Abstract
Even though phenazines have been extensively reported as anticancer molecules, the molecular target of these compounds is severely lagging behind. Our study consequently focuses on the anticancer target of a phenazine analogue (CPUL1) for its potently antitumor activities in initial stage. Along with redox status courses of Hep G2 cells, thioredoxin reductase I (TrxR1) was speculated as anticancer target of CPUL1. By virtue of zymologic, immunological and molecular biological experiments, we demonstrated that TrxR1 could be the anticancer target of CPUL1. The knowledge on phenazine targeting to TrxR1 have not been reported previously. Thus, it can provide valuable information for further development of the TrxR1 inhibitors.
Keywords: Anticancer target, Hep G2, inhibitors, phenazines, thioredoxin reductase I
Introduction
Thioredoxin reductases (TrxR, EC 1.8.1.9) are dimeric flavoproteins belonging to the family of pyridine nucleotide-disulphide oxidoreductases, which is found in different types of cancer cells cytoplasm. TrxR overexpression is essential to maintain the phenotypes of cancer cells, and emerging evidence has demonstrated the physiological and pathological significance of TrxR1 in cellular redox signalling networks, which are involved in virtually all aspects of cell functions, such as differentiation, proliferation and death1. As a key member of the system, TrxR1 is the only known reductase to keep Trx1 in a reduced state, which is required for most functions of Trx1. Thus, the discovery of TrxR1 small molecular inhibitors is of importance leads to potential therapeutic agents that interfere with the cellular redox network2,3.
Phenazines, a large group of natural or synthetic nitrogen-containing heterocyclic compounds4,5, have been associated with anticancer activities since 19596. Previous work suggested that the anticancer modes of the existing analogues were reported as dual topoisomerase I/II7 and GSH depletion8, respectively. Besides those targets, the knowledge on modes of action and molecular targets of phenazine analogues are severely lagging behind9,10.
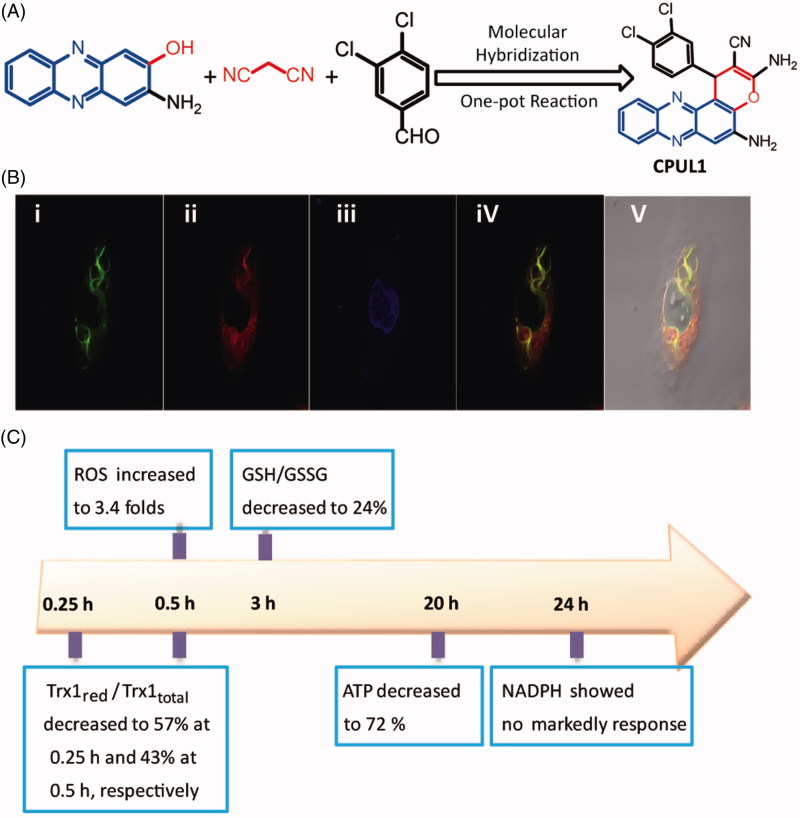
In our previous study, a preliminary mechanism research indicated that a phenazine analogue (Figure 1(A)), namely CPUL1, which demonstrated antitumor activities against both mouse liver carcinoma cell lines (H22–H8D8) implanted xenografted mice in vivo and human liver carcinoma cell line (Hep G2) in vitro, might be acting as dual topoisomerase I and II inhibitors and apoptosis activator11. Intriguingly, with our ongoing study on the detailed antitumor molecular mechanism of compound CPUL1, we found that the compound was prevailingly distributed thoroughly in Hep G2 cell plasma not in cytoplast (Figure 1(B)), which was confirmed by laser scanning confocal microscopy (LSCM). This freakishly phenomenon was distinguishing from typical topoisomerase I/II inhibitors, such as doxorubicin12, etoposide13 and 10-hydroxycamptothecin14, which were reported as locating at nucleus in cancer cell lines by LSCM methods. The discrepant results of CPUL1 between the LSCM and topoisomerase I/II inhibition experiments aroused a suspicion that the CPUL1 might not targeting to the topoisomerase I/II in Hep G2 cell lines. Considering the controversial role of the CPUL1 against Hep G2 cells, the target of CPUL1 against Hep G2 cells becomes the crux of the scene to be unveiled. Thus, we attempted to discover and identify the anticancer target of CPUL1 in this study.
