Abstract
Transgenic technology is a powerful tool for gene functional characterization, and poplar is a model system for genetic transformation of perennial woody plants. However, the poplar genetic transformation system is limited to a number of model genotypes. Herein, we developed a transformation system based on efficient Agrobacterium-mediated transformation for the hybrid poplar Populus Alba × Populus glandulosa Uyeki, which is a fast-growing poplar species that is suitably grown in the northern part of China. Importantly, we optimized many independent factors and showed that the transformation efficiency was improved significantly using juvenile leaf explants. Explants were infected by an Agrobacterium suspension with the OD600 = 0.6 for 15 min and then co-cultured in dark conditions for 3 days. Using the improved transformation system, we obtained the transgenic poplar with overexpression of β-glucuronidase (GUS) via direct organogenesis without callus induction. Furthermore, we analyzed the GUS gene in the transgenic poplars using PCR, qRT-PCR, and GUS staining. These analyses revealed that the GUS gene was efficiently transformed, and it exhibited various expression levels. Taken together, these results represent a simple, fast, and efficient transformation system of hybrid poplar plants. Our findings may facilitate future studies of gene functions in perennial woody plants and tree breeding via transgenic technology assisted design.
Keywords: Agrobacterium tumefaciens, leaf explants, regeneration transformation, P. alba × P. glandulosa Uyeki
1. Introduction
Poplar is an important group of cultivated tree species used in both ecological and economic plantations. Its popularity is largely due to its adaptability to diverse environmental conditions and its capacity for rapid growth [1]. Tree breeding by transgenic technology assisted design is the major technique for improving varieties of poplar with superior hereditary characteristics. This technique can overcome the difficulties associated with the breeding of woody plants, which require many years to produce progeny [2]. The genetic transformation system can introduce foreign genes into plants for understanding and controlling plant gene expression, which provide a powerful tool for analyzing the function of genes and the functional characterization of gene products. Then the in-depth knowledge of gene function is effective for making more purposeful and systematic breeding programs directed at transgenic technology assisted breeding of poplar.
Strategies for variety-independent genetic transformation of plants have been developed, including Agrobacterium-mediated transformation, viral transformation, electroporation of protoplasts, and particle bombardment [3,4,5,6]. Although both biological and physical methods have been used, the Agrobacterium-mediated transformation is the predominant method for poplar [7]. Since the first successful poplar transformation [3], a series of Agrobacterium-mediated poplar transformations have been reported, such as P. tremula, P. tremuloides, P. alba, P. nigra, P. tremula × P. tremuloides, P. trichocarpa, P. trichocarpa × P. deltoids, P. deltoides × P. euramericana [8,9,10,11,12,13,14,15].
The in vitro tissue-to-plant regeneration system is an integral part of genetic transformation procedures. Two tissue culture regeneration systems have been used in poplar: organogenesis and somatic embryogenesis. Regeneration through organogenesis is an efficient method for in vitro production of whole plants [16]. For in vitro regeneration of poplar through organogenesis, different explants have been generally used for transformation, including leaves [15,17,18], petiole [19], stems [20,21,22], roots [20], and shoot tips [23]. Interestingly, the leaf disc transformation method is the predominant method used in poplar transformation systems. In the past, new transgenic poplars with resistance to pests [24], resistance to stress [25], and modifications to wood properties [26] have been bred from different poplar species with various transformation systems. Efficient and reproducible Agrobacterium-mediated transformation and regeneration systems have been obtained in many poplar species. However, these have been reported only for a limited number of model genotypes, and the process is still difficult in many poplar species [16,27].
In the present study, we developed a simple and efficient system for transformation, selection, and regeneration based on Agrobacterium-mediated transformation of hybrid poplar P. alba × P. glandulosa Uyeki, known as suwon poplar. This is one of the major poplar species in Korea, and suitable for reforestation of hillsides. P. alba × P. glandulosa was introduced to China in 1984, and commonly used in China due to its high wood quality, fast growth, and relatively high drought tolerance. P. alba × P. glandulosa is ideal as a model plant and as a source of laboratory research materials due to its easy cultivation, rapid growth, and strong in vitro regeneration frequency characteristics. Herein, we optimized many independent factors to achieve a stable regeneration efficiency of poplar. We used β-glucuronidase (GUS) as a reporter gene, which has proved to be efficient in vivo and achieved the transgenic poplar with this method. Furthermore, we used PCR, quantitative real-time PCR (qRT-PCR), and β-glucuronidase (GUS) staining to examine the expression levels of the target GUS gene in the wild-type and transgenic poplar. The purpose of this study is to establish a simple, fast, and stable transformation system for P. alba × P. glandulosa. The results of this study should provide a powerful tool for the study of gene functions in perennial woody plants.
