Abstract
Gestational diabetes mellitus (GDM), an important public health problem that affects mothers and offspring, is a common metabolic disorder. We evaluated the effect of the pre-pregnancy Mediterranean diet (MD) level of exposure on the odds of GDM development. A case-control study (291 GDM cases and 1175 controls without GDM) was conducted in pregnant women. Pre-pregnancy dietary intake was assessed using a validated food frequency questionnaire to calculate an MD adherence index (range score 0–9: low ≤ 2; middle 3–4; high 5–6; very high ≥ 7). Adjusted odds ratios (aOR) and their 95% confidence intervals (CI) were estimated using multivariable logistic regression models including age, BMI, family history of diabetes mellitus, previous GDM, miscarriages, and gravidity. Overall, middle-high MD adherence was 216/291 (74.2%) and very high adherence was 17/291 (5.8%) in cases. In controls the corresponding figures were 900/1175 (76.6%) and 73/1175 (6.2%), respectively. Compared to low adherence, high MD adherence was associated with GDM reduction (aOR 0.61, 95% CI 0.39,0.94; p = 0.028), and very high MD adherence was even more strongly associated (aOR 0.33, 95% CI 0.15, 0.72; p = 0.005). The protective effect of adherence to the MD prior to pregnancy should be considered as a preventive tool against the development of GDM.
Keywords: Mediterranean diet (MD), gestational diabetes mellitus (GDM), pregnancy, maternal nutrition, lifestyles
1. Introduction
Gestational diabetes mellitus (GDM), a state of carbohydrate intolerance which develops or is first recognized in the second or third trimester of pregnancy [1], is an important public health problem that affects both mother and offspring. The complications of GDM include spontaneous abortion, fetal anomalies, preeclampsia, stillbirth, macrosomia, hypoglycaemia, and neonatal hyperbilirubin, amongst others [1,2]. It is estimated that the prevalence of GDM has been increasing worldwide, growing in parallel with obesity [1]. Its prevalence ranges between 2.5% and 14% influenced by racial, geographic and dietary factors [3,4], reaching almost 20% in some Asian countries [5].
The maternal diet composition affects the metabolic patterns of both mother and offspring [6,7,8,9,10]. The Mediterranean diet (MD) is associated with improved health outcomes [11], with a greater adherence to a MD pattern linked to lower cardiovascular disease [12,13] and risk factors (i.e., reduced obesity [14], hypertension [15]), the prevention of some cancers (i.e., breast, endometrium, ovary, prostate, and stomach [16]) and reduced incidence of micronutrient deficiencies [17]. There is also current scientific evidence regarding the protective effects of the MD pattern on type-2 diabetes [18]. As GDM shares the physiopathological mechanisms of diabetes mellitus, a MD may act as a protective factor for its development. Studies of dietary advice including a MD during pregnancy [7,19,20,21,22,23,24], suggest a posible benefit.
Previous studies have analyzed the association between adherence to a MD, or other dietary compositions during pregnancy, and GDM development [19,20,21,22,23,24], yet few have assessed the relationship between pre-pregnancy adherence to a MD and the development of GDM. Those that do have inconsistent results and show a less clear association, which may be due to the fact that some of these studies have been carried out on the MD in non-Mediterranean populations [25,26,27] or with different anthropometric, sociodemographic characteristics and culinary habits [19,20,21,22,23,24,25,26,27,28]. Thus, this association has not yet been demonstrated consistently or conclusively. We evaluated the effect of the pre-pregnancy Mediterranean diet (MD) level of exposure on the odds of GDM development.
2. Material and Methods
2.1. Study Design and Setting
This study was a case-control study consisting of pregnant women with GDM (cases) and those without (controls) in the catchment area of Virgen de las Nieves University Hospital of Granada, Spain (Project of Excellence of the Junta de Andalucía CTS 05/942). Ethical approval was obtained through the Ethics and Research Committees of the University of Granada and the Virgen de las Nieves University Hospital of Granada. One in five women who attended the antenatal visit for the screening ultrasound scan at 20–22 week of gestation were systematically informed about the study and informed consent was obtained for participation. The antenatal protocol in the South of Spain includes a systematic visit to the obstetrician at 20 weeks for all pregnant women [29]. Sample size was calculated considering all the following assumptions: case to control ratio 1:4, percentage of controls exposed to a moderate/high adherence to a MD pattern of 50% [30], an odds ratio (OR) greater than or equal to 1.5 for a population that does not have a MD, accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test. The sample size was calculated using Fleiss’s formula with correction of continuity, and a total of 255 cases and 1020 controls was the estimation [31].
