davey Smith G, Melbye M, Jacobsson B, Muglia LJ. 2017. Genetic Associations with Gestational Duration and Spontaneous Preterm Birth. N Engl J Med 377: 1156–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vilches M, Nieto A. 2015. Analysis of Pregnancy-Induced Anti-HLA Antibodies Using Luminex Platform. Transplant Proc 47: 2608–10 [DOI] [PubMed] [Google Scholar]
- 142.Regan L, Braude PR, Hill DP. 1991. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod 6: 294–8 [DOI] [PubMed] [Google Scholar]
- 143.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. 2009. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion 49: 1825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. 1954. EVIDENCE FOR ACTIVELY ACQUIRED TOLERANCE TO Rh ANTIGENS. Proc Natl Acad Sci U S A 40: 420–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barton BM, Xu R, Wherry EJ, Porrett PM. 2017. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J Leukoc Biol 101: 975–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bromberger B, Spragan D, Hashmi S, Morrison A, Thomasson A, Nazarian S, Sawinski D, Porrett P. 2017. Pregnancy-Induced Sensitization Promotes Sex Disparity in Living Donor Kidney Transplantation. J Am Soc Nephrol 28: 3025–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jameson SC, Masopust D. 2018. Understanding Subset Diversity in T Cell Memory. Immunity 48: 214–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trupin LS, Simon LP, Eskenazi B. 1996. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology 7: 240–4 [DOI] [PubMed] [Google Scholar]
- 149.Li DK, Wi S. 2000. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am J Epidemiol 151: 57–62 [DOI] [PubMed] [Google Scholar]
- 150.Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. 1999. Change in paternity: a risk factor for preeclampsia in multiparous women? J Reprod Immunol 45: 81–8 [DOI] [PubMed] [Google Scholar]
- 151.Feeney JG, Scott JS. 1980. Pre-eclampsia and changed paternity. Eur J Obstet Gynecol Reprod Biol 11: 35–8 [DOI] [PubMed] [Google Scholar]
- 152.Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, Papiernik E. 1993. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J Reprod Immunol 24: 1–12 [DOI] [PubMed] [Google Scholar]
- 153.Nelson RW, McLachlan JB, Kurtz JR, Jenkins MK. 2013. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J Immunol 190: 2828–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. 2004. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med 200: 201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mold JE, McCune JM. 2012. Immunological tolerance during fetal development: from mouse to man. Adv Immunol 115: 73–111 [DOI] [PubMed] [Google Scholar]
- 156.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322: 1562–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. 1988. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science 241: 1815–7 [DOI] [PubMed] [Google Scholar]
- 158.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, Sollinger HW, Bean MA. 1998. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med 339: 1657–64 [DOI] [PubMed] [Google Scholar]
- 159.Ichinohe T, Uchiyama T, Shimazaki C, Matsuo K, Tamaki S, Hino M, Watanabe A, Hamaguchi M, Adachi S, Gondo H, Uoshima N, Yoshihara T, Hatanaka K, Fujii H, Kawa K, Kawanishi K, Oka K, Kimura H, Itoh M, Inukai T, Maruya E, Saji H, Kodera Y, Japanese Collaborative Study Group for N-CHSCT. 2004. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood 104: 3821–8 [DOI] [PubMed] [Google Scholar]
- 160.van Rood JJ, Loberiza FR Jr., Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringden O, Hows JM, Horowitz MH. 2002. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood 99: 1572–7 [DOI] [PubMed] [Google Scholar]
- 161.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, Tam RC, Illigens BM, Anosova N, Benichou G, Burlingham WJ. 2003. Tolerance to noninherited maternal MHC antigens in mice. J Immunol 171: 5554–61 [DOI] [PubMed] [Google Scholar]
- 162.Matsuoka K, Ichinohe T, Hashimoto D, Asakura S, Tanimoto M, Teshima T. 2006. Fetal tolerance to maternal antigens improves the outcome of allogeneic bone marrow transplantation by a CD4+ CD25+ T-cell-dependent mechanism. Blood 107: 404–9 [DOI] [PubMed] [Google Scholar]
- 163.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. 2011. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant 11: 1296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kinder JM, Jiang TT, Ertelt JM, Xin L, Strong BS, Shaaban AF, Way SS. 2015. Cross-Generational Reproductive Fitness Enforced by Microchimeric Maternal Cells. Cell 162: 505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kinder JM, Jiang TT, Clark DR, Chaturvedi V, Xin L, Ertelt JM, Way SS. 2014. Pregnancy-induced maternal regulatory T cells, bona fide memory or maintenance by antigenic reminder from fetal cell microchimerism? Chimerism 5: 16–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campbell DA Jr., Lorber MI, Sweeton JC, Turcotte JG, Niederhuber JE, Beer AE. 1984. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation 37: 340–4 [DOI] [PubMed] [Google Scholar]
- 167.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. 1999. Microchimerism of maternal origin persists into adult life. J Clin Invest 104: 41–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ. 2009. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 114: 3578–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, Burlingham WJ. 2007. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol 179: 6749–61 [DOI] [PubMed] [Google Scholar]
- 170.Patas K, Engler JB, Friese MA, Gold SM. 2013. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J Reprod Immunol 97: 140–6 [DOI] [PubMed] [Google Scholar]
- 171.Coyle PK. 2012. Pregnancy and multiple sclerosis. Neurol Clin 30: 877–88 [DOI] [PubMed] [Google Scholar]
- 172.Mor G, Aldo P, Alvero AB. 2017. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17: 469–82 [DOI] [PubMed] [Google Scholar]
- 173.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. 2015. Variation in the human immune system is largely driven by non-heritable influences. Cell 160: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matzinger P. 1994. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045 [DOI] [PubMed] [Google Scholar]
- 175.Bonney EA. 2016. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet Gynecol Clin North Am 43: 679–98 [DOI] [PMC free article] [PubMed] [Google Scholar]