Abstract
A growing body of evidence supports a role for tissue-to-diet 15N and 13C discrimination factors (Δ15N and Δ13C), as biomarkers of metabolic adaptations to nutritional stress, but the underlying mechanisms remain poorly understood. In obese rats fed ad libitum or subjected to gradual caloric restriction (CR), under a maintained protein intake, we measured Δ15N and Δ13C levels in tissue proteins and their constitutive amino acids (AA) and the expression of enzymes involved in the AA metabolism. CR was found to lower protein mass in the intestine, liver, heart and, to a lesser extent, some skeletal muscles. This was accompanied by Δ15N increases in urine and the protein of the liver and plasma, but Δ15N decreases in the proteins of the heart and the skeletal muscles, alongside Δ13C decreases in all tissue proteins. In Lys, Δ15N levels rose in the plasma, intestine, and some muscles, but fell in the heart, while in Ala, and to a lesser extent Glx and Asx, Δ13C levels fell in all these tissues. In the liver, CR was associated with an increase in the expression of genes involved in AA oxidation. During CR, the parallel rises of Δ15N in urine, liver, and plasma proteins reflected an increased AA catabolism occurring at the level of the liver metabolic branch point, while Δ15N decreases in cardiac and skeletal muscle proteins indicated increased protein and AA catabolism in these tissues. Thus, an increased protein and AA catabolism results in opposite Δ15N effects in splanchnic and muscular tissues. In addition, the Δ13C decrease in all tissue proteins, reflects a reduction in carbohydrate (CHO) oxidation and routing towards non-indispensable AA, to achieve fuel economy.
Keywords: caloric restriction, obesity, amino acid oxidation, dietary nutrient routing, 13C and 15N natural isotope abundance
1. Introduction
Natural abundances of the stable carbon and nitrogen heavy isotopes (δ13C and δ15N) have been used for decades, in ecological studies, to assess the positions of different organisms in the food chain, and more recently in nutritional epidemiology, as biomarkers of dietary exposure [1,2,3,4,5,6]. Their utilization is based on the observation that the isotopic composition of the body reflects that of the diet, plus a small fractionation factor called the trophic step or discrimination factor, which on average is equal to 1‰ for 13C and 3–4‰ for 15N [7]. This 13C and 15N bioaccumulation is mostly due to discrimination against these heavy isotopes during the formation of respiratory CO2 (by carbonic anhydrase [8]) and N waste (particularly during urea production by AA deamination and oxidation [9]), along with marked variations in δ13C and δ15N values, between different metabolic pools and tissues [1,7,10,11]. The variability of δ13C within the body, is due to the differential routing of macronutrients, which generally have distinct δ13C values, to different tissues and metabolic pools [12,13]. Using a multi-compartmental analysis of multi-pool and multi-tissue δ15N measurements in rats, we previously showed that 15N bioaccumulation and δ15N variability within the body actually result from isotope effects occurring in multiple metabolic pathways, including AA oxidation and protein turnover [10].
A growing body of evidence suggests that beyond their usefulness as dietary proxies, δ13C and δ15N also vary between subjects, depending on their particular metabolic status, orientations or imbalances [10,14,15]. In particular, studies in different species have suggested that δ15N reflects fluctuations of the nitrogen balance induced by nutritional stress, with δ15N increasing in tissues and urine during fasting periods or episodes of negative nitrogen or energy balances [16,17,18,19]. In humans, such increases have been traced in the hair stem during nutritional stress. Fuller et al. showed that in pregnant women, weight loss due to morning sickness could be traced by an increase in δ15N values in the hair [20]. Similarly, hair δ15N levels rise during episodes of starvation in patients suffering from anorexia nervosa, and fall during nutritional rehabilitation [21,22]. Fewer data are available regarding δ13C variations during metabolic stress, but studies in humans and mice have suggested lower levels of δ13C in exhaled breath and hair, during catabolic episodes induced by fasting, starvation, or acute inflammation [22,23,24]. Taken together, these data suggest that δ13C and δ15N might represent biomarkers of the catabolic states.
However, the mechanisms involved in the increases in δ15N and decreases in δ13C during nutritional stress and caloric restriction (CR) remain poorly understood. It has been suggested that during CR-induced protein losses, increased tissue proteolysis could result in an increased contribution of 15N-enriched endogenous AA to the free AA pool used for protein synthesis or ureogenesis, thus leading to a further 15N enrichment of tissue proteins and urine [25]. Alternatively, the increase in δ15N might also result from CR-induced activation of the metabolic pathways of AA transamination and deamination, which favor 14N elimination from body AA [10]. Regarding δ13C, its CR-induced decrease could result from the increased oxidation of 13C-depleted body lipids, leading to a 13C depletion of respiratory CO2 and the keto-acid precursors used for AA synthesis [24,26]. However, because most of the aforementioned studies are observational, with little or no control of the isotopic composition of the diet [16,17,20,21,22,26], changes to δ15N and δ13C values in tissues and urine might also partly reflect their changes in the diet. Experimental studies under strictly controlled nutritional conditions and a constant dietary isotopic environment are, therefore, necessary to understand how tissue and urinary δ15N and δ13C are affected by nutritional stress.
Based on data from the literature and on our previous studies [10,27], we hypothesized that δ13C and δ15N in tissue protein, respectively, reflect the relative contribution of macronutrients for tissue AA synthesis and the relative orientation of tissue AA towards oxidations versus protein synthesis, which are both expected to change during CR. To test this hypothesis, we investigated the CR-induced tissue protein δ15N and δ13C variations, in rats maintained under strictly controlled conditions of dietary intake and isotopic exposure. To gain further insight into the underlying mechanisms, we investigated the relationships between δ15N and protein mass variations in different tissues, and measured the expression of genes coding for the enzymes involved in AA metabolism that might affect their δ15N.
2. Materials and Methods
2.1. Diet and Animals
A detailed description of the study design can be found in the article by Galmiche et al. [28]. This study was approved by the local ethical committee for animal experiment (COMETHEA) and authorized by the office for animal experimentation from the French Ministry of Research (authorization # 00734.01). Briefly, 24-week old male Wistar rats weighing 489 ± 6 g were individually housed, acclimatized for one week, and then randomly allocated to four groups, which were fed different sources of lipids (high-oleic sunflower oil, rapeseed oil, or fish oil). In this ancillary study, we only considered the two groups of rats that received high-oleic sunflower oil.
During the first 4-week induction period, rats from both groups had ad libitum access to the same high-fat induction diet containing (as energy) 16% protein, 32% carbohydrate (CHO), and 52% lipids. Thereafter, rats from the first group continued to have ad libitum access to food for eight consecutive weeks (ad libitum (AL) group, n = 11), while those of the second group were subjected to gradual CR as follows—75% of the spontaneous energy intake measured during the last week of the induction period for one week, 60% for the next two weeks, and 50% for the remaining five weeks (CR group, n = 12). All diets were customarily prepared by the local experimental feed preparation unit (UPAE, INRA). CR was achieved by adjusting both the amount of food supplied to the rats, according to their body weight, and the composition of the diet, keeping the same contribution of lipids to dietary energy throughout the experiment (~55%, as in the high-fat induction diet), but increasing the protein contribution during the gradual CR (from 21% to 30%) (Table 1). Under these conditions, the daily intake of micronutrients and protein (0.56 g/100 g body weight) remained constant throughout the experiment.
Table 1.