2.2. Participants
The participants consisted of Spanish women over 18 years of age with a low risk pregnancy. Women with a diagnosis of type 1 or 2 diabetes, or carbohydrate intolerance prior to pregnancy, as well as high risk pregnancies and those that needed to modify their diet or physical activity level in the previous year or during the first half of gestation for a medical reason were excluded.
Following the universal 50 g glucose challenge test in gestational weeks 24–28, women who had a venous plasma glucose ≥140 mg/dL were scheduled for a diagnostic 3 h, 100 g, oral glucose tolerance test. The National Diabetes Data Group (NDDG) criteria (fasting, 105 mg/dL; 1 h, 190 mg/dL; 2 h, 165 mg/dL; 3 h, 145 mg/dL) were considered [32]. GDM (cases) was defined as at least two plasma glucose measurements equal to or higher than the cutoff points. The control group had a negative 50 g glucose challenge test (<140 mg/dL) or positive 50 g glucose challenge test (≥140 mg/dL) and negative diagnostic oral glucose tolerance test.
2.3. Data Sources and Variables
Information about the pregnant women was collected on: anthropometric data, sociodemographic variables and personal, obstetric and family history, as well as her current work situation.
2.3.1. Dietary Assessment
To collect information on the dietary pattern of the women, the food consumption frequency questionnaire (FFQ) developed by Martín-Moreno et al. was used. This questionnaire has been translated, adapted, and validated in the Spanish population [33] and records the intake of 118 different foods. We collected the frequency of consumption and average amount for different food groups during the year prior to pregnancy. The interviews were always carried out prior to the visit to the obstetrician and by personnel trained for that purpose, with an approximate duration of 45 min.
To measure the adherence to the MD, the index developed by Trichopoulou et al. [34] was used. This index considers the following nine components: vegetables, legumes, fruits and nuts, cereals, fish, meat, dairy products, the ratio of monounsaturated lipids to saturated lipids and ethanol consumption. The median for each food group was estimated using the control group. For consumption of each typical Mediterranean food higher than the median of the consumption distribution in the control group, a person received 1 point; consumption lower received zero points. For consumption of non-Mediterranean foods lower than the median 1 point was awarded; consumption higher than the median received zero points. For ethanol consumption, only the intake of wine was taken into account, if it was between 5 and 25 g/day, women received 1 point and 0 if the value was higher or lower than that figure. The total score ranged from 0 (minimum adherence to a traditional MD pattern) to 9 (maximum adherence). Subsequently, this MD adherence variable was categorized as: low adherence (0–2), middle (3–4), high (5–6) and very high (≥7 points).
2.3.2. Other Variables
Current smokers were defined as those who smoked at least one cigarette per day in the last six months. The educational level of women was registered as: primary studies (eight years or less of basic education); secondary (four years of secondary education) and university (university or postgraduate studies). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Both, weight and height just before pregnancy, were obtained from the woman’s medical records where it had been recorded by their doctor or nurse. The cutoff points of the World Health Organization were used to determine overweight and obesity in the participants. Women with a BMI ≥ 30 kg/m2 were classified as obese and those with a BMI ≥ 25 kg/m2 but <30 kg/m2 as overweight [35].
2.4. Statistical Analysis
In the descriptive analysis of the sample, the mean, standard deviation (SD) and range of the continuous quantitative variables were calculated: age, previous BMI, energy intake. Food intakes were adjusted for total energy intake using the residuals method for cases and controls as recommended by Willet et al. [36]. For the qualitative variables of interest, the distribution of absolute and relative frequencies was calculated. We identified the relationship between each of the components of the MD and the development of GDM using multivariable logistic regression models and calculated crude (cOR) and adjusted odds ratios (aOR), and their 95% confidence intervals (CI). We used information from previous studies and directed acyclic graph (DAG) to identify potential confounding, thus epidemiological and statistical criteria were used to construct the models. Age, BMI, family history diabetes mellitus, previous GDM, previous miscarriages, gravidity, total energy intake, and leisure time physical activity were taken into account as possible confounding factors. The statistical program Stata v.14 (Stata Corp., 2015, College Station, TX, USA) was used.