2. Results
2.1. Optimization of the Transformation Procedure
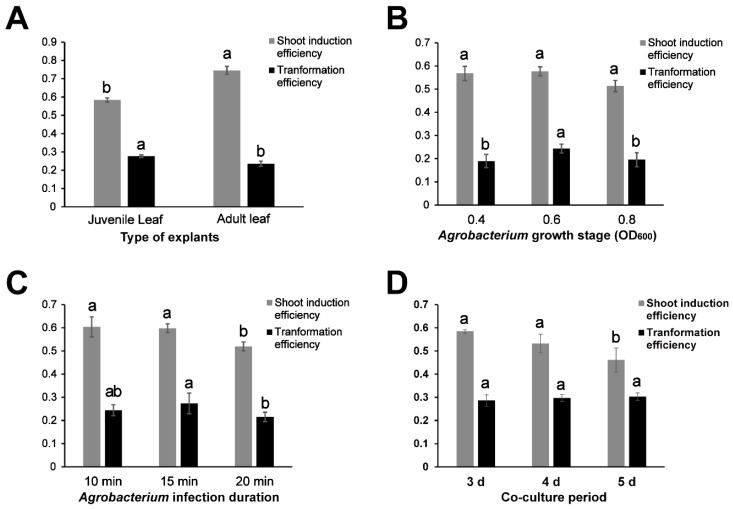
To establish the transformation system of P. alba × P. glandulosa, we optimize several operations in the transformation procedure, including the age of leaf explants (Figure 1A), the growth stage of bacterium (Figure 1B), Agrobacterium infection duration (Figure 1C), and co-culture period (Figure 1D). The transformation efficiency was significantly increased with juvenile leaf explants, but the frequency of shoot induction was simultaneously decreased (Figure 1A). The transformation efficiency was significantly increased when Agrobacterium infection with an OD600 of 0.6, and the transformation efficiency was decreased when the growth time of Agrobacterium was excessively long or short (Figure 1B). The highest transformation efficiency was obtained with an Agrobacterium incubation time of 15 min (Figure 1C). The transformation efficiency was not significantly different with different co-culture periods, but the shoot induction efficiency significantly decreased with the extension of the co-culture period (Figure 1D).
Figure 1.
Factors that affect the shoot induction efficiency and transformation efficiency of P. alba × P. glandulosa. (A) Ages of leaves. (B) Growth stages of Agrobacterium bacteria. (C) Agrobacterium infection duration. (D) Co-culture period. a and b showed the statistical significance of differences that determined using the Student’s t-test (p < 0.05).
2.2. Simple and Fast Transformation System
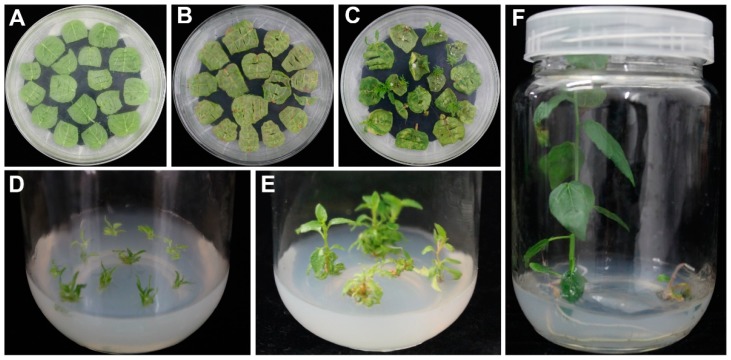
A simple and fast Agrobacterium-mediated transformation system was developed for P. alba × P. glandulosa using leaf explants and the following protocol: 2 days of pre-culture (Figure 2A), 3 days of co-culture, 4 to 5 weeks of shoot induction (Figure 2B,C), and 4 to 5 weeks for root induction (Figure 2D,E) and seedlings development (Figure 2F). In addition, to determinate the stability of kanamycin-resistance in regenerated poplar plants, the regenerated poplar plants were subcultured on M4 for kanamycin-resistance plants propagation and showed that 96% of the explants from regenerated poplar plants developed into whole poplar plants. Furthermore, to ensure that the regenerated poplar plants were free from Agrobacterium, we subcultured the regenerated poplar plants on M1 for plant propagation free from kanamycin and showed no Agrobacterium occurred on the culture medium or plants in the whole process of subculture. During transformation experiments, a total of 115 leaf segments of P. alba × P. glandulosa were infected with Agrobacterium, 63 shoots were induced cultured on shoot-inducting medium M3, 39 independent kanamycin-resistant plants were obtained cultured on root-inducting medium M4, and 37 positive transgenic poplar lines were finally obtained after being subcultured on M4. The results indicate that the transformation frequency of the system was 32.18%.