Composition of the experimental diets.
| Composition | Caloric Restriction Diets * | |||
|---|---|---|---|---|
| Ad libitum Diet | 75% Restriction | 60% Restriction | 50% Restriction | |
| Nutrients composition (% of diet weight) | ||||
| Casein HCl | 19.4 | 25.6 | 32.4 | 35.4 |
| L-Cystine | 0.3 | 0.3 | 0.4 | 0.4 |
| Corn starch | 25.5 | 20.1 | 14.1 | 11.6 |
| Sucrose | 14.4 | 11.4 | 8.0 | 6.5 |
| High-Oleic sunflower oil | 27.0 | 27.1 | 27.2 | 27.2 |
| Soybean oil | 2.0 | 2.1 | 2.2 | 2.3 |
| AIN 93N mineral mix | 4.8 | 6.3 | 8.0 | 8.7 |
| AIN 93VX vitamin mix | 1.4 | 1.8 | 2.3 | l2.5 |
| Alpha-cellulose | 5.0 | 5.0 | 5.0 | 5.0 |
| Choline bitartrate | 0.2 | 0.3 | 0.4 | 0.4 |
| Energy composition (% of diet energy) | ||||
| Protein | 16 | 21 | 27 | 30 |
| CHO | 32 | 26 | 18 | 15 |
| Lipids | 52 | 53 | 55 | 55 |
| Isotope composition (‰) ** | ||||
| δ15N | 5.8 | 5.8 | 5.8 | 5.8 |
| δ13C | −22.3 | −22.6 | −22.8 | −23.0 |
* The ad libitum diet was given during the first 4-week period of obesity induction in all rats, and then during the consecutive 8-week period in half of the rats (AL group, n = 11) while the other half (CR group, n = 12) were subjected to gradual caloric restriction, receiving a 75% restricted diet for 1 week, a 60% restricted diet for the next 2 weeks, and a 50% restricted diet for the remaining 5 weeks. ** The diets comprised proteins with a δ15N of 5.8‰ and a δ13C of −22.1‰, carbohydrates, with a δ13C of −15.7‰ and lipids with a δ13C of −29.1‰. In order to account for the preferential routing of dietary protein carbon to tissue protein carbon, the global δ13C values of the diets were calculated by considering that the dietary proteins supply 75% of the tissue protein carbon, and the remaining 25% come from non-protein macronutrients [12,13].
Food intake and body weight were monitored on a weekly basis throughout the study. At completion of the ad libitum/restriction period, the rats were fasted overnight, deeply anesthetized by isoflurane, and euthanized by exsanguination. Urine was collected from the bladder and plasma was prepared by centrifugation from blood collected on heparin. The following organs and tissues were dissected, weighed, and snap-frozen in liquid nitrogen—small intestine, liver, heart, hind-limb skeletal muscles (gastrocnemius, soleus and tibialis anterior), epididymal adipose tissue, and subcutaneous adipose tissue. All samples were stored at –80 °C until subsequent analysis.
2.2. Sample Preparation
Protein fractions were prepared from frozen tissues and plasma using sulfosalicylic acid precipitation and delipidation, as previously described [27].
To analyze protein-bound AA in tissues, samples were prepared at the UC Davis stable isotope facility, as previously described [29]. Briefly, 5–10 mg of delipidated tissue or dietary protein were hydrolyzed with 6 M HCl at 150 °C, for 70 min, under nitrogen. After hydrolysis, the samples were dried in a heating block at 60 °C, under a stream of nitrogen, and were re-suspended in 200 µL 0.1 M HCl. Methoxycarbonyl AA methyl esters (AA-MCF) were prepared by reacting 100 µL of the AA solution and 20 µL of the internal reference (norleucine) with 15 µL methyl chloroformate. AA-MCF were extracted in 100 µL chloroform and water traces were removed with 0.1 mg of sodium sulfate.
2.3. Elemental and Isotope Determinations
The nitrogen and carbon elemental and isotope compositions of the dietary macronutrients (protein, CHO, and lipids), urine, and delipidated protein fraction from the sampled tissues, were measured by elemental analysis/isotope ratio mass spectrometry (EA-IRMS), using an elemental analyzer (EA Vario Micro Cube, Elementar, Germany) coupled with an isotope-ratio mass spectrometer (Isoprime, VG instruments, Manchester, UK). Tyrosine was used for calibration and drift correction. The compound-specific isotopic composition of AA-MCF from tissue proteins were measured by the gas-chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS), at the UC Davis stable isotope facility, as described by Walsh et al. [29]. AA-MCF were separated on an Agilent DB-23 column (30 m × 0.25 mm ID, 0.25 µm film thickness) and converted to CO2 and N2 in a combustion reactor, at 1000 °C. Water was removed through a nafionTM dryer and the isotope ratios of N2 and CO2 were measured using a Delta V Advantage isotope ratio mass spectrometer (Thermofisher, Waltham, MA, USA). The isotopic calibration of AA-MCF was performed using the internal standard, norleucine.
The natural abundances of 15N and 13C in the samples were expressed, relative to the standards (atmospheric N2 for 15N/14N and Vienna Pee Dee Belemnite for 13C/12C, respectively), using the delta notation, according to the following equation: δ (‰) = 1000 × (Rsample − Rstandard)/Rstandard where δ is the parts-per-thousand difference in ratio between heavy and light isotopes (15N/14N and 13C/12C), in the samples (Rsample) and the standards (Rstandard).
Typical replicate measurement errors for the δ15N and δ13C values of the standards were 0.1‰ in EA-IRMS and 0.3–1.6‰, depending on individual AA, in GC-C-IRMS.
Discrimination factors (Δ15N and Δ13C) for a given tissue protein or its individual AA were calculated by subtracting the δ15N and δ13C values of the dietary protein or its individual AA from the δ15N and δ13C values of the tissue protein or its individual AA, respectively (for X = 15N or 13C, ∆Xtissue proteins = δXtissue proteins − δXdiet proteins, ∆Xtissue AA = δXtissue AA − δXdiet AA). The δ15N and δ13C values of the dietary CHO, lipid, protein, and protein-bound individual AA used to calculate the discrimination factors are presented in Supplementary Table S1.
2.4. Gene Expression
The expression of genes coding for enzymes involved in amino acid oxidation was determined in the liver using quantitative reverse-transcription polymerase chain reaction (Q-RT-PCR). Total RNA was extracted from liver samples (50 mg), using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and complementary DNA (cDNA) was synthetized as previously described [28].
The target genes were those involved in nitrogen transfer between AA and in AA oxidation—glutamate-pyruvate-transaminase (Gpt 1), arginase 1 (Arg1), glutaminase (Gls2), histidase (Hal), tyrosine amino-transferase (Tat), serine/threonine deaminase (Sds), ornithine aminotransferase (Oat), branched chain ketoacid dehydrogenase α-polypeptide (Bckdha), asparagine synthetase (Asns), aminoadipate–semialdehyde synthase (Aass), and pyrroline-5-carboxylate reductase (Pycr2). The primers (Supplementary Table S2) were used for quantitative PCR on a 7300 real-time PCR system, as previously described [28]. Gene expression levels were calculated using the 2-ΔCT formula with 18S mRNA as the reference gene, and expressed as a percentage of the expression level measured in the AL group.
2.5. Statistical Analysis
All results were expressed as means ± standard error. Statistical analysis was performed using analysis of variance with the group and tissue, as fixed effects, and rat as a random factor (GLM procedure, SAS 9.1, SAS Institute, Cary, NC, USA). For multiple Pearson’s correlations and post-hoc comparisons, the false discovery rate (FDR) was controlled at 10%, using the Benjamini–Hochberg procedure [30].
3. Results
3.1. Body Weight and Composition
As expected from randomization, weight gain during the first ad libitum feeding period was similar in both groups (data not shown), with the animals weighing 566 ± 8 g at the end of this first period of obesity induction. During the second period, rats fed ad libitum (AL group) continued to put on weight, while the restricted rats (CR group) experienced a ~17% reduction in body weight, which by the end of the experiment resulted in a ~20% lower body weight, among CR rats, compared to AL rats (Table 2).
Table 2.