3. Results
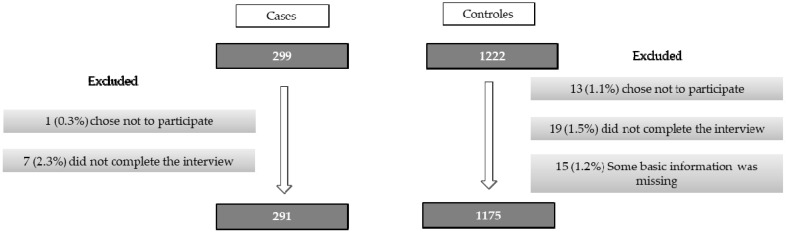
There were 299 cases of pregnant women diagnosed with GDM and 1,222 controls without. Among the cases, one (0.3%) did not have the correct tests and seven (2.4%) decided not to participate after recruitment. Thus, eight cases (2.7%) were excluded from analysis. Among the controls, 13 (1.1%) did not participate, 19 (1.5%) did not complete the interview and 15 (1.2%) had data missing for other variables. Therefore, the final sample analysed included 291 cases and 1175 controls (Figure 1).
Figure 1.
Flow diagram of the women included in the study and analyses.
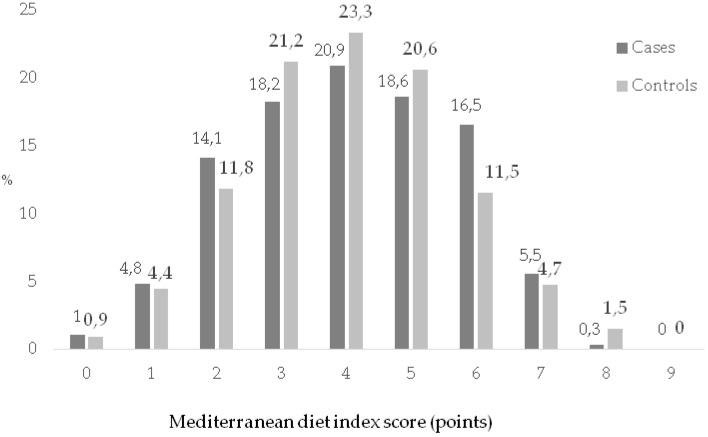
The age range of the participants was between 18–45 years (Table 1). The average age in cases was higher than in controls (33.50 years (SD 5.5) vs. 29.80 years (SD 5.1)). A greater frequency of antecedents of diabetes mellitus and previous GDM was observed among the cases than controls. The BMI was higher among cases than controls: 27.62 kg/m2 (SD 6.2) vs. 24.22 kg/m2 (SD 4.5), respectively. The cases had more frequent extreme scores on the global index (Figure 2). Middle-high adherence to the MD was 216/291 (74.2%) in cases and 900/1175 (76.6%) in controls (very high adherence of 5.8% vs. 6.2%, respectively).
Table 1.
Description of cases with gestational diabetes mellitus and the controls without gestational diabetes mellitus.
| Cases (n = 291) |
Controls (n = 1175) |
p Value | |
|---|---|---|---|
| Age (Mean; SD) | 33.50; SD = 5.5 | 29.80; SD = 5.1 | <0.001 |
| (Years) | n (%) | n (%) | |
| <25 | 18 (6.2) | 178 (15.2) | |
| 25–29 | 49 (16.8) | 345 (29.4) | |
| 30–34 | 91 (31.3) | 436 (37.1) | |
| ≥35 | 133 (45.7) | 216 (18.3) | |
| Education | 0.140 | ||
| University | 80 (27.5) | 358 (30.5) | |
| Secondary | 74 (25.4) | 339 (28.8) | |
| Primary | 137 (47.1) | 478 (40.7) | |
| Employment | <0.001 | ||
| Work outside the home | 94 (32.4) | 558 (47.5) | |
| Unemployment | 24 (8.3) | 84 (7.2) | |
| Sick leave in pregnancy | 61 (21.0) | 105 (8.9) | |
| Retired | 2 (0.7) | 6 (0.5) | |
| Housewife | 109 (37.6) | 421 (35.9) | |
| Antecedents of Diabetes Mellitus | 135 (46.4) | 300 (25.5) | <0.001 |
| Previous Gestational Diabetes Mellitus | 58 (19.9) | 23 (1.9) | <0.001 |
| Gravidity | <0.001 | ||
| 0 | 106 (36.4) | 555 (47.2) | |
| 1 | 89 (30.6) | 365 (31.1) | |
| 2 | 57 (19.6) | 168 (14.3) | |
| 3 | 22 (7.6) | 61 (5.2) | |
| ≥4 | 17 (5.8) | 26 (2.2) | |
| Parity | <0.001 | ||
| 0 | 146 (50.2) | 631 (53.7) | |
| 1 | 85 (29.2) | 416 (35.4) | |
| 2 | 42 (14.4) | 108 (9.2) | |
| ≥3 | 18 (6.2) | 20 (1.7) | |
| Miscarriage | <0.001 | ||
| 0 | 201 (69.1) | 933 (79.4) | |
| 1 | 69 (23.7) | 199 (16.9) | |
| ≥2 | 21 (7.2) | 43 (3.7) | |
| History of macrosomia | 10 (3.4) | 37 (3.1) | 0.062 |
| Body Mass Index (kg/m2) | <0.001 | ||
| (Mean; SD) | 27.62; SD = 6.2 | 24.22; SD = 4.5 | |
| 18.5–24.9 | 117 (40.2) | 789 (67.2) | |
| 25–29.9 | 83 (28.5) | 268 (22.8) | |
| ≥30 | 91 (31.8) | 118 (10.0) | |
| Smoking | 0.161 | ||
| Never | 110 (37.8) | 504 (42.9) | |
| Ex-smoker | 73 (25.1) | 242 (20.6) | |
| Current smoker | 108 (37.1) | 429 (36.5) | |
Figure 2.