Figure 2.
The flowchart of the transformation system for P. alba × P. glandulosa. (A) Pre-culture of leaf explants on M1. (B) Shoot induction of leaf explants on M3 after co-cultivated with Agrobacterium bacteria. (C) Shoot induction of leaf explants on M3 for 4 weeks. (D) Shoots cultured on M4 for root induction. (E) Root induction on M4 for 2 weeks. (F) Transgenic poplar seedlings culture on M4.
2.3. Positive Plant Identification by PCR Analysis
To determine whether the transgenic poplar plants were GUS-positive, we selected 20 transgenic poplar plants and isolated the genomic DNA from leaves for PCR analysis. The 35S promoter and part of the GUS gene were PCR-amplified using a specific primer pair and the expected band size of 680 bp, the actin gene that existing in poplar was used as an internal control. The bands were observed at the expected size from all of the samples, but they were not observed for the wild-type poplar plant (Figure 3A). These results showed that all 20 poplar plants are transgenic plants. To ensure that the regenerated poplar plants were free from Agrobacterium, we used the tzs gene that does not belong to the T-DNA region in Agrobacterium strain as a positive control. The bands of tzs gene were observed from Agrobacterium strain, but they were not observed in all of the poplar plant samples, including the wild-type poplar plant and all of the transgenic poplar plant. (Figure 3A).
Figure 3.
PCR, qRT-PCR, and GUS staining analysis of transgenic poplars. (A) PCR product of 35S-GUS with 680 bp was detected in Atu, and all of the transgenic poplars, PCR product of actin gene with 250 bp was detected in all of the poplars, and PCR product of tzs gene with 660 bp was detected in Atu. M, Marker; Atu, Agrobacterium tumefaciens; WT, wild type of P. alba × P. glandulosa; 1–20, transgenic poplars. (B) GUS staining of different transgenic poplar and WT plants. WT, wild type; Line 1–Line 7, different lines of transgenic poplars. Bars = 0.5 cm. (C) GUS staining of different explants of Line 6. TB terminal bud; L, leaf; JS, juvenile stem; AS, adult stem; R, root. Bars = 0.5 cm. (D) qRT-PCR analysis of transgenic poplar and WT plants. WT, wild type; Line 1–Line 7, different lines of transgenic poplars.
2.4. Quantitative Assay of GUS Expression by qRT-PCR
To analyze the GUS expression level of different lines, we isolated the genomic RNA from the randomly selected leaves of the seven positive transgenic poplar plants and wild-type plants. We then used qRT-PCR to analyze the relative expression of GUS. The expression of GUS varied from 10 times higher to 427 times higher than the level of wild-type plants. This result confirmed that the expression of GUS differed among the various transgenic lines (Figure 3D).
2.5. β-Glucuronidase Histochemical Assays
We further stained the leaves of the seven poplar plants and wild-type plants that were analyzed via qRT-PCR. The GUS staining of leaf explants of transgenic poplars showed various levels of intensity, which suggests that the expression of GUS was different in various transgenic poplar plants (Figure 3B). In addition, we stained different explants of the transgenic poplar line 6, including terminal bud, leaf, juvenile stem, adult stem, and root. All of the explants showed positive GUS staining (Figure 3C).