Effect of caloric restriction on body weight and composition.
| AL (n = 11) | CR (n = 12) | CR vs. AL | |||
|---|---|---|---|---|---|
| Whole body weight (g) | 602 ± 20 | 482 ± 10 | −21% | ** | |
| Adiposity (%) | 29.0 ± 0.7 | 23.6 ± 0.7 | −19% | ** | |
| Epididymal adipose tissue | Tissue mass (g) | 26.7 ± 1.5 | 15.1 ± 1.1 | −43% | ** |
| Protein mass (mg N) | 15.3 ± 0.8 | 10.4 ± 1.0 | −32% | ** | |
| Subcutaneous adipose tissue | Tissue mass (g) | 148.1 ± 8.3 | 99.4 ± 4.3 | −33% | ** |
| Protein mass (mg N) | 71.0 ± 6.1 | 52.0 ± 4.6 | −27% | * | |
| Small Intestine | Tissue mass (g) | 6.43 ± 0.21 | 5.15 ± 0.10 | −20% | ** |
| Protein mass (mg N) | 173.7 ± 6.0 | 142.4 ± 4.9 | −18% | ** | |
| Liver | Tissue mass (g) | 12.71 ± 0.57 | 11.14 ± 0.29 | −12% | * |
| Protein mass (mg N) | 507.5 ± 20.8 | 422.9 ± 13.9 | −17% | ** | |
| Heart | Tissue mass (g) | 1.07 ± 0.03 | 0.92 ± 0.02 | −14% | ** |
| Protein mass (mg N) | 35.1 ± 1.3 | 29.1 ± 0.7 | −17% | ** | |
| Gastrocnemius muscle | Tissue mass (g) | 5.57 ± 0.08 | 5.31 ± 0.13 | ||
| Protein mass (mg N) | 190.0 ± 3.8 | 176.6 ± 3.4 | −7% | * | |
| Tibialis anterior muscle | Tissue mass (g) | 1.80 ± 0.04 | 1.71 ± 0.04 | ||
| Protein mass (mg N) | 60.4 ± 1.5 | 59.0 ± 1.6 | |||
| Soleus muscle | Tissue mass (g) | 0.39 ± 0.01 | 0.36 ± 0.01 | ||
| Protein mass (mg N) | 14.3 ± 0.5 | 12.8 ± 0.4 | −10% | * | |
| Total | Tissue mass (g) | 202.7 ± 9.9 | 139.1 ± 5.5 | −31% | ** |
| Protein mass (mg N) | 1067 ± 30 | 905 ± 19 | −15% | ** | |
Data are means ± standard error for rats with ad libitum access to food (AL, n = 11) or those subjected to gradual caloric restriction (CR, n = 12). Adiposity (%) is the weight of adipose tissues (epidydimal and subcutaneous) divided by the whole-body weight. For bilateral tissues (muscles and epididymal and subcutaneous fat), both left and right tissues were weighed. ** and * indicate significant differences between groups at p < 0.01 and p < 0.05, respectively, when controlling the false discovery rate at 10%.
This lower whole-body weight in CR compared to AL rats was mainly due to a lower adipose tissue mass (−34% for the sum of epidydimal and subcutaneous adipose tissues) and, thus, lower adiposity (−19% for the adipose tissue mass divided by the whole-body weight). To a lesser degree, it was also associated with a lower protein mass in the small intestine, liver, and heart (−17%). In skeletal muscles, the differences were even smaller, with a protein mass that was only 10% lower in the oxidative soleus muscle and 7% in the mixed (both oxidative and glycolytic) gastrocnemius muscle, while no difference was observed for the glycolytic tibialis anterior muscle.
3.2. Δ15N Values in Tissue Protein and AA
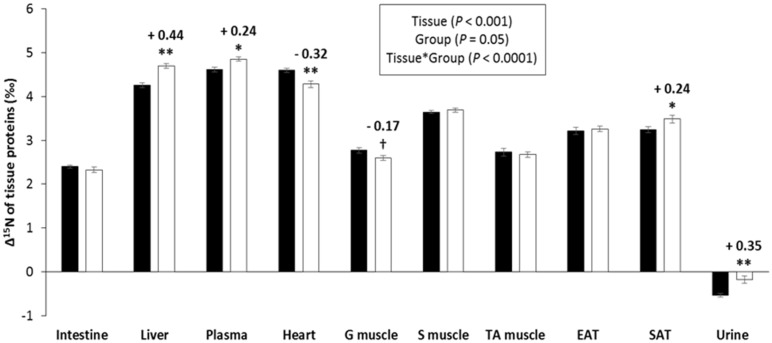
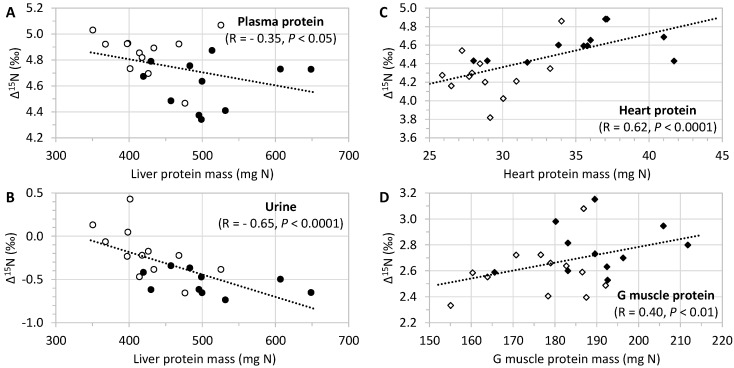
The proteins in different tissues were 15N-enriched versus dietary protein, with a mean Δ15N of 3.52 ± 0.06‰. Body protein Δ15N varied considerably between tissues, being higher in the liver, plasma, and the heart (4.4‰ to 4.7‰) than in skeletal muscles and adipose tissue (2.7‰ to 3.7‰), and in the intestine (2.4‰) (Figure 1). Unlike tissue protein, the urine was slightly but significantly 15N-depleted, compared to dietary protein (Δ15N = −0.34 ± 0.06‰). CR was associated with contrasting effects on tissue Δ15N, with a significant rise in levels in the liver, plasma, and subcutaneous adipose tissue proteins, as well as in urine (+0.24‰ to +0.44‰), and on the contrary, a significant fall in heart proteins (−0.32‰) and a marginally significant (p = 0.074) fall in gastrocnemius muscle proteins (−0.17‰), while no change was observed in other skeletal muscles (soleus and tibialis anterior), the small intestine or epididymal adipose tissue proteins (Figure 1). When the AL and CR rats were considered together, we found negative associations between the liver protein mass and Δ15N levels in the urine and plasma proteins (−0.65 ≤ R ≤ −0.35), and on the contrary, positive associations were found between the muscle protein mass and Δ15N levels that were highly significant (p < 0.01) in cardiac and gastrocnemius muscles (0.40 ≤ R ≤ 0.62), but were only marginally significant in the soleus muscle (R = 0.27, p = 0.077) (Figure 2).
Figure 1.
Effect of caloric restriction on the natural abundances of nitrogen stable isotopes in tissue proteins (Δ15Ntissue protein = δ15Ntissue protein − δ15Ndiet protein, ‰). Results are means ± standard error for rats with ad libitum access to food (AL group, ■, n = 11) or subjected to gradual energy restriction (CR group, □, n = 12). G—gastrocnemius; S—soleus; TA—tibialis anterior; EAT—epididymal adipose tissue; SAT—subcutaneous adipose tissue. p values in the box refer to the main effects and their interaction in the analysis of variance. **, *, and + indicate significant group effects at p < 0.01, p < 0.05, and p < 0.10, respectively, when controlling for the false discovery rate at 10%.
Figure 2.
Associations between tissue protein masses and natural abundances in nitrogen stable isotopes (Δ15Ntissue protein = δ15Ntissue protein − δ15Ndiet protein, ‰) in metabolic pools of splanchnic (panel A & B) and peripheral (panel C & D) origins in rats with ad libitum access to food (AL group, n = 11, filled symbols) or subjected to gradual caloric restriction (CR group, n = 12, empty symbols). G—gastrocnemius; S—soleus. In the liver, protein mass was negatively associated (Pearson’s R) with the Δ15N of plasma proteins that are mainly synthesized in the liver (panel A) and with the Δ15N of urine that mainly derive from liver ureagenesis (panel B). By contrast, in the cardiac (panel C) and skeletal G (panel D) muscles, protein mass was positively associated with Δ15N.