Distribution of adherence to Mediterranean diet in the study population.
Table 2 shows the average consumption of the components of the diet in each group of participants, with the average consumption of legumes in both being very similar. When the relationship between the consumption of each component of the MD pattern and the development of GDM was analyzed (Table 3), a statistically significant association was found only between the consumption of meat products and their derivatives and the development of GDM (aOR = 0.56; 95% CI 0.42, 0.74).
Table 2.
Consumption of the components of the Mediterranean diet in the cases with gestational diabetes mellitus and the controls without gestational diabetes mellitus.
| Components of the MD | Cases (n = 291) Mean (SD) 95% CI p25, p50, p75 |
Controls (n = 1175) Mean (SD) 95% CI p25, p50, p75 |
p Value |
|---|---|---|---|
| Vegetables (g/day) | 584.14 (294.03) | 588.72 (314.88) | 0.082 |
| 550.22–618.07 | 570.69–606.74 | ||
| 355.95, 560.71, 738.09 | 345.24, 540.48, 795.24 | ||
| Fruits (g/day) | 241.34 (185.86) | 217.86 (150.69) | 0.023 |
| 219.89–262.78 | 209.24–226.49 | ||
| 125.16, 207.44, 313.86 | 111.72, 191.30, 291.36 | ||
| Legumes (g/day) | 0.23 (0.12) | 0.23 (0.13) | 0.954 |
| 0.22–0.24 | 0.22–0.24 | ||
| 0.17, 0.22, 0.27 | 0.17, 0.22, 0.27 | ||
| Cereals (g/day) | 236.47 (98.84) | 227.90 (89.02) | 0.151 |
| 225.07–247.88 | 222.80–232.99 | ||
| 173.50, 227.14, 287.14 | 162.86, 227,14, 278.57 | ||
| Fish (g/day) | 89.86 (61.59) | 80.75 (50.18) | 0.008 |
| 82.76–96.97 | 77.88–83.63 | ||
| 47.26, 74.19, 121.43 | 47.26, 70.71, 107.62 | ||
| Dairy products (g/day) | 474.31 (287.95) | 492 (283.38) | 0.342 |
| 441.09–507.53 | 475.78–508.22 | ||
| 275, 397.62, 632.26 | 286.90, 439.52, 648.80 | ||
| Meat and derivatives (g/day) | 172.92 (76.02) | 149.91 (70.65) | <0.001 |
| 164.15–181.69 | 145.87–153.95 | ||
| 119.28, 163.45, 208.69 | 103.09, 141.07, 184.28 | ||
| Ratio of monounsaturated/saturated lipids | 0.98 (0.18) | 0.92 (0.14) | 0.695 |
| 0.95–1.00 | 0.92–0.93 | ||
| 0.85, 0.94, 1.06 | 0.83, 0.91, 1.00 | ||
| Ethanol (g/day) | 0.55 (1.37) | 0.60 (1.83) | <0.001 |
| 0.39–0.71 | 0.49–0.70 | ||
| 0, 0, 0.66 | 0, 0, 0.33 |
g/day: grams/day. p: percentile.
Table 3.