Figure 1.
The preliminary experiment, including design strategy, LSCM and time course of the redox related key factor for investigating the target of CPUL1. (A) Design of ROS inducer molecule CPUL1 with molecular hybridization strategy. (B) The distribution of CPUL1 in the Hep G2 cells. Hep G2 cells were stained with 2 μM CPUL1, 0.1 μM Mito Tracker Red CMXROS, and 0.1 μ Dihydrochloride (DAPI) for 30 min. (i) Ex = 488 nm for CPUL1. (ii) Ex = 580 nm for Mito Tracker Red CMXROS. (iii) Ex = 360 nm for DAPI. (iv) Merged images of (i) and (iii) in dark field. (v) Merged images of (i) and (iii) in bright field. (C) A summary plot displays the time relationships between the Trx1red/Trx1total ratio, ROS levels, GSH/GSSG ratio, NADPH lifetimes and ATP contents in Hep G2 cells treated with 2 μM of CPUL1.
Materials and methods
The general procedures, the details concerning the experiment steps and the analytical data are provided in the Supplementary Material.
Results and discussion
Since we observed visible apoptosis of Hep G2 cells after treated with CPUL1, we sought to find clues from the process of redox status. We tested the time courses of redox related key factors in Hep G2 cells, among them ROS levels, GSH/GSSG ratios, NAPDH levels and ATP levels before and after treated with CPUL1 at different time, respectively (Figure 1(C) and Figures S1–S4, see Supplementary Material). In these results, most unexpectedly, the ROS levels were dramatically increased at the first 15 min (Listed in Figure 1(C) and Figure S1). However, NADPH (Figure S4) and ATP levels (Figure S2) did not show significant differences with control groups before 18 h, respectively. It is widely recognized that the depletion of NADPH and ATP is associated with the pace of apoptosis15,16. However, the stable NADPH and ATP levels in the first 4 h after treated with CPUL1 can deduce a result that ATP mediating the ROS produce process did rather not take place in HepG2 cells after treated by CPUL1. Combined the results of the redox related key factors time-course study, a conjectural apoptosis process was hypothesized as following: (1) CPUL1 could trigger apoptosis mainly through elevating the ROS level rather than inhibiting the topoisomerase I/II; and (2) deleting ROS function instead of accelerating ROS production might be inhibited by CPUL1 in apoptosis cells.
In mammalian cells, there are two major thiol-dependent antioxidant systems, the thioredoxin- (Trx) and the glutathione- (GSH) dependent enzyme systems which may act in concert17,18. In the next experiment, we tried to verify if there were significant differences between Trx1red/Trx1total and GSH/GSSG levels under treatment of CPUL1 in Hep G2 cell lines. Amazingly, Trx1red/Trx1total levels decreased to 57% at 0.25 h and 43% at 0.5 h (Figure 2(G)), whereas, GSH/GSSG ratios are markedly decreasing after 2 h (Figure S3), respectively. These results can be elucidated that the reductive Trx1 level decreased dramatically at the first 0.5 h, and the ROS level simultaneously increased by 3.4-folds, then the GSH compensation mechanism had come into force and decreased to 24% after 2 h. Harris18 and Mandal19 have also demonstrated homoplastically standpoint that the Trx1 and GSH can work synergistically as antioxidant roles, as long as the GSH metabolism could compensate the lack of reductive Trx1 in tumour cells.