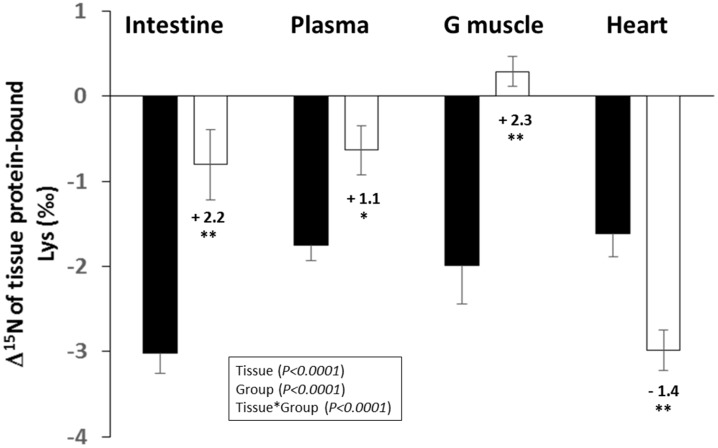
δ15N was also measured for 11 transaminating (Ala, Asx, Glx, Gly, Pro, Ile, Leu, and Val), non-transaminating, and indispensable (Met, Phe and Lys) AA in tissue proteins from the small intestine, plasma, heart, and the gastrocnemius muscle; Δ15N was calculated by subtracting the δ15N value of their dietary counterpart. There were important inter-tissue differences in the Δ15N values of each individual AA, except for one of the non-transaminating and indispensable AA, Phe (Supplementary Table S3). CR was associated with major changes to the Δ15N of Lys; this was higher in the intestine, the plasma, and the gastrocnemius muscle, and lower in the heart of CR, compared to the AL rats (Figure 3). CR was also associated with some changes to the Δ15N of the branched-chain AA (Ile, Leu, and Val) that were lower in the intestine and plasma of the CR, compared to the AL rats, with no significant effect in the other AA (Supplementary Table S3).
Figure 3.
Effect of caloric restriction on the natural abundances of nitrogen stable isotopes in tissue protein-bound Lys (Δ15Ntissue Lys = δ15Ntissue Lys − δ15Ndiet Lys, ‰). Data are means ± standard error for rats with ad libitum access to food (AL group, ■, n = 8) or subjected to gradual caloric restriction (CR group, □, n = 8). G, gastrocnemius. p values in the box refer to the main effects and their interaction in the analysis of variance. ** and * indicate significant group effects at p < 0.01 and p < 0.05, respectively, when controlling the false discovery rate at 10%.
3.3. Δ13C Values in Tissue Protein and AA
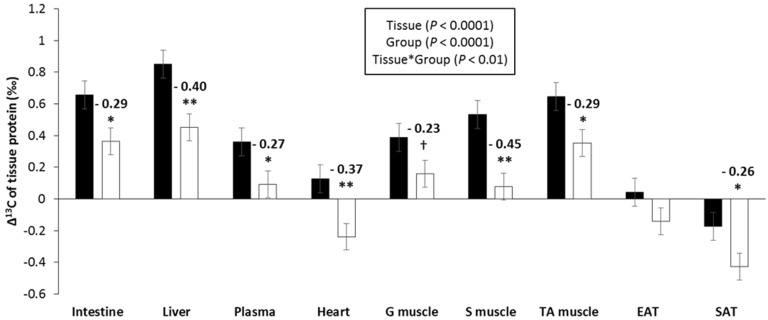
In the same way as for 15N, there was a slight but a significant enrichment in 13C in tissue proteins compared to dietary protein (Δ13C = 0.22 ± 0.03‰, p < 0.01), which differed between tissues—the highest Δ13C levels were observed in the liver and small intestine (0.5‰ to 0.6‰) and the lowest was observed in the heart and adipose tissues (−0.3‰ to −0.1‰) (Figure 4). Compared to Δ15N, the magnitude of Δ13C variations between tissues, was much smaller, with a 0.9‰ difference between the highest and lowest mean values, compared to 2.4‰ for Δ15N. CR was associated with significantly lower Δ13C levels in almost all tissues (mean difference = −0.30 ± 0.03‰).
Figure 4.
Effect of caloric restriction on the natural abundances of carbon stable isotopes in tissue proteins (Δ13Ctissue protein = δ13Ctissue protein − δ13Cdiet protein, ‰). Data are means ± standard error for ad libitum fed rats (AL, ■, n = 11) or rats subjected to caloric restriction (CR, □, n = 12). G muscle—gastrocnemius; S muscle—soleus; TA muscle—tibialis anterior; EAT—epididymal adipose tissue; SAT—subcutaneous adipose tissue. p values in the box refer to the main effects and their interaction in the analysis of variance. **, *, and + indicate significant group effects at p < 0.01, p < 0.05, and p < 0.10, respectively, when controlling the false discovery rate at 10%.
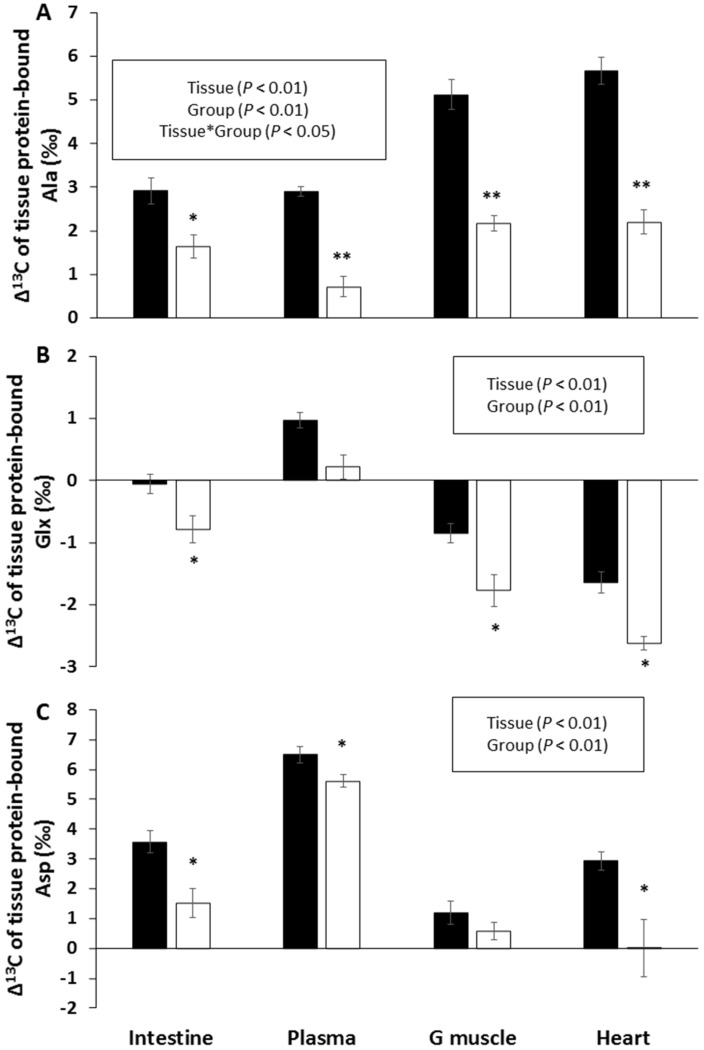
The δ13C of individual AA were also measured for 11 non-indispensable (Ala, Asx, Glx, Gly, and Pro) and indispensable (Ile, Leu, Val, Met, Phe, and Lys) AA in tissue proteins of the small intestine, plasma, heart, and gastrocnemius muscle, and their Δ13C was calculated by subtracting the δ13C of the corresponding AA in dietary protein. There were marked inter-tissue differences in the Δ13C values of all AAs, except for two indispensable AAs—Val and Lys (Supplementary Table S4). CR was associated with significantly lower Δ13C values for the three non-indispensable AAs (Ala, Asx, and Glx) in all four tissues (Figure 5), with only a moderate or non-significant effect for the other AAs (Supplementary Table S4).
Figure 5.
Effect of caloric restriction on the natural abundances of carbon stable isotopes in tissue protein-bound alanine (Ala, panel A), aspartate and asparagine (Asx, panel B), and glutamate and glutamine (Glx, panel C). Data are means ± standard error for ad libitum fed rats (AL group, ■, n = 8) or rats subjected to caloric restriction (CR group, □, n = 8). G muscle—gastrocnemius. p values in the box refer to the main effects and their interaction in the analysis of variance. ** and * indicate significant group effects at p < 0.01 and p < 0.05, respectively, when controlling the false discovery rate at 10%.