3. Discussion
Genetic transformation is a powerful tool in plant molecular biology and functional genomics research. Since the first successful poplar transformation was established [3], poplars have been transformed for genetic improvement and gene function research far more than any other forest tree species [7,27]. A large number of factors affect the transformation efficiency and the regeneration during the process of poplar genetic transformation, including the explant source, the growth stage of bacterium, co-culture time period, and Agrobacterium infection duration. Juvenile sources of explant tissues have superior regeneration potential compared with other sources due to their lower lignin content [17]. Moreover, Agrobacterium concentration and infection time significantly affect the transformation efficiency [15,17]. In this study, we analyzed the poplar transformation efficiency by varying the developmental stage of leaf explants, Agrobacterium concentrations, the duration for the co-culture period, and duration of Agrobacterium infection. These results indicated that the development stage of leaf explants was the main influencing factor for transformation efficiency of P. alba × P. glandulosa. In addition, the best results for P. alba × P. glandulosa genetic transformation were obtained using juvenile leaf explants that were dipped into Agrobacterium suspension (OD600 = 0.6) for 15 min and then co-cultured in dark conditions for 3 days (Figure 1).
A major problem for the poplar transformation system is inducing shoots from transformed cells, and there are two major approaches for shoot induction in most of the existing poplar transformation systems. One method is inducing cell division and callus growth on the cut surface of poplar explants infected with Agrobacterium and then inducing shoots from induced calluses [22]. Callus induction is a time-consuming and complex operation, and methods that include the callus phase may lead to plants that differ from the mother plant due to somaclonal variations [28]. Another method is directly inducing shoots from the cut poplar explants infected with Agrobacterium [18,29]. In the present study, we developed a fast poplar transformation system for P. alba × P. glandulosa via direct organogenesis without callus induction (Figure 2). The whole genetic transformation process was completed within 3 months. In addition, the regenerated poplar plants were subcultured on M4 and M1, respectively, showing that the kanamycin-resistance transgenic poplar plants were subcultured steady and the transgenic poplar plants were free from Agrobacterium contamination. These results suggested that the Agrobacterium-mediated transformation regeneration system was adopted as the fast and effective for Populus. alba × P. glandulosa. Nonetheless, the transformation system of P. alba × P. glandulosa exhibits a relatively higher transformation frequency at 32.18%.
In plant genetic transformation, the transgenic tissues will form chimeric shoots that contain transgenic and non-transgenic sections with a certain probability [30,31]. PCR is a powerful and direct tool for detecting a target gene that has been inserted into the genome, and it is routinely used in the identification of transgenic plants [18]. In the present study, transgenic poplar plants that subcultured two times were detected by the previously optimized vector-specific genomic PCR, This indicates that the GUS gene was inserted into the poplar genome successfully in the 20 transgenic poplar lines. (Figure 3A).
Transgene expression is usually correlated to the transgene copy number that was inserted into the host plants genome [32,33]. The qRT-PCR method has been validated for accurate estimation of transgene expression in various transgenic plants [34]. The current results showed various expressions of GUS in the seven samples. These levels are significantly higher than levels for wild-type plants, revealing that this transformation system can transform the exogenous gene into P. alba × P. glandulosa efficiently, and increased the gene expression significantly (Figure 3D). In addition, the GUS histochemical analysis showed that the high expression of the GUS gene led to high staining intensity for different transgenic poplar plants and different explants, indicating that the staining intensity was consistent with the GUS gene expression (Figure 3B,C). Together, these results provide strong evidence that transformation systems are efficient for P. alba × P. glandulosa. Additionally, the transformed exogenous gene is suitable to achieve high expression levels of the target gene and protein.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Explants were collected from P. alba × P. glandulosa plants, sterilized with 0.2% NaClO for 15 min, rinsed three times with sterile water, and transferred to the culture bottle. Seedlings of poplar (P. alba × P. glandulosa) were cultured on M1 (Table 1) and subjected to a 16-h light:8-h dark photoperiod with 500 to 600 μmol m−2 s−1 of photosynthetically active radiation. Plants were kept at 25 °C during the day and at 22 °C at night.
Table 1.