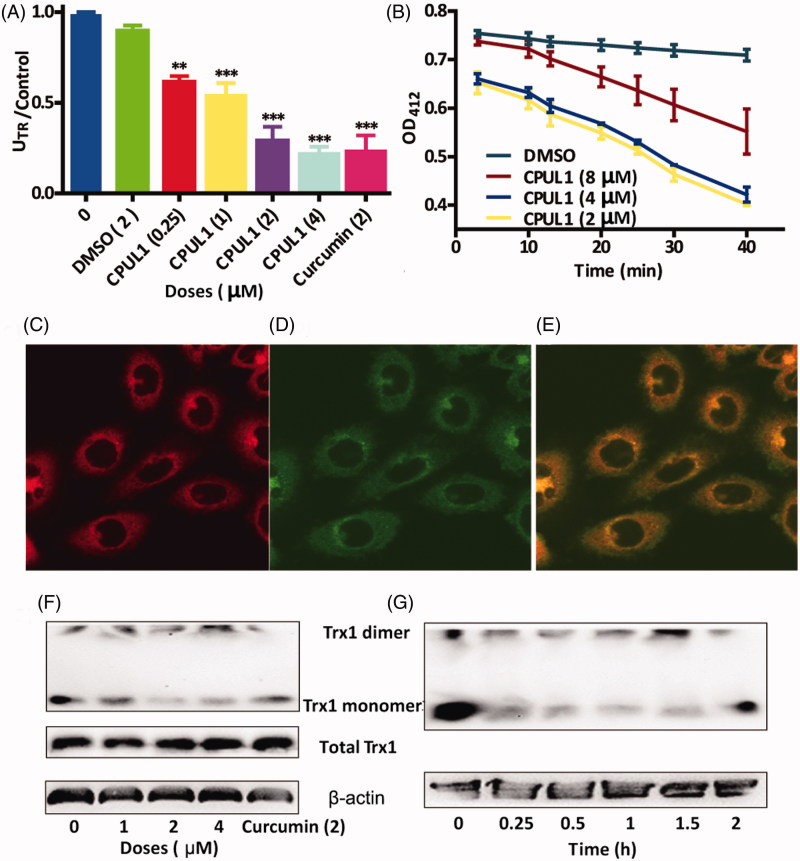
Figure 2.
The evidences for CPUL1 acted as TrxR1 inhibitors based on enzymatic reaction, immunofluorescence and non-reduced western blot. (A) TrxR1 activities vs control after treated with different doses of CPUL1 (0.25, 1, 2 and 4 μM) for 0.5 h. (B) The time course NADPH consumption after treat with different doses of CPUL1 (2, 4 and 8 μM) for (10, 20, 30 and 40 min), respectively. (C–E) The immune-fluorescence staining of Hep G2 after treated with TrxR1 antibody (C, 580 nm), CPUL1 (D, 448 nm) and merged images (E), respectively. (F, G) The non-reduced western blot assays of Trx1, including the Hep G2 cells were treated with CPUL1 at different doses (1, 2 and 4 μM) for 0.5 h (F) and treated with 4 μM CPUL1 for different time (0, 0.25, 0.5, 1, 1.5 and 2 h) (G), respectively. Curcumin used as positive control. Values represent the mean ± SD obtained from three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from the value of control (untreated).
In the following section, we focused on the crucial proteins in Trx enzyme system including TrxR1 and Trx1, which are widely scattered in cytoplasm of cells. We tried to detect the mRNA levels of TrxR1 and Trx1 treated with CPUL1 by RT-PCR, in order to see whether the gene expression of both proteins were disturbed by CPUL1 at the very beginning. The results showed that the mRNA levels of the two groups were not significantly different from those of the negative control group (the data were not shown).