3.4. Expression of Enzymes Involved in Liver AA Metabolism
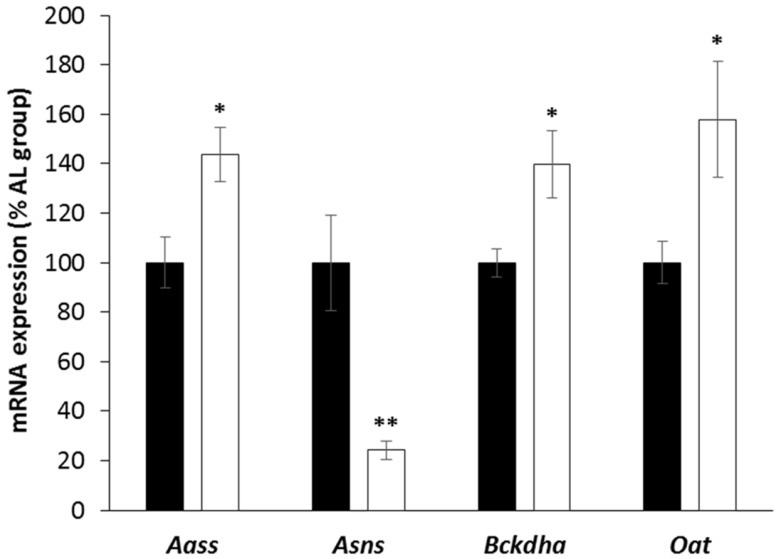
In the livers of CR rats compared to AL rats, we found a dramatic reduction (−75%, p < 0.001) in the mRNA levels of Asns (that converts aspartate and glutamine to asparagine and glutamate) but marked increases (+40% to +60%, p < 0.05) in the mRNA levels of Aass (responsible for removing the εNH2 of Lys in mitochondria), Bckdha (the rate-limiting step in branched chain AA catabolism), and Oat (involved in glutamate catabolism) (Figure 6). No differences were observed for the other enzymes involved in AA catabolism and measured during this study (data not shown).
Figure 6.
Effect of caloric restriction on the expression level of mRNA coding for enzymes involved in amino acid metabolism in the liver. Aass—aminoadipate-semialdehyde synthase; Asns—asparagine synthetase; Bckdha—branched chain ketoacid dehydrogenase; Oat—ornithine aminotransferase. Data are means ± standard error for rats with ad libitum access to food (AL group, ■, n = 10) or subjected to gradual caloric restriction (CR group, □, n = 10). ** and * indicate significant group effects at p < 0.01 and p < 0.05, respectively, when controlling the false discovery rate at 10%.
4. Discussion
Whereas a growing body of evidence suggests that the natural abundances of 13C and 15N in the body might represent new biomarkers of nutritional stress [16,17,18,19,20,21,22,23,26], the underlying mechanisms are still largely elusive because most of the data gathered to date have come from observational studies with little or no control of the nutritional and isotopic conditions. The present results provide the first in vivo demonstration, under carefully controlled nutritional and isotopic conditions, that CR induces changes to the Δ15N and Δ13C of body proteins, and their constitutive AA. In our particular CR context, which consisted of CHO restriction with a maintained protein intake, the adaptive response was fuel economy. This was achieved by decreased CHO oxidation, compensated for by an increased AA oxidation, which translated into lower Δ13C values, in all tissues, and different Δ15N variations, depending on the tissues concerned, respectively.
4.1. CR Effects on Body Composition and AA Oxidation
When compared to the maintained ad libitum diet, the main effect of CR was a considerable loss of fat mass (−34% in total for adipose tissues), whereas lean mass was more moderately affected (−15% overall loss of protein). This limited extent of protein loss was probably due to the fact that the protein intake was maintained throughout a gradual CR, ultimately achieving a high-protein diet, such as those used for body weight loss in obese humans where losing weight during CR usually involved a loss of both fat and lean mass, and high-protein diets are considered to be beneficial in limiting muscle mass losses [31]. Here, our finding of a limited but significant loss of protein mass, after CR, despite a similar nitrogen intake between groups, indicated a lower nitrogen balance due to an increased AA oxidation at the whole body level.
We also observed that the most marked decrease in tissue protein masses concerned the splanchnic organs (small intestine, liver) and cardiac muscle, while only little or no difference was observed in the skeletal muscles. A previous study in rodents has already shown that during CR, the liver and heart are more prone to protein loss than skeletal muscles [32]. As the use of AA for protein synthesis in muscles is preserved during CR on a high-protein diet [33,34,35], the moderate decrease in protein mass likely resulted from an increase in proteolysis and AA allocation to oxidation. In the present study, we observed that only the oxidative soleus and mixed gastrocnemius, but not the glycolytic tibialis anterior, experienced a moderate protein mass loss. This was probably because when compared to the glycolytic muscles, oxidative muscles have a greater capacity for branched-chain AA transamination and oxidation [36,37], producing the glucogenic AA (Gln and Ala) required to sustain gluconeogenesis and to meet energy needs during CR [38,39].
An additional evidence for an increased AA oxidation, during CR, is the modification of the expression level of genes encoding enzymes involved in the AA metabolism. The expression of these genes has only been measured in the liver, known to be the main site of AA catabolism. During CR, the increased expression of mRNA coding for enzymes directly involved in AA oxidation, such as OAT (ammonia detoxication via glutamate/glutamine synthesis), BCKDHa (branched-chain keto-acid oxidation), and AASS (Lys mitochondrial oxidation), supported a change in the relative allocation of liver AA, towards greater oxidation. Furthermore, the decreased expression of mRNA coding for ASNS (asparagine synthetase), a nitrogen sparing enzyme upregulated during AA starvation [40], is an additional indirect evidence of an increased AA oxidation, during CR.
Taken together, these data evidence that in the present study, CR induced an increased oxidation of varying magnitude, depending on the tissues. This increased AA oxidation fulfilled energy needs and provided substrates to fuel the hepatic and renal gluconeogenesis, in the context of insufficient CHO intake.
4.2. Tissue Δ15N Are Fingerprints of CR-Induced Effects on Tissue AA Allocation to Oxidation
We further explored the Δ15N capacity to reflect the relative allocation of AA to oxidation in the different tissues. Using a multi-compartmental model of the complex functioning of Δ15N in the body, we had previously predicted that tissue protein ∆15N values vary as a function of the proportion of tissue AA directed towards oxidation rather than being used for protein synthesis, with an enhanced relative allocation of AA to catabolism that translated into a ∆15N increase in all tissues except muscles, where it caused a reduction in ∆15N [10]. These opposite relationships between ∆15N and the relative allocation of AA to catabolism, which were positive in the liver and negative in muscles, were due to the contrasted signs of isotopic fractionation associated with AA catabolism in these tissues. Indeed, in the liver, AA oxidation favored 14N elimination and, hence, 15N retention, owing to the isotope effects associated with transamination and deamination, whereas, under catabolic conditions, the muscles released some of the most 15N-enriched AA (such as Ala and Gln) into the bloodstream, which might favor 15N elimination from the muscles [10]. In the present study, we observed that CR induced a marked loss of protein in the liver (−17%), which was associated with a considerable increase in Δ15N (+0.25 to +0.45‰) in the urine and liver-synthesized proteins (i.e., both constitutive liver proteins and plasma-exported proteins), together with a moderate protein loss in oxidative skeletal muscles and the heart (−7% to −17%) associated with a moderate reduction of Δ15N in the corresponding proteins (−0.17 to −0.32‰). These results, therefore, confirmed the predictions of our previously developed model [10], showing that the CR-induced protein losses observed in the liver, heart, and oxidative skeletal muscles, involved an increase in the relative allocation of AA to oxidation.