Composition of media for cultivation, transformation, selection, and regeneration of hybrid poplar P. alba × P. glandulosa.
| Components | M1 (Plant Propagation and Pre-Culture) |
M2 (Co-Culture) |
M3 (Shoot Induction) |
M4 (Root Induction) |
|---|---|---|---|---|
| MS | 4.43 g/L | 4.43 g/L | 4.43 g/L | 2.215 g/L |
| Sucrose | 30 g/L | 30 g/L | 30 g/L | 30 g/L |
| Agar | 5.8 g/L | 5.8 g/L | 5.8 g/L | 5.8 g/L |
| NAA | — | 0.05 mg/L | 0.05 mg/L | 0.02mg/L |
| 6-BA | — | 0.5 mg/L | 0.5 mg/L | — |
| IBA | — | — | — | 0.05 mg/L |
| Kanamycin | — | — | 30 mg/L | 30 mg/L |
| Cefotaxime | — | — | 200 mg/L | 200 mg/L |
| Timentin | — | — | 200 mg/L | 200 mg/L |
| pH | 5.8 | 5.8 | 5.8 | 5.8 |
4.2. Agrobacterium Culture and Transformation Procedure
We constructed the 35S:GUS vector and transformed to Agrobacterium tumefaciens GV3101 [35]. A single colony of Agrobacterium tumefaciens was grown in LB medium (containing 50 mg/L kanamycin and 50 mg/L rifampicin) overnight at 28 °C with shaking until the culture density reached an OD600 of 0.6. The Agrobacterium tumefaciens cells were harvested by centrifugation at 5000 rpm for 10 min. The cells were then suspended with 1/2 MS solution (pH 5.8 to 6.0) containing 5% (w/v) sucrose and acetosyringone (100 μM) as the transformation solution.
For the whole process of genetic transformation, the explants were cultured on Murashige and Skoog medium with different supplementary components, as shown in Table 1. The leaf explants excised from 3-week-old plantlets were cut with three wounds perpendicular to main veins and cultured on M1 for 2 days. The cut leaves were incubated with the suspended Agrobacterium tumefaciens for 15 min with slow shaking every 5 min. The cut leaves were blotted with sterile filter paper to remove the excess bacteria and then cultured on M2 in dark conditions for 3 days. The co-cultivated leaves were washed 2 times in sterilized distilled water for 5 min, blotted with sterile filter paper to remove the excess water, and transferred to M3 for shoot induction. The co-cultivated leaves were transferred to fresh M3 every 10 days, and the growth conditions were similar to the growth conditions of the P. alba × P. glandulosa plants. The shoots grew to 1 to 2 cm after 4 weeks, and they were then cut off and transferred to M4. In this medium, the roots formed and the whole poplar plants were developed. The transgenic poplar plants were grown on M1 for 3 weeks to ensure that plants were free from Agrobacterium contamination.
4.3. Plant Genomic DNA Isolation and PCR Analysis
The genomic DNA was isolated from leaves of transgenic and wild-type poplar plants via the cetyltrimethylammonium bromide (CTAB) procedure described previously, with some modifications [36]. For PCR analyses, a forward primer (5′-CCTCTGCCGACAGTGGTC-3′) was designed from the 35S promoter, and a reverse primer (5′-CATCGGCTTCAAATGGCGTATAGC-3′) was designed from GUS. For an internal control, actin gene that existing in poplar was used [37], and its primers were forward 5′-AAGGTTGTTGCACCACCAGA-3′ and reverse 5′-AACACACAGTCCATCACCGC-3′. The primers of tzs gene were forward 5′-TCTGGCCACTGAGGAAAATC-3′ and reverse 5′-ATCTACGGACCGACTTGCAG-3′ [38].
4.4. Quantitative Analysis of GUS Expression by qRT-PCR
Whole seedlings of poplar were collected and stored in liquid nitrogen. The total RNA of these plants was isolated using an RNA Isolation Kit (TIANGEN, Beijing, China). Reverse transcription was performed using the First-Strand cDNA Synthesis Kit (TIANGEN, Beijing, China). qRT-PCR was performed using the SYBR Green Mix (TAKARA) in an optical 96-well plate. The primers for GUS were forward 5′-CTGTGGAATTGATCAGCGTTGGTGG-3′ and reverse 5′-AAGACTTCGCGCTGATACCAGACG-3′. For an internal control, 18SrRNA was used, and its primers were forward 5′-CGAAGACGATCAGATACCGTCCTA-3′ and reverse 5′-TTTCTCATAAGGTGCTGGCGGAGT-3′. The initial denaturing time was 94 °C for 30 s, followed by 40 cycles at 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 15 s, with a final extension at 72 °C for 10 min.