Relationship between the components of the Mediterranean diet and the development of gestational diabetes mellitus.
| Components of the MD | cOR | (95 % CI) | aOR | (95% CI) | p Value |
|---|---|---|---|---|---|
| Vegetables | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.15 | (0.89 , 1.49) | 0.95 | (0.69 , 1.29) | 0.753 |
| Fruits | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.17 | (0.91, 1.52) | 0.84 | (0.62, 1.14) | 0.282 |
| Legumes | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 0.87 | (0.67, 1.13) | 0.75 | (0.55, 1.01) | 0.066 |
| Cereals | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.00 | (0.78, 1.30) | 0.79 | (0.58, 1.06) | 0.125 |
| Fish | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.00 | (0.78, 1.30) | 0.81 | (0.61, 1.08) | 0.163 |
| Dairy products | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.28 | (0.99, 1.66) | 1.25 | (0.95, 1.64) | 0.104 |
| Meat and derivatives | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 0.53 | (0.41, 0.70) * | 0.56 | (0.42, 0.74) * | 0.000 |
| Ratio of monounsaturated/saturated lipids | |||||
| ≥Median | 1 | Reference | 1 | Reference | |
| <Median | 1.35 | (1.04, 1.74) * | 1.13 | (0.85, 1.51) | 0.381 |
| Ethanol | |||||
| <5 and >25 g/day | 1 | Reference | 1 | Reference | |
| 5–25 g/day | 0.67 | (0.26, 1.73) | 0.61 | (0.21, 1.74) | 0.361 |
cOR: crude odds ratio; aOR: adjusted odds ratio, adjusted for age, BMI, family history DM, previous GDM, miscarriages, gravidity, total energy intake, and leisure time physical activity. * Significant association p < 0.05. g/day: grams/day.
Middle-high adherence was very similar in both cases (74.2%) and controls (76.6%), with only a very high adherence of 5.8% vs. 6.2%, respectively (Table 4). In the crude analysis, the level of adherence to the MD was observed to increase the protective effect on the development of GDM. In the adjusted analysis, it was found that the strength of the association became more intense, so the aOR was increasingly protective as the level of adherence to the MD increased. The aOR = 0.61 (95% CI 0.39, 0.94); p = 0.028 and aOR = 0.33 (95% CI 0.15, 0.72), p = 0.005 for a high and very high adherence to the MD, respectively.
Table 4.
Relationship between adherence to the Mediterranean diet and gestational diabetes mellitus.
| Adherence to the MD (level) | Cases (n = 291) |
Controls (n = 1175) |
cOR | 95% CI | aOR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ||||||
| Low (0–2) | 58 (19.9) | 202 (17.2) | 1 | Reference | 1 | Reference | Reference |
| Middle (3–4) | 114 (39.1) | 523 (44.5) | 0.75 | (0.53, 1.08) | 0.67 | (0.44, 1.01) | 0.060 |
| High (5–6) | 102 (35.1) | 377 (32.1) | 0.94 | (0.65, 1.35) | 0.61 | (0.39, 0.94) * | 0.028 |
| Very high (≥7) | 17 (5.8) | 73 (6.2) | 0.81 | (0.44, 1.48) | 0.33 | (0.15, 0.72) * | 0.005 |
| p trend | 0.014 |
cOR: crude odds ratio; aOR: adjusted odds ratio, adjusted for age, BMI, family history DM, previous GDM, miscarriages, gravidity, total energy intake, and leisure time physical activity. GDM: Gestational Diabetes Mellitus; * Significant association p < 0.05.
4. Discussion
Our results show a protective effect of adherence to the MD prior to pregnancy for preventing GDM, with a temporal association. Very high adherence to the MD was more strongly associated with a reduction in GDM suggesting a dose-response. In addition, we observed the protective role of low consumption of meat and derivatives on the development of GDM.
The strengths of our study include the large representative sample from a reference population healthy pregnant women in the South of Spain. Only a small number of participants were lost to the antenatal care protocol. There was approximately a 99% coverage of the population of pregnant women in the public hospital. The analysis of overall dietary patterns offered a global assessment using a validated FFQ in the Spanish population [33]. This approach is superior to evaluating individual food groups [37,38,39]. To minimize selection bias, the sample was recruited through systematic sampling, using the antenatal ultrasound which is mandated as part of routine care. The collection of dietary information pre-pregnancy made it possible to study a temporal relationship.