The existing TrxR1 inhibitors18,20–22 had inspired us to detect the TrxR1 activities, including free-cell and in-cell experiments, after treated with CPUL1, respectively. Surprisingly, the CPUL1 could inhibit the TrxR1 activities in both dose- and time-dependent manners against negative and positive groups (Figure 2(A,B)), respectively. In free-cell experiment (Figure 2(A)), the TrxR1 activities was decreased to 19.5% against control groups after treated with 4 μM CPUL1. In the time-course experiment, we chosen the in-cell experiment to detect the variation of TrxR1 activities along with time of CPUL1 treament, in which the consumption of NADPH was in direct proportion to the activities of TrxR123. Figure 2(B) demonstrated time course of NADPH consumption (OD412 nm) in the biomedical reaction system, in which we could see that the OD412 nm of NADPH was dramatically decreased along with the doses of CPUL1 decreased from 2 to 8 μM; it simultaneously means that the TrxR1 reductive activites was decreased along with the increased doses of CPUL1.
Secondly, the immunofluorescence staining experiment was performed to prove the combination of CPUL1 and TrxR1, in which the TrxR1 was traced by its fluorescent antibody by incubated with Hep G2 cells for 2 h. Figure 2(C,D) indicated the fluorecence of TrxR1 antibody and CPUL1 in the Hep G2 cells at absorption of 580 and 448 nm, respectively. When combining Figure 2(C,D) together in Figure 2(E), we can see that the fluorescence of TrxR1 antibody was perfectly merged with CPUL1, which adamantly suported the evidence of that CPUL1 could bind to TrxR1 in the cells.
Lastly, Trx1, the crucial downstream protein of TrxR1, was further investigated to evidence the TrxR1 was inhibited by the compound. To the best of our knowledge, the oxidative status of Trx1, the downstream protein of TrxR1, could be demonstrated by non-reducing westernblot24. As we can see from Figure 2(F,G), the total Trx1 protein expression showed no significant difference between the treated groups and untreated groups, so total Trx1 of the HepG2 cells were not affected by treatment of the CPUL1; however, the monomer of Trx1 ratio was decreased and Trx1 dimer ratio was increased after treated with CPUL1 in both dose- and time-dependent fashion against negative and positive control groups (Figure 2(F,G)), respectively. The non-reduced western blot straitforwardly demonstrated that the oxidative status of Trx1 was increased after treated with CPUL1. These results indicated that redox homeostasis of the Hep G2 cells was broken to trigger the apoptosis of the cancer cells.
The TrxR1 activties might be inhibited at the very beginning, then the preponderance of Trx1, the crucial downstream protein of TrxR1, was following transfer to oxidative status, which disequilibrated redox status of the Hep G2 cells. In order to figure out the anti-proliferation funtion of the CPUL1 in the HepG2 cells, the downstream protein molecules of the Trx1 were further investigated in the following western blot experiments (Figure 3(A)).
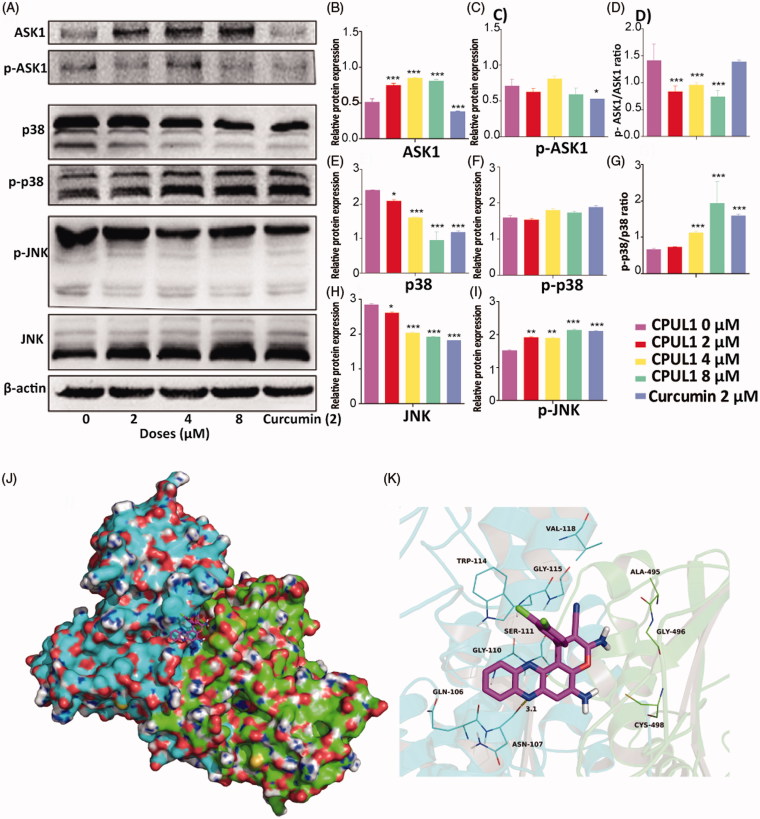
Figure 3.