4.5. β-Glucuronidase Histochemical Assay
The samples were thoroughly rinsed with distilled water and vacuumed for 20 min while immersed in GUS staining solution and then incubated overnight at 37 °C. The GUS staining solution consisted of 10 mM Na2EDTA, 100 mM phosphate buffer (pH 7.0), 2 mM K4Fe(CN)6, 2 mM K3Fe(CN)6, and 1 mg/mL of X-Gluc (5-bromo-4-chloro-3-indolyl β-d-glucuronide). The stained samples were soaked in 70% (v/v) ethanol to remove chlorophyll.
5. Conclusions
In summary, we optimized many factors in the genetic transformation process of P. alba × P. glandulosa, and the transformation efficiency was improved significantly. We developed a simple and fast poplar transformation system for P. alba × P. glandulosa with directly induced shoots. Furthermore, the PCR, qRT-PCR, and GUS staining results showed that the transformation systems are efficient for P. alba × P. glandulosa with a 32.2% transformation frequency.
Acknowledgments
We thank Deyou Qiu for presenting P. alba × P. glandulosa plants. We are grateful to Jinxing Lin for his suggestions on experimental design.
Author Contributions
Conceived and designed the experiments: R.L.; Performed the experiment: C.S., L.L., Y.G.; Contributed reagents/materials/analysis tools: C.S., L.L., H.X., R.L.; Wrote the manuscript: C.S.; Revised manuscript for submission: R.L.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (2019ZY29), the State ‘13.5’ Key Research Program of China (No. 2016YFD0600102), the National Natural Science Foundation of China (31670182, 31761133009, 31530084, 31401149, 3180030099), the China Postdoctoral Science Foundation (2018M631246), and the Program of Introducing Talents of Discipline to Universities (111 project, B13007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Balatinecz J.J., Kretschmann D.E., Leclercq A. Achievements in the utilization of poplar wood-guideposts for the future. For. Chron. 2001;77:265–269. doi: 10.5558/tfc77265-2. [DOI] [Google Scholar]
- 2.Confalonieri M., Belenghi B., Balestrazzi A., Negri S., Facciotto G., Schenone G., Delledonne M. Transformation of elite white poplar (Populus alba L.) cv. ‘Villafranca’ and evaluation of herbicide resistance. Plant Cell Rep. 2000;19:978–982. doi: 10.1007/s002990000230. [DOI] [PubMed] [Google Scholar]
- 3.Parsons T.J., Sinkar V.P., Stettler R.F., Nester E.W., Gordon M.P. Transformation of poplar by Agrobacterium tumefaciens. Biotechnology. 1986;4:533–536. doi: 10.1038/nbt0686-533. [DOI] [Google Scholar]
- 4.Vainstein A., Marton I., Zuker A., Danziger M., Tzfira T. Permanent genome modifications in plant cells by transient viral vectors. Trends Biotechnol. 2011;29:363–369. doi: 10.1016/j.tibtech.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Miao Y., Jiang L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2007;2:2348–2353. doi: 10.1038/nprot.2007.360. [DOI] [PubMed] [Google Scholar]
- 6.Taylor N.J., Fauquet C.M. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002;21:963–977. doi: 10.1089/104454902762053891. [DOI] [PubMed] [Google Scholar]
- 7.Victor B.B., Amy M.B., Richard M., Serge F., Lisa G., Sonali G., Steven H.S. Genetic transformation: A powerful tool for dissection of adaptive traits in trees. New Phytol. 2005;167:9–18. doi: 10.1111/j.1469-8137.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- 8.Tzfira T., Jensen C.S., Wang W., Zuker A., Vincour B., Altman A., Vainstein A. Transgenic Populus tremula: A step-by-step protocol for its Agrobacterium-mediated transformation. Plant Mol. Biol. 1997;15:219–235. doi: 10.1023/A:1007484917759. [DOI] [Google Scholar]