The possible limitations of the study include concern about recall accuracy but this is likely to be non-differential between cases and controls as the participants were interviewed before being evaluated for GDM. There may also be concern about social desirability bias [40], depending on what women think they should consume, but this would be directed toward the null avoiding invalidation of our observed results. In observational epidemiologic studies, effect sizes can be caused by residual confounding due to the presence of unknown factors. In the present study this has been addressed by using multivariable analyses, however, it can not be completely ruled out when interpreting our results. Additionally, RCT evidence during pregnancy is consistent with our findings [41,42,43].
Other studies that evaluated adherence to the MD have used different methods. For example, Tobias et al. [25] used the Trichopoulou index, adapting it by not including dairy products. They studied women without GDM in previous pregnancies. Exclusion of women with previous diabetes mellitus may concentrate nulliparous women in the dataset. In our study there were patients with a history of previous GDM both among cases and controls, increasing the generalisability. Although pregnant women with previous GDM may modify their dietary patterns before another pregnancy, we took into account the dietary pattern during the year prior to pregnancy.
In the assessment of the quality of diet, other studies have separated the effects of different foods or meals. However, we eat nutrients through food, and dietary patterns rich in one nutrient tend to be associated with greater or lesser consumption of others [44]. The demonstrated benefits of a MD are probably not due to the isolated effect of some specific component of it, but it is due to synergistic effects and complex interactions between all the rations components. This is probably why when comparing each one of the MD components individually, no significant results are obtained, except when the consumption of meat is analyzed. This result is consistent with Schoenaker et al. [27], who state that the pattern `meats, sandwiches and sweets´ was associated with an increased risk of GDM after adjustment for socioeconomic, reproductive and lifestyle factors. Other studies corroborate the association between the consumption of meat products and an increase in the risk of development of diabetes mellitus [21,45].
5. Conclusions
The protective effect of adherence to a MD pattern prior to pregnancy should be considered as a preventive tool against the development of GDM. The MD should be promoted during the pre-pregnancy period for maternal and offspring health. Health care providers should keep this conclusion in mind to encourage adherence to the MD in women.
Acknowledgments
The authors thank Ingrid de Ruiter, MBChB PhD, for English language and editing support. The results of this study are part of the doctoral thesis of Julia Gómez-Fernández.
Author Contributions
J.J.J.-M. and J.M.-M. conceived and designed the study. R.O.-R., J.M.-M., and J.J.J.-M. analyzed data. R.O.-R., J.G.-F., and C.A.-P. coordinated data collection. The first draft of the paper was written by R.O.-R., J.G.-F., C.A.-P., and K.S.K. All authors discussed, made contributions to the article and approved the final version of the article.
Funding
The work has been made from the information collected in two research projects (PI03/1207 of the Health Research Fund of the Ministry of Health and Consumption, and continued by the CTS Excellence Project 05/942 of the Andalusian Government).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.American Diabetes Association American Diabetes Association Standards of Medical Care in Diabetes. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd E., Gomersall J.C., Tieu J., Han S., Crowther C.A., Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017;11:CD010443. doi: 10.1002/14651858.CD010443.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Ramos M., Escolar-Pujolar A., Mayoral-Sánchez E., Corral-San Laureano F., Fernández-Fernández I. Diabetes mellitus in Spain: Death rates, prevalence, impact, costs and inequalities. Gac. Sanit. 2006;20:15–24. doi: 10.1157/13086022. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez-Moleon J.J., Bueno-Cavanillas A., Luna-Del-Castillo J.D., Garciá-Martín M., Lardelli-Claret P., Gálvez-Vargas R. Prevalence of gestational diabetes mellitus: Variations related to screening strategy used. Eur. J. Endocrinol. 2002;146:831–837. doi: 10.1530/eje.0.1460831. [DOI] [PubMed] [Google Scholar]