According to the predictions of our model [10], an increase of the relative allocation of AA to oxidation in the liver could be expected to result in a parallel increase in the Δ15N of urine and, both, the constitutive and exported liver proteins. In line with this, we showed relatively parallel increases in the Δ15N of urine and plasma proteins as the liver protein mass decreased in all the AL and CR rats. We had already validated this model prediction by combining AA tracers and arteriovenous balance methods in ruminants, showing that the ∆15N of both plasma proteins and urine, increased linearly with liver AA oxidation and urea production [41]. During CR, an increase in urine or urinary urea Δ15N levels has also been reported in other animal models, including the reindeer, artic squirrel, or Bonobos [17,18,19]. In addition to the ∆15N in the bulk plasma proteins, we measured the ∆15N of their constitutive AA, and observed parallel CR-induced increases in the ∆15N of Lys in the plasma proteins and the ∆15N of plasma proteins. As Lys accounts for only 10–15% of tissue nitrogen, increases in the Δ15N of other individual plasma protein AA were also required to explain the Δ15N increase in bulk plasma protein after CR, but as we did not observe any other significant increases in Δ15N in the 11 AA that we were able to measure using GC-C-IRMS, this pointed to other AA being involved. Arg, Gln, and Asn, which participate in nitrogen transfer and contribute to 20–25% of protein nitrogen, were the best candidates to explain the increase in Δ15N in the liver constitutive and plasma-exported proteins induced by CR. However, unfortunately, these three AA were not amenable to an analytical measurement of Δ15N, during our study. This CR-induced increase in the Δ15N of Lys in plasma protein was paralleled by an increase in the liver AASS expression. AASS is a bifunctional enzyme that catalyzes the removal of the ε-NH2 group of Lys, and both, its lysine-ketoglutarate reductase (amine oxidoreductase), and its saccharopine dehydrogenase activities were likely to favor the light nitrogen isotope, resulting in a gradual 15N enrichment of Lys [9,42]. The CR-induced increase in AASS liver expression might also account for the increase in the Δ15N of Lys in proteins from the small intestine and skeletal muscles, in accordance with the prominent role of the liver in the whole-body Lys catabolism [43].
In cardiac and skeletal muscles, and in contrast with the liver, we observed significant Δ15N decreases in tissue proteins that experienced a significant loss after CR (i.e., heart and gastrocnemius muscle proteins). More generally, in both AL and CR rats, positive relationships were seen between protein Δ15N values and protein mass for nearly all of the muscles studied (except for the glycolytic tibialis anterior muscle). These results were in line with our model predictions regarding the effect of CR [10]. They were also in line with the experimental findings showing a positive association between muscle protein ∆15N and lean mass in rats, in a different dietary context—that of over-nutrition [27]. As for the constitutive AA in muscle proteins, only Lys displayed significant Δ15N variations after CR, which, again, could not explain the Δ15N variations observed in the bulk proteins. Among the different muscles, the heart experienced the greatest Δ15N decrease and protein loss after CR, which was as marked as the protein loss seen in the splanchnic tissues (intestine and liver). Consistent with this, it is known that the heart is not spared during prolonged CR, which can indeed result in cardiac muscle loss and impaired functioning [44,45]. The skeletal muscles that experienced a CR-induced protein loss associated with a Δ15N decrease were the oxidative and mixed muscles that might be more prone to an increased AA release, than the glycolytic muscles, for fueling gluconeogenesis in our context of CR, with a maintained protein intake.
4.3. Effects of CR on Dietary CHO Routing to Tissue Proteins
Unlike the heavier stable N isotope (15N) that bioaccumulates significantly, due to the preferential catabolism of 15N-depleted AA, so that its natural abundance in tissue proteins reflects tissue AA partitioning between anabolic and oxidative pathways, the heavier stable C isotope (13C) bioaccumulates only moderately, so that its natural abundance in the body, globally reflects that of the diet [1]. However, δ13C varies within the body because macronutrients generally differ in their δ13C values and are differentially routed to different tissues and metabolic pools [12,13]. Indeed, although the carbon in tissue proteins originate mainly from dietary proteins, it might also derive from the CHO and lipids that fuel non-indispensable AA synthesis, through intermediary metabolic pathways, to an extent that depends on nutritional conditions [12,27,46,47]. Knowing the specific δ13C signatures of the different macronutrients, δ13C levels in tissue proteins reflect macronutrient use for AA synthesis and their subsequent routing to tissue proteins, and it has, thus, been possible to estimate the respective contributions of macronutrients to C in tissue proteins [12,13,27,46,47]. Data compiled from numerous studies involving a wide variety of dietary conditions have suggested a relatively fixed contribution of around 75% of dietary protein to C in tissue proteins, under a broad range of dietary compositions. However, this did not include diets markedly deficient or excessive in protein [12], which could have applied to our CR diet whose protein content rose markedly (with up to 30% of diet energy in the form of protein, during the last five weeks). A correct estimation of the respective contributions of macronutrients to C in tissue proteins requires that sufficient time has elapsed for the complete turnover of tissue proteins since the last dietary shift, thus, enabling a δ13C steady state. In our study, the dietary δ13C value was only constant during the last five weeks, which was less than the ~seven weeks required for the δ13C to reach a steady state in the small intestine, the liver, and the plasma, and far less than the ~15 weeks required for the muscles [10,46,48,49,50,51]. Nonetheless, the CR-induced reduction in tissue protein δ13C clearly indicated less routing of the macronutrient with the highest δ13C (dietary CHO), to the benefit of nutrients with lower δ13C values (proteins and lipids). According to Neuberger et al. [26], the breakdown of 13C-depleted adipose tissues would be the main contributor to the decrease in the δ13C of tissue protein observed during starvation. However, in the present study, while CR did induce a significant loss of fat mass, the rats were not starved, as protein intake was kept constant and the contribution of dietary lipids to energy intake remained elevated. Thus, in the present study, in addition to the fatty acids released from the adipose tissues, both the dietary protein and the dietary lipid might also have provided carbon for the de novo AA synthesis and contributed to the CR-induced decrease in protein δ13C.
Interestingly, during the present study, the reduction in CR-induced tissue protein Δ13C levels in the four studied tissues was always associated with a decrease in the Δ13C levels of three of the non-indispensable protein-bound AA (Ala, Glx, and Asx), while those in the other non-indispensable AA (Pro and Gly) and indispensable AA remained unchanged. Ala is generally synthetized in the muscle via the transamination of pyruvate produced from glucose oxidation [52], and its δ13C has been shown to partly reflect that of dietary CHO, making it a biomarker for the intake of sugar-sweetened beverages [3]. Therefore, the decrease in Ala Δ13C in all tissues mirrored the reduction in muscle glucose oxidation resulting from CHO restriction. The same reason also explained the drop in Asx and Glx Δ13C levels, but these changes were less marked, because their keto-acid precursors (oxaloacetate and ketoglutarate) were synthetized from acetyl-CoA produced during oxidation of the three macronutrients, and not just glucose. By contrast, the Δ13C of Pro and Gly did not vary during CR, because these AA were not produced via keto-acid transamination, but were mostly synthetized from Glu for Pro, and from Ser, Thr, and choline for Gly.
5. Conclusions
In conclusion, this study was able to demonstrate that CR led to changes in the natural abundances of the heavier stable C and N isotopes in tissue protein and protein-bound AA, which represent the isotopic signatures of the metabolic adaptations necessary to cope with a reduced energy intake. As an adaptive response ensuring fuel economy during CHO restriction with a maintained protein intake, these carbon isotopic footprints revealed a decreased CHO oxidation in all tissues, while nitrogen isotopic footprints indicated an increased catabolic use of AA, which was prominent in the liver and the heart. CR is frequently associated with a decrease in protein intake and although it is quite difficult to speculate on what could have happened if the protein intake had not been maintained, it seems likely that this would have induced more pronounced effects on the tissue protein losses and associated isotopic signatures, particularly in muscles that were relatively preserved in the present study.