- 9.Cseke L.J., Cseke S.B., Podila G.K. High efficiency poplar transformation. Plant Cell Rep. 2007;26:1529–1538. doi: 10.1007/s00299-007-0365-0. [DOI] [PubMed] [Google Scholar]
- 10.Delledonne M., Allegro G., Belenghi B., Balestrazzi A., Picco F., Levine A., Zelasco S., Calligari P., Confalonieri M. Transformation of white poplar (Populus alba L.) with a novel Arabidopsis thaliana cysteine proteinase inhibitor and analysis of insect pest resistance. Mol. Breed. 2001;7:35–42. doi: 10.1023/A:1009605001253. [DOI] [Google Scholar]
- 11.Biswas K.K., Mohri T., Kogawara S., Hase Y., Oono Y. An improved system for shoot regeneration from stem explants of Lombardy poplar (Populus nigra L. var. italica Koehne) Am. J. Plant Sci. 2012;3:1181–1186. doi: 10.4236/ajps.2012.39143. [DOI] [Google Scholar]
- 12.Eriksson M.E., Israelsson M., Olsson O., Moritz T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000;18:784–788. doi: 10.1038/77355. [DOI] [PubMed] [Google Scholar]
- 13.Song J., Lu S., Chen Z.Z., Lourenco R., Chiang V.L. Genetic transformation of Populus trichocarpa genotype Nisqually-1: A functional genomic tool for woody plants. Plant Cell Physiol. 2006;47:1582–1589. doi: 10.1093/pcp/pcl018. [DOI] [PubMed] [Google Scholar]
- 14.Han K.H., Meilan R., Ma C., Strauss S.H. An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus) Plant Cell Rep. 2000;19:315–320. doi: 10.1007/s002990050019. [DOI] [PubMed] [Google Scholar]
- 15.Movahedi A., Zhang J., Amirian R., Zhuge Q. An efficient Agrobacterium-mediated transformation system for poplar. Int. J. Mol. Sci. 2014;15:10780–10793. doi: 10.3390/ijms150610780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña L., Séguin A. Recent advances in the genetic transformation of trees. Trends Biotechnol. 2001;19:500–506. doi: 10.1016/S0167-7799(01)01815-7. [DOI] [PubMed] [Google Scholar]
- 17.Han X., Ma S., Kong X., Takano T., Liu S. Efficient Agrobacterium-mediated transformation of hybrid poplar Populus davidiana Dode × Populus bollena Lauche. Int. J. Mol. Sci. 2013;14:2515–2528. doi: 10.3390/ijms14022515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H.H., Wang C.T., Liu H., Tang R.J., Zhang H.X. An efficient Agrobacterium-mediated transformation and regeneration system for leaf explants of two elite aspen hybrid clones Populus alba × P. berolinensis and Populus davidiana × P. bolleana. Plant Cell Rep. 2011;30:2037–2044. doi: 10.1007/s00299-011-1111-1. [DOI] [PubMed] [Google Scholar]
- 19.Thakur A.K., Sarita S., Srivastava D.K. Plant regeneration and genetic transformation studies in petiole tissue of Himalayan poplar (Populus ciliata Wall) Curr. Sci. 2005;89:664–668. [Google Scholar]
- 20.Yadav R., Arora P., Kumar D., Katyal D., Dilbaghi N., Chaudhury A. High frequency direct plant regeneration from leaf, internode, and root segments of Eastern Cottonwood (Populus deltoides) Plant Biotechnol. Rep. 2009;3:175–182. doi: 10.1007/s11816-009-0088-5. [DOI] [Google Scholar]
- 21.Mohri T., Yamamoto N., Shinohara K. Agrobacterium-mediated transformation of lombardy poplar (Populus nigra L. var. italica Koehne) using stem segments. J. For. Res. 1996;1:13–16. doi: 10.1007/BF02348333. [DOI] [Google Scholar]
- 22.Yevtushenko D.P., Misra S. Efficient Agrobacterium-mediated transformation of commercial hybrid poplar Populus nigra L. × P. maximowiczii A. Henry. Plant Cell Rep. 2010;29:211–221. doi: 10.1007/s00299-009-0806-z. [DOI] [PubMed] [Google Scholar]
- 23.Kang B., Osburn L., Kopsell D., Tuskan G.A., Cheng Z.M. Micropropagation of Populus trichocarpa ‘Nisqually-1’: The genotype deriving the Populus reference genome. Plant Cell Tiss. Org. Cult. 2009;99:251–257. doi: 10.1007/s11240-009-9596-9. [DOI] [Google Scholar]