- 5.Zhu W.W., Yang H.X., Wang C., Su R.N., Feng H., Kapur A. High Prevalence of Gestational Diabetes Mellitus in Beijing: Effect of Maternal Birth Weight and Other Risk Factors. Chin. Med. J. (Engl.) 2017;130:1019–1025. doi: 10.4103/0366-6999.204930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatzi L., Garcia R., Roumeliotaki T., Basterrechea M., Begiristain H., Iñiguez C., Vioque J., Kogevinas M., Sunyer J., INMA Study Group et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2013;110:2058–2068. doi: 10.1017/S0007114513001426. [DOI] [PubMed] [Google Scholar]
- 7.Delnord M., Blondel B., Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Curr. Opin. Obstet. Gynecol. 2015;27:133–142. doi: 10.1097/GCO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison J.L., Regnault T.R. Nutrition in pregnancy: Optimising maternal diet and fetal adaptations to altered nutrient supply. Nutrients. 2016;8:342. doi: 10.3390/nu8060342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy M.M., Stettler N., Smith K.M., Reiss R. Associations of consumption of fruits and vegetables during pregnancy with infant birth weight or small for gestational age births: A systematic review of the literature. Int. J. Womens Healt. 2014;6:899–912. doi: 10.2147/IJWH.S67130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmermans S., Steegers-Theunissen R.P., Vujkovic M., den Breeijen H., Russcher H., Lindemans J., Mackenbach J., Hofman A., Lesaffre E.E., Jaddoe V.V., et al. The Mediterranean diet and fetal size parameters: The Generation R Study. Br. J. Nutr. 2012;108:1399–1409. doi: 10.1017/S000711451100691X. [DOI] [PubMed] [Google Scholar]
- 11.Dietary Nutrition and Your Health: Dietary Guidelines for Americans 2015–2020. [(accessed on 24 January 2019)]; Available online: http://health.gov/dietaryguidelines/2015/guidelines/
- 12.Estruch R., Ros E., Salas-Salvado J., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., Lamuela-Raventos R.M., et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Gonzalez M.A., Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr. Opin. Lipidol. 2014;25:20–26. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 14.Romaguera D., Norat T., Mouw T., May A.M., Bamia C., Slimani N., Travier N., Besson H., Luan J., Wareham N., et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J. Nutr. 2009;139:1728–1737. doi: 10.3945/jn.109.108902. [DOI] [PubMed] [Google Scholar]
- 15.Toledo E., de A Carmona-Torre F., Alonso A., Puchau B., Zulet M.A., Martinez J.A., Martinez-Gonzalez M.A. Hypothesis-oriented food patterns and incidence of hypertension: 6-year follow-up of the SUN (Seguimiento Universidad de Navarra) prospective cohort. Public Health Nutr. 2010;13:338–349. doi: 10.1017/S1368980009991066. [DOI] [PubMed] [Google Scholar]
- 16.Verberne L., Bach-Faig A., Buckland G., Serra-Majem L. Association between the Mediterranean diet and cancer risk: A review of observational studies. Nutr. Cancer. 2010;62:860–870. doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Quezada I., Roman-Vinas B., Serra-Majem L. The Mediterranean diet and nutritional adequacy: A review. Nutrients. 2014;6:231–248. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salas-Salvadó J., Guasch-Ferre M., Lee C.H., Estruch R., Clish C.B., Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J. Nutr. 2016;146:920S–927S. doi: 10.3945/jn.115.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J.R., Yuan M.Y., Chen N.N., Lu J.H, Hu C.Y., Mai W.B., Zhang R.F., Pan Y.H., Qiu L., Wu Y.F., et al. Maternal dietary patterns and gestational diabetes mellitus: A large prospective cohort study in China. Br. J. Nutr. 2015;113:1292–1300. doi: 10.1017/S0007114515000707. [DOI] [PubMed] [Google Scholar]
- 20.Mijatovic-Vukas J., Capling L., Cheng S., Stamatakis E., Louie J., Cheung N.W., Markovic T., Ross G., Senior A., Brand-Miller J.C., et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: A systematic review and meta-analysis. Nutrients. 2018;10:698. doi: 10.3390/nu10060698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin D., Lee K.W., Song W.O. Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients. 2015;7:9369–9382. doi: 10.3390/nu7115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tryggvadottir E.A., Medek H., Birgisdottir B.E., Geirsson R.T., Gunnarsdottir I. Association between healthy maternal dietary pattern and risk for gestational diabetes mellitus. Eur. J. Clin. Nutr. 2016;70:237–242. doi: 10.1038/ejcn.2015.145. [DOI] [PubMed] [Google Scholar]
- 23.Izadi V., Tehrani H., Haghighatdoost F., Dehghan A., Surkan P.J., Azadbakht L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition. 2016;32:1092–1096. doi: 10.1016/j.nut.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Karamanos B., Thanopoulou A., Anastasiou E., Assaad-Khalil S., Albache N., Bachaoui M., Slama C.B., El Ghomari H., Jotic A., Lalic N., et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014;68:8–13. doi: 10.1038/ejcn.2013.177. [DOI] [PubMed] [Google Scholar]
