Abstract
Curcumin is a natural polyphenol component of Curcuma longa Linn, which is currently considered one of the most effective nutritional antioxidants for counteracting free radical-related diseases. Several experimental data have highlighted the pleiotropic neuroprotective effects of curcumin, due to its activity in multiple antioxidant and anti-inflammatory pathways involved in neurodegeneration. Although its poor systemic bioavailability after oral administration and low plasma concentrations represent restrictive factors for curcumin therapeutic efficacy, innovative delivery formulations have been developed in order to overwhelm these limitations. This review provides a summary of the main findings involving the heme oxygenase/biliverdin reductase system as a valid target in mediating the potential neuroprotective properties of curcumin. Furthermore, pharmacokinetic properties and concerns about curcumin’s safety profile have been addressed.
Keywords: curcumin, free radicals, heme oxygenase, neuroprotection, safety profile
1. Introduction
Curcumin (1,7-bis[4-hydroxy 3-methoxy phenyl]-1,6-heptadiene-3,5-dione) is a polyphenol compound contained in the rhizome of Curcuma longa Linn. Indeed, turmeric contains several polyphenols, the most abundant being curcumin (~77%), demethoxycurcumin (~15%), and bis-demethoxycurcumin (~3%) [1]. Considering that curcumin prevails over the other congeners, most of the literature in this field has explored the beneficial effects of this compound, although a few papers have studied the physical and biological properties of related curcuminoids [2,3].
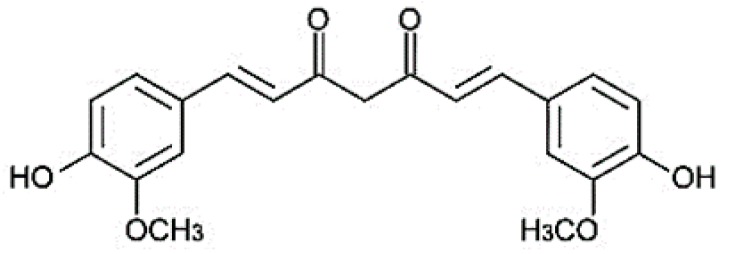
In addition to the culinary use due to its spicy and pleasant taste, curcumin has been considered for thousands of years, by traditional Indian medicine, as an effective remedy in the treatment of several diseases [4,5,6]. Chemically speaking, the curcumin structure presents two aromatic rings holding o-methoxy phenolic groups, linked by an α,β-unsaturated β-diketone moiety (Figure 1) [7].
Figure 1.
Chemical structure of curcumin.
These three reactive functional sites are responsible for the multiple different biological effects of curcumin. Indeed, literature data have reported that the antioxidant activity of curcumin as a free radical scavenger is mediated primarily by the phenolic groups, which undergo oxidation through electron transfer and hydrogen abstraction mechanisms (reviewed in [8]). On the other hand, many studies have demonstrated that curcumin exerts beneficial effects by enhancing the cell stress response in several experimental models, thus supporting the adjuvant role proposed for this dietary supplement in free radical-derived disorders, mainly neurodegenerative diseases [6,9]. In this light, several research studies underlined the pivotal role played by the heme oxygenase/biliverdin reductase system (HO/BVR) as a determinant of curcumin’s neuroprotective effects (see below). Unfortunately, despite the