The results of Western blotting key ROS stress proteins and CPUL1 docked into the binding site of the TrxR1. (A) Western blotting results of ASK1, p-ASK1, p38, p-p38, p-JNK and JNK after treated by different doses of CPUL1 (2, 4 and 8 μM). Curcumin used as positive control. (B–I) The levels of proteins in (A) were normalized to β-actin. Values represent the mean ±SD obtained from three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from the value of control (untreated). (J) Total view of CPUL1 was docked into the binding site of the TrxR1. (K) Detailed view of CPUL1 was docked into the binding site of the TrxR1.
At the begining of the protein profiles of the downstream protein molecules, ASK1, a member of mitogen-activated protein kinase (MAPK) family, was chosen as the first molecule to be investigated, which is not only combined to reduce Trx1 directly but also can induce apoptosis in cancer cells at free molecule status25. Since reduced Trx1, but not the oxided Trx1 is known to bind with ASK-1 to repress its activities26, we firstly obtained the potein levels of ASK-1 before and after treated with CPUL1 by western blot analysis. In agreement with this, increased levels (1.6 folds) of ASK1 were found in HepG2 cells after exposed to 4 µM of CPUL1 for 24 h (Figure 3(A,B)), which is consistent with the increased oxidation levels of Trx1 previously observed in Figure 2(D).
Next, we tried to detect the phosphorylation levels of ASK1 which could indicate ASK1 activities in the cells, wheras the phosphorylation levels of ASK1 did not show significant difference versus negative control groups (Figure 3(A,C)). However, to our surprise, the pASK1/ASK1 ratio decreased to 68% after incubated 4 µM of CPUL1 after 24 h compared to negative control groups (Figure 3(D)), implying that the activities of ASK1 were engaged after exposed to CPUL1.
Then, JNK and p38, the downstream proteins of ASK1 in mammalian cells27, were selected to gain further insight into the molecular mechanism of anti-tumor activity of CPUL1 in the following western blotting experiments, respectively. Our results showed that 4 µM of CPUL1 activated the phosphorylation of JNK (1.3-folds vs. blank control) and decreased JNK levels to 29% in concentration-dependent manner (Figure 3(A,H and I)), respectively. The expression levels of p38 were decreased to 67% comparing to blank control groups (Figure 3(E)), wheras the phosphorylation of p38 (Figure 3(F)) did not show significant differences between the experiment groups and blank control groups. However, the ratio of p-p38/p38 were increased singnificantly along with the doses of CPUL1 rising (Figure 3(G)). These results suggested that the ROS levels fortified by the CPUL1 appeared to enhance JNK and p38 signals to sensitize Hep G2 cell apoptosis.
We and others28 have implicated JNK and p38 MAPK pathway in the apoptotic effects in many human cancer cells, which were similarly caused by TrxR inhibition. Besides that, Al-Gayyar29 demonstrated that up-regulation of thioredoxin interacting protein (TXNIP), an endogenous inhibitor of Trx, could decrease Trx1 activities and activate the pro-apoptotic p38 MAPK/JNK pathway. Combined above results of the protein profiles, these effects implied that the anticancer mechanism phenomena of CPUL1 is identical to those existing TrxR1 inhibitors26,28.