Acknowledgments
The authors would like to thank N. Khodorova for her technical assistance with isotopic measurements, and M. Sebilo and V. Vaury (UMR 7618 BIOEMCO, Université Pierre et Marie Curie, Paris, France) for supplying the internal standard for isotopic measurements.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/5/1087/s1, Table S1: Natural abundances of nitrogen and carbon stable isotopes (δ15N and δ13C, ‰) in dietary macronutrients and in dietary protein-bound amino acids (means of triplicate determination). Table S2: qPCR primers used for cDNA amplifications; Table S3: Natural abundances of nitrogen stable isotopes in tissue protein-bound amino acids (AA); Table S4: Natural abundances of carbon stable isotopes in tissue protein-bound amino acids.
Author Contributions
J.-F.H., D.H., and H.F.; Data curation, J.-F.H., O.L.M., D.H., and H.F.; Funding acquisition, D.H.; Investigation, J.-F.H., O.L.M., D.H., V.M., and G.G.; Methodology, J.-F.H., O.L.M., V.M., G.G., and H.F.; Project administration, D.H.; Supervision, J.-F.H. and H.F.; Validation, J.-F.H., F.M., and H.F.; Writing—original draft, J.-F.H. and H.F.; Writing—review & editing, J.-F.H., O.L.M., D.H., V.M., F.M., and H.F.
Funding
The original study from which the animals were derived was funded by Terres Univia. Stable isotope measurements were in part funded by the “Groupe Lipides Nutrition” (GLN). O.L.M. was supported by a PhD grant from the ABIES doctoral school (ED 581).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.O’Brien D.M. Stable isotope ratios as biomarkers of diet for health research. Annu. Rev. Nutr. 2015;35:565–594. doi: 10.1146/annurev-nutr-071714-034511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash S.H., Kristal A.R., Hopkins S.E., Boyer B.B., O’Brien D.M. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a yup’ik study population. J. Nutr. 2014;144:75–80. doi: 10.3945/jn.113.182113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choy K., Nash S.H., Kristal A.R., Hopkins S., Boyer B.B., O’Brien D.M. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J. Nutr. 2013;143:878–884. doi: 10.3945/jn.112.172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel P.S., Cooper A.J.M., O’Connell T.C., Kuhnle G.G.C., Kneale C.K., Mulligan A.M., Luben R.N., Brage S., Khaw K.T., Wareham N.J., et al. Serum carbon and nitrogen stable isotopes as potential biomarkers of dietary intake and their relation with incident type 2 diabetes: The epic-norfolk study. Am. J. Clin. Nutr. 2014;100:708–718. doi: 10.3945/ajcn.113.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien D.M., Kristal A.R., Nash S.H., Hopkins S.E., Luick B.R., Stanhope K.L., Havel P.J., Boyer B.B. A stable isotope biomarker of marine food intake captures associations between n-3 fatty acid intake and chronic disease risk in a yup’ik study population, and detects new associations with blood pressure and adiponectin. J. Nutr. 2014;144:706–713. doi: 10.3945/jn.113.189381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhouri T.H.I., Jahren A.H., Appel L.J., Chen L.W., Alavi R., Anderson C.A.M. Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. J. Nutr. 2014;144:902–905. doi: 10.3945/jn.113.186213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCutchan J.H., Lewis W.M., Kendall C., McGrath C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 2003;102:378–390. doi: 10.1034/j.1600-0706.2003.12098.x. [DOI] [Google Scholar]
- 8.Paneth P., Oleary M.H. Carbon isotope effect on dehydration of bicarbonate ion catalyzed by carbonic-anhydrase. Biochemistry. 1985;24:5143–5147. doi: 10.1021/bi00340a028. [DOI] [PubMed] [Google Scholar]
- 9.Macko S.A., Estep M.L.F., Engel M.H., Hare P.E. Kinetic fractionation of stable nitrogen isotopes during amino-acid transamination. Geochim. Cosmochim. Acta. 1986;50:2143–2146. doi: 10.1016/0016-7037(86)90068-2. [DOI] [Google Scholar]
- 10.Poupin N., Mariotti F., Huneau J.F., Hermier D., Fouillet H. Natural isotopic signatures of variations in body nitrogen fluxes: A compartmental model analysis. PLoS Comput. Biol. 2014;10:e1003865. doi: 10.1371/journal.pcbi.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez del Rio C., Wolf B.O. Mass-balance models for animal isotopic ecology. In: Starck J.M., Wang T., editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Science Publishers, Inc.; Enfield, NH, USA: 2005. pp. 141–174. [Google Scholar]
- 12.Fernandes R., Nadeau M.J., Grootes P.M. Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol. Anthropol. Sci. 2012;4:291–301. doi: 10.1007/s12520-012-0102-7. [DOI] [Google Scholar]
- 13.Kurle C.M., Koch P.L., Tershy B.R., Croll D.A. The effects of sex, tissue type, and dietary components on stable isotope discrimination factors (delta c-13 and delta n-15) in mammalian omnivores. Isotopes. Environ. Health Stud. 2014;50:307–321. doi: 10.1080/10256016.2014.908872. [DOI] [PubMed] [Google Scholar]
- 14.Reitsema L.J. Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition. Am. J. Hum. Biol. 2013;25:445–456. doi: 10.1002/ajhb.22398. [DOI] [PubMed] [Google Scholar]
- 15.Petzke K.J., Fuller B.T., Metges C.C. Advances in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:532–540. doi: 10.1097/MCO.0b013e32833c3c84. [DOI] [PubMed] [Google Scholar]
- 16.Hobson K.A., Alisauskas R.T., Clark R.G. Stable-nitrogen isotope enrichment in avian-tissues due to fasting and nutritional stress—Implications for isotopic analyses of diet. Condor. 1993;95:388–394. doi: 10.2307/1369361. [DOI] [Google Scholar]
- 17.Barboza P.S., Parker K.L. Body protein stores and isotopic indicators of n balance in female reindeer (rangifer tarandus) during winter. Physiol. Biochem. Zool. 2006;79:628–644. doi: 10.1086/502811. [DOI] [PubMed] [Google Scholar]
- 18.Deschner T., Fuller B.T., Oelze V.M., Boesch C., Hublin J.J., Mundry R., Richards M.P., Ortmann S., Hohmann G. Identification of energy consumption and nutritional stress by isotopic and elemental analysis of urine in bonobos (pan paniscus) Rapid Commun. Mass Spectrom. 2012;26:69–77. doi: 10.1002/rcm.5312. [DOI] [PubMed] [Google Scholar]
- 19.Lee T.N., Buck C.L., Barnes B.M., O’Brien D.M. A test of alternative models for increased tissue nitrogen isotope ratios during fasting in hibernating arctic ground squirrels. J. Exp. Biol. 2012;215:3354–3361. doi: 10.1242/jeb.068528. [DOI] [PubMed] [Google Scholar]
- 20.Fuller B.T., Fuller J.L., Sage N.E., Harris D.A., O’Connell T.C., Hedges R.E.M. Nitrogen balance and delta n-15: Why you’re not what you eat during pregnancy. Rapid Commun. Mass Spectrom. 2004;18:2889–2896. doi: 10.1002/rcm.1708. [DOI] [PubMed] [Google Scholar]
- 21.Hatch K.A., Crawford M.A., Kunz A.W., Thomsen S.R., Eggett D.L., Nelson S.T., Roeder B.L. An objective means of diagnosing anorexia nervosa and bulimia nervosa using n-15/n-14 and c-13/c-12 ratios in hair. Rapid Commun. Mass Spectrom. 2006;20:3367–3373. doi: 10.1002/rcm.2740. [DOI] [PubMed] [Google Scholar]
- 22.Mekota A.M., Grupe G., Ufer S., Cuntz U. Serial analysis of stable nitrogen and carbon isotopes in hair: Monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 2006;20:1604–1610. doi: 10.1002/rcm.2477. [DOI] [PubMed] [Google Scholar]