- 25.Tobias D.K., Zhang C., Chavarro J., Bowers K., Rich-Edwards J., Rosner B., Mozaffarian D., Hu F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012;96:289–295. doi: 10.3945/ajcn.111.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gicevic S., Gaskins A.J., Fung T.T., Rosner B., Tobias D.K., Isanaka S., Willett W.C. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PloS ONE. 2018;13:e0195103. doi: 10.1371/journal.pone.0195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenaker D.A., Soedamah-Muthu S.S., Callaway L.K., Mishra G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia. 2015;58:2726–2735. doi: 10.1007/s00125-015-3742-1. [DOI] [PubMed] [Google Scholar]
- 28.Donazar-Ezcurra M., Lopez-Del Burgo C., Martinez-Gonzalez M.A., Basterra-Gortari F.J, de Irala J., Bes-Rastrollo M. Pre-pregnancy adherences to empirically derived dietary patterns and gestational diabetes risk in a Mediterranean cohort: The Seguimiento Universidad de Navarra (SUN) project. Br. J. Nutr. 2017;118:715–721. doi: 10.1017/S0007114517002537. [DOI] [PubMed] [Google Scholar]
- 29.Junta de Andalucıa . Manual de atencion al embarazo, parto y puerperio [Guidelines for Pregnancy, Delivery and Puerperium Care] Direccion General de Salud Pública; València, Spain,: 2006. [Google Scholar]
- 30.Gonzalez C.A., Argilaga S., Agudo A., Amiano P., Barricarte A., Beguiristain J.M., Chirlaque M.D., Dorronsoro M., Martinez C., Navarro C., et al. Sociodemographic differences in adherence to the Mediterranean dietary pattern in Spanish populations. Gac. Sanit. 2002;16:214–221. doi: 10.1016/s0213-9111(02)71664-6. [DOI] [PubMed] [Google Scholar]
- 31.Kelsey J.L., Thompson W.D., Evans A.S. Methods in Observational Epidemiology. Oxford University Press; New York, NY, USA: 1986. [Google Scholar]
- 32.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Moreno J.M., Boyle P., Gorgojo L., Maisonneuve P., Fernandez-Rodriguez J.C., Salvini S., Willett W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993;22:512–519. doi: 10.1093/ije/22.3.512. [DOI] [PubMed] [Google Scholar]
- 34.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 35.Salas-Salvadó J., Rubio M.A., Barbany M., Moreno B., Grupo Colaborativo de la SEEDO SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. (Barc.) 2007;10:184–196. doi: 10.1016/S0025-7753(07)72531-9. [DOI] [PubMed] [Google Scholar]
- 36.Willett W., Stampfer M. Implications of total energy intake for epidemiologic analyses. In: Willett W., editor. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York, NY, USA: 1998. [Google Scholar]
- 37.Cespedes E.M., Hu F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015;101:899–900. doi: 10.3945/ajcn.115.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kant A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004;104:615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Roman-Viñas B., Ribas Barba L., Ngo J., Martínez-González M.A, Wijnhoven T.M., Serra-Majem L. Validity of dietary patterns to assess nutrient intake adequacy. Br. J. Nutr. 2009;101:S12–S20. doi: 10.1017/S0007114509990547. [DOI] [PubMed] [Google Scholar]
- 40.Hebert J.R., Clemow L., Pbert L., Ockene I.S., Ockene J.K. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int. J. Epidemiol. 1995;24:389–398. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 41.Al Wattar B.H., Dodds J., Placzek A., Spyreli E., Moore A., Hooper R., Beresford L., Roseboom T.J., Bes-Rastrollo M., Hitman G., et al. Effect of simple, targeted diet in pregnant women with metabolic risk factors on maternal and fetal outcomes (ESTEEM): Study protocol for a pragmatic multicentre randomised trial. BMJ Open. 2016;6:e013495. doi: 10.1136/bmjopen-2016-013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assaf-Balut C., Garcia de la Torre N., Duran A., Fuentes M., Bordiú E., Del Valle L., Familiar C., Ortolá A., Jiménez I., Herraiz M.A., et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PloS ONE. 2017;12:e0185873. doi: 10.1371/journal.pone.0185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Ferre N., Del Valle L., Torrejon M.J., Barca I., Calvo M.I., Matía P., Rubio M.A., Calle-Pascual A.L. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: A three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clin. Nutr. 2015;34:579–585. doi: 10.1016/j.clnu.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Jacques P.F., Tucker K.L. Are dietary patterns useful for understanding the role of diet in chronic disease? Am. J. Clin. Nutr. 2001;73:1–2. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Aune D., Ursin G., Veierod M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]