From above evidences, we preliminarily verified the CPUL1 could combine with TrxR1 and inhibit it at enzymes levels, cell levels and molecular levels, respectively. As far as we known, there are three main classes of TrxR1 inhibitors according to past research results30. The first class of TrxR1 inhibitors is binding to Sec-498 in TrxR1, such as Auranofin18 and iron(II) complexes22. The second type of TrxR inhibitors, for instance Curcumin21 and Brevetoxin 220, can attack both Sec-498 and Cys-58 active sites of TrxR. 4-hydroxy-2-nonenal31, quinols32 and Se compounds33, the third genre of TrxR inhibitors, could inhibit TrxR as well as Trx without selectivities.
In order to investigate the binding mode between CPUL1 and TrxR1, the theoretical binding mode of CPUL1 in the binding site of the TrxR1 was illustrated in Figure 3(J,K). CPUL1 adopted a compact conformation to bind in the site of the TrxR1. CPUL1 was located at the hydrophobic pocket, surrounded by the residues Trp-114, Val-118, Ala-495 and Sec-498, forming a stable hydrophobic binding. Detailed analysis showed that the 3,4-dichlorophenyl group of CPUL1 formed CH–π interaction with the residue Trp-114. One of the chlorine atom of CPUL1 formed Cl–π interaction with the residue Trp-114. Importantly, one hydrogen bond was observed between the CPUL1 and residue Asn-107, with bond lengths of 3.1 Å, which was the main interactions between the CPUL1 and the TrxR1. To our surprise, the key double bond was oriented to the residue Sec-498, which was convenient for the selenocysteine to be attacked. All these interactions helped CPUL1 to anchor in the binding site of the TrxR1. The above molecular simulations gave us rational explanation of the interactions between CPUL1 and TrxR1, which provided valuable information for further development of the TrxR1 inhibitors.
All together, with the time course of the redox related key factor strategy, we disclosed phenazine analogue CPUL1 might target to the TrxR1, which was further evidenced by zymological, immunological and molecular biological experiments. To the best of our knowledge, phenazine analogues have not been reported as TrxR1 inhibitor in previous study. Lastly, we tried to cope with the characteristic of the combination between CPUL1 and TrxR1, and the results showed that CPUL1 was formed a stable hydrophobic domain between the residues Trp-114, Val-118, Ala-495 and Sec-498, which is valuable for further development of the TrxR1 inhibitors.
Supplementary Material
Funding Statement
The study received financial support from National Key R&D Program of China (2018YFC0311003), the Project Program of National Nature Science Foundation of China (Grant No. 81872757), the Project Program of Nature Science Foundation of Jiangsu Province of China for Young Scientists (Grant No. BK20130648), the Priority Academic Program Development of Jiangsu Higher Education Institution and Qing Lan Project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Zhang J, Li X, Han X, et al. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci 2017;38:794–808. [DOI] [PubMed] [Google Scholar]
- 2.Stafford WC, Peng X, Olofsson MH, et al. Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy. Sci Transl Med 2018;10:eaaf7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Zhang J, Peng S, et al. Thioredoxin reductase inhibitors: a patent review. Expert Opin Ther Pat 2017;27:547. [DOI] [PubMed] [Google Scholar]
- 4.Laursen JB, Nielsen J. Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev 2004;104:1663–86. [DOI] [PubMed] [Google Scholar]
- 5.Price-Whelan A, Dietrich LEP, Newman DK. Rethinking 'secondary' metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2006;2:71–8. [DOI] [PubMed] [Google Scholar]
- 6.Pierson IIS, Pierson AE. Metabolism and function of phenazines in bacteria: impacts on the behaviour of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 2010;86:1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamage SA, Spicer JA, Rewcastle GW, et al. Structure − activity relationships for pyrido-, imidazo-, pyrazolo-, pyrazino-, and pyrrolophenazinecarboxamides as topoisomerase-targeted anticancer agents. J Med Chem 2002;45:740–3. [DOI] [PubMed] [Google Scholar]
- 8.Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic Biol Med 2002;33:1527–33. [DOI] [PubMed] [Google Scholar]