- 23.Butz D.E., Cook M.E., Eghbalnia H.R., Assadi-Porter F., Porter W.P. Changes in the natural abundance of (co2)-c-13/(co2)-c-12 in breath due to lipopolysacchride-induced acute phase response. Rapid Commun. Mass Spectrom. 2009;23:3729–3735. doi: 10.1002/rcm.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whigham L.D., Butz D.E., Johnson L.K., Schoeller D.A., Abbott D.H., Porter W.P., Cook M.E. Breath carbon stable isotope ratios identify changes in energy balance and substrate utilization in humans. Int. J. Obes. 2014;38:1248–1250. doi: 10.1038/ijo.2014.7. [DOI] [PubMed] [Google Scholar]
- 25.Gannes L.Z., del Rio C.M., Koch P. Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998;119:725–737. doi: 10.1016/S1095-6433(98)01016-2. [DOI] [PubMed] [Google Scholar]
- 26.Neuberger F.M., Jopp E., Graw M., Puschel K., Grupe G. Signs of malnutrition and starvation-reconstruction of nutritional life histories by serial isotopic analyses of hair. Forensic Sci. Int. 2013;226:22–32. doi: 10.1016/j.forsciint.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Mantha O.L., Polakof S., Huneau J.F., Mariotti F., Poupin N., Zalko D., Fouillet H. Early changes in tissue amino acid metabolism and nutrient routing in rats fed a high-fat diet: Evidence from natural isotope abundances of nitrogen and carbon in tissue proteins. Br. J. Nutr. 2018;119:981–991. doi: 10.1017/S0007114518000326. [DOI] [PubMed] [Google Scholar]
- 28.Galmiche G., Huneau J.F., Mathe V., Mourot J., Simon N., Le Guillou C., Hermier D. N-3 fatty acids preserve muscle mass and insulin sensitivity in a rat model of energy restriction. Br. J. Nutr. 2016;116:1141–1152. doi: 10.1017/S0007114516003111. [DOI] [PubMed] [Google Scholar]
- 29.Walsh R.G., He S.N., Yarnes C.T. Compound-specific delta c-13 and delta n-15 analysis of amino acids: A rapid, chloroformate-based method for ecological studies. Rapid Commun. Mass Spectrom. 2014;28:96–108. doi: 10.1002/rcm.6761. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y., Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 31.Carbone J.W., McClung J.P., Pasiakos S.M. Skeletal muscle responses to negative energy balance: Effects of dietary protein. Adv. Nutr. 2012;3:119–126. doi: 10.3945/an.111.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson J.A., Yu C.H., Yang M.U., Pi-Sunyer F.X. Effect of age on protein conservation during very-low-energy diet in obese sprague-dawley rats. Obes. Res. 1998;6:448–457. doi: 10.1002/j.1550-8528.1998.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 33.Chevalier L., Bos C., Azzout-Marniche D., Fromentin G., Mosoni L., Hafnaoui N., Piedcoq J., Tome D., Gaudichon C. Energy restriction only slightly influences protein metabolism in obese rats, whatever the level of protein and its source in the diet. Int. J. Obes. 2013;37:263–271. doi: 10.1038/ijo.2012.19. [DOI] [PubMed] [Google Scholar]
- 34.Miller B.F., Robinson M.M., Reuland D.J., Drake J.C., Peelor F.F., Bruss M.D., Hellerstein M.K., Hamilton K.L. Calorie restriction does not increase short-term or long-term protein synthesis. J. Gerontol. A-Biol. 2013;68:530–538. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zangarelli A., Chanseaume E., Morio B., Brugere C., Mosoni L., Rousset P., Giraudet C., Patrac V., Gachon P., Boirie Y., et al. Synergistic effects of caloric restriction with maintained protein intake on skeletal muscle performance in 21-month-old rats: A mitochondria-mediated pathway. FASEB J. 2006;20:2439–2450. doi: 10.1096/fj.05-4544com. [DOI] [PubMed] [Google Scholar]
- 36.Faure M., Glomot F., Papet I. Branched-chain amino acid aminotransferase activity decreases during development in skeletal muscles of sheep. J. Nutr. 2001;131:1528–1534. doi: 10.1093/jn/131.5.1528. [DOI] [PubMed] [Google Scholar]
- 37.Yang Q., Birkhahn R.H. Branched-chain transaminase and keto acid dehydrogenase activities in burned rats: Evidence for a differential adaptation according to sex. Nutrition. 1997;13:640–645. doi: 10.1016/S0899-9007(97)83006-7. [DOI] [PubMed] [Google Scholar]
- 38.Hagopian K., Ramsey J.J., Weindruch R. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp. Gerontol. 2003;38:267–278. doi: 10.1016/S0531-5565(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 39.Owen O.E., Smalley K.J., D’Alessio D.A., Mozzoli M.A., Dawson E.K. Protein, fat, and carbohydrate requirements during starvation: Anaplerosis and cataplerosis. Am. J. Clin. Nutr. 1998;68:12–34. doi: 10.1093/ajcn/68.1.12. [DOI] [PubMed] [Google Scholar]
- 40.Jousse C., Averous J., Bruhat A., Carraro V., Mordier S., Fafournoux P. Amino acids as regulators of gene expression: Molecular mechanisms. Biochem. Biophys. Res. Commun. 2004;313:447–452. doi: 10.1016/j.bbrc.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Cantalapiedra-Hijar G., Ortigues-Marty I., Sepchat B., Agabriel J., Huneau J.F., Fouillet H. Diet-animal fractionation of nitrogen stable isotopes reflects the efficiency of nitrogen assimilation in ruminants. Br. J. Nutr. 2015;113:1158–1169. doi: 10.1017/S0007114514004449. [DOI] [PubMed] [Google Scholar]
- 42.Handley L.L., Raven J.A. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ. 1992;15:965–985. doi: 10.1111/j.1365-3040.1992.tb01650.x. [DOI] [Google Scholar]
- 43.Benevenga N.J., Blemings K.P. Unique aspects of lysine nutrition and metabolism. J. Nutr. 2007;137:1610s–1615s. doi: 10.1093/jn/137.6.1610S. [DOI] [PubMed] [Google Scholar]
- 44.Webb J.G., Kiess M.C., Chanyan C.C. Malnutrition and the heart. Can. Med. Assoc. J. 1986;135:753–758. [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Q.J., Zhao K., Han X.F., Huff A.F., Cui Q., Babcock S.A., Yu S.Q., Zhang Y.M. Inhibition of ampk accentuates prolonged caloric restriction-induced change in cardiac contractile function through disruption of compensatory autophagy. BBA-Mol. Basis Dis. 2015;1852:332–342. doi: 10.1016/j.bbadis.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 46.MacAvoy S.E., Arneson L.S., Bassett E. Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia. 2006;150:190–201. doi: 10.1007/s00442-006-0522-0. [DOI] [PubMed] [Google Scholar]
- 47.Wolf N., Newsome S.D., Peters J., Fogel M.L. Variability in the routing of dietary proteins and lipids to consumer tissues influences tissue-specific isotopic discrimination. Rapid Commun. Mass Spectrom. 2015;29:1448–1456. doi: 10.1002/rcm.7239. [DOI] [PubMed] [Google Scholar]
- 48.Arneson L.S., MacAvoy S.E. Carbon, nitrogen, and sulfur diet–tissue discrimination in mouse tissues. Can. J. Zool. 2005;83:989–995. doi: 10.1139/z05-083. [DOI] [Google Scholar]
- 49.Kraeer K., Arneson L.S., MacAvoy S.E. The intraspecies relationship between tissue turnover and metabolic rate in rats. Ecol. Res. 2014;29:937–947. doi: 10.1007/s11284-014-1182-x. [DOI] [Google Scholar]
- 50.Kurle C.M. Interpreting temporal variation in omnivore foraging ecology via stable isotope modelling. Funct. Ecol. 2009;23:733–744. doi: 10.1111/j.1365-2435.2009.01553.x. [DOI] [Google Scholar]
- 51.MacAvoy S.E., Macko S.A., Arneson L.S. Growth versus metabolic tissue replacement in mouse tissues determined by stable carbon and nitrogen isotope analysis. Can. J. Zool. 2005;83:631–641. doi: 10.1139/z05-038. [DOI] [Google Scholar]
- 52.Palmer T.N., Caldecourt M.A., Snell K., Sugden M.C. Alanine and inter-organ relationships in branched-chain amino and 2-oxo acid metabolism. Biosci. Rep. 1985;5:1015–1033. doi: 10.1007/BF01119623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.