- 24.Klocko A.L., Meilan R., James R.R., Viswanath V., Ma C., Payne P., Miller L., Skinner J.S., Oppert B., Cardineau G.A., et al. Bt-Cry3Aa transgene expression reduces insect damage and improves growth in field-grown hybrid poplar. Can. J. For. Res. 2013;44:28–35. doi: 10.1139/cjfr-2013-0270. [DOI] [Google Scholar]
- 25.Harfouche A., Meilan R., Altman A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014;34:1181–1198. doi: 10.1093/treephys/tpu012. [DOI] [PubMed] [Google Scholar]
- 26.Coleman H.D., Yan J., Mansfield S.D. Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc. Natl. Acad. Sci. USA. 2009;106:13118–13123. doi: 10.1073/pnas.0900188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J., Wang L., Yan D., Lu M.Z. Research and application of transgenic poplar in China, in challenges and opportunities for the world’s forests in the 21st century. Springer Dordr. 2014;81:567–584. [Google Scholar]
- 28.Ramanathan T., Gurudeeban S., Satyavani K. In vitro plant regeneration from leaf primordia of gum-bearing tree Aegle marmelos. J. For. Res. 2010;4:208–212. doi: 10.3923/rjf.2010.208.212. [DOI] [Google Scholar]
- 29.Cavusoglu A., Ipekci-Altas Z., Bajrovic K., Gozukirmizi N., Zehir A. Direct and indirect plant regeneration from various explants of eastern cottonwood clones (Populus deltoides Bartram ex Marsh.) with tissue culture. Afr. J. Biotechnol. 2011;10:3216–3221. [Google Scholar]
- 30.Husaini A.M. Pre-and post-agroinfection strategies for efficient leaf disk transformation and regeneration of transgenic strawberry plants. Plant Cell Rep. 2010;29:97–110. doi: 10.1007/s00299-009-0801-4. [DOI] [PubMed] [Google Scholar]
- 31.Li B., Xie C., Qiu H. Production of selectable marker-free transgenic tobacco plants using a non-selection approach: Chimerism or escape, transgene inheritance, and efficiency. Plant Cell Rep. 2009;28:373–386. doi: 10.1007/s00299-008-0640-8. [DOI] [PubMed] [Google Scholar]
- 32.Cervera M., Pina J.A., Juarez J., Navarro L., Pena L. A broad exploration of a transgenic population of citrus: Stability of gene expression and phenotype. Theor. Appl. Genet. 2000;11:1683–1685. doi: 10.1007/s001220051338. [DOI] [Google Scholar]
- 33.Tang J., Scarth R., Fristensky B. Effects of genomic position and copy number of acyl-ACP thioesterase transgenes on the level of the target fatty acids in Brassica napus L. Mol. Breed. 2003;12:71–81. doi: 10.1023/A:1025495000264. [DOI] [Google Scholar]
- 34.Zheng L., Liu G., Meng X., Li Y., Wang Y. A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem. Genet. 2012;50:761–769. doi: 10.1007/s10528-012-9518-0. [DOI] [PubMed] [Google Scholar]
- 35.Gururaj H.B., Padma M.N., Giridhar P., Ravishankar G.A. Functional validation of Capsicum frutescens aminotransferase gene involved in vanillylamine biosynthesis using Agrobacterium mediated genetic transformation studies in Nicotiana tabacum and Capsicum frutescens calli cultures. Plant Sci. 2012;195:96–105. doi: 10.1016/j.plantsci.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Porebski S., Bailey L.G., Baum B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997;15:8–15. doi: 10.1007/BF02772108. [DOI] [Google Scholar]
- 37.Zhang D., Du Q., Xu B., Zhang Z., Li B. The actin multigene family in Populus: Organization, expression and phylogenetic analysis. Mol. Genet. Genom. 2010;284:105–119. doi: 10.1007/s00438-010-0552-5. [DOI] [PubMed] [Google Scholar]
- 38.Han Z.F., Hunter D.M., Sibbald S., Zhang J.S., Tian L. Biological activity of the tzs gene of nopaline Agrobacterium tumefaciens GV3101 in plant regeneration and genetic transformation. Mol. Plant Microbe Interact. 2013;26:1359–1365. doi: 10.1094/MPMI-04-13-0106-R. [DOI] [PubMed] [Google Scholar]