- 9.Cimmino A, Evidente A, Mathieu V, et al. Phenazines and cancer. Nat Prod Rep 2012;29:487–501. [DOI] [PubMed] [Google Scholar]
- 10.Guttenberger N, Blankenfeldt W, Breinbauer R. Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg Med Chem 2017;25:6149–66. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Yan Y, Wang L, et al. Design, facile synthesis and biological evaluations of novel pyrano[3,2-α]phenazine hybrid molecules as antitumor agents. Eur J Med Chem 2017;127:928–43. [DOI] [PubMed] [Google Scholar]
- 12.Shen Z, Chen T, Ma X, et al. Multifunctional theranostic nanoparticles based on exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging and chemotherapy. ACS Nano 2017;11:10992–1004. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Xie X, Zhou Q, et al. EGF-functionalized single-walled carbon nanotubes for targeting delivery of etoposide. Nanotechnology 2012;23:045104. [DOI] [PubMed] [Google Scholar]
- 14.Wu W, Li R, Bian X, et al. Covalently combining carbon nanotubes with anticancer agent: preparation and antitumor activity. ACS Nano 2009;3:2740–50. [DOI] [PubMed] [Google Scholar]
- 15.Wang HW, Wei YH, Guo HW. Reduced nicotinamide adenine dinucleotide (NADH) fluorescence for the detection of cell death. Anticancer Agents Med Chem 2009;9:1012–7. [DOI] [PubMed] [Google Scholar]
- 16.Yu JS, Guo HW, Wang HW, et al. Increase of reduced nicotinamide adenine dinucleotide fluorescence lifetime precedes mitochondrial dysfunction in staurosporine-induced apoptosis of HeLa cells. J Biomed Optics 2011;16:036008. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 2014;66:75–87. [DOI] [PubMed] [Google Scholar]
- 18.Harris IS, Treloar AE, Inoue S, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cells 2015;27:211–22. [DOI] [PubMed] [Google Scholar]
- 19.Mandal PK, Schneider M, Kolle P, et al. Loss of thioredoxin reductase 1 renders tumors highly susceptible to pharmacologic glutathione deprivation. Cancer Res 2010;70:9505–14. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Tuladhar A, Rolle S, et al. Brevetoxin-2, is a unique inhibitor of the C-terminal redox center of mammalian thioredoxin reductase-1. Toxicol Appl Pharm 2017;329:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 2005;280:25284–90. [DOI] [PubMed] [Google Scholar]
- 22.Xie LN, Luo ZD, Zhao ZN, Chen TF. Anticancer and antiangiogenic iron (ii) complexes that target thioredoxin reductase to trigger cancer cell apoptosis. J Med Chem 2017;60:202–14. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Sandalova T, Lindqvist Y, Arnér E. Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J Biol Chem 2009;284:3998–4008. [DOI] [PubMed] [Google Scholar]
- 24.Cox AG, Brown KK, Arner ESJ, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharm 2008;76:1097–109. [DOI] [PubMed] [Google Scholar]
- 25.Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal 2009;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branco V, Coppo L, Solá S, et al. Impaired cross-talk between the thioredoxin and glutathione systems is related to ASK-1 mediated apoptosis in neuronal cells exposed to mercury. Redox Biol 2017;13:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 2010;429:403–17. [DOI] [PubMed] [Google Scholar]
- 28.Zeng XS, Ji JJ, Kwon Y, et al. The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radic Biol Med 2014;67:10–8. [DOI] [PubMed] [Google Scholar]
- 29.Al-Gayyar MMH, Abdelsaid MA, Matragoon S, et al. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br J Pharmacol 2011;164:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Zhang B, Li X, et al. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: an update. Med Res Rev 2019;39:5–39. [DOI] [PubMed] [Google Scholar]
- 31.Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc 2006;128:1879–85. [DOI] [PubMed] [Google Scholar]
- 32.Chew EH, Lu J, Bradshaw TD, Holmgren A. Thioredoxin reductase inhibition by antitumor quinols: a quinol pharmacophore effct correlating to anti proliferative activity. FASEB J 2008;22:2072–83. [DOI] [PubMed] [Google Scholar]
- 33.Benstoem C, Goetzenich A, Kraemer S, et al. Selenium and its supplementation in cardiovascular disease: what do we know? Nutrients 2015;7:3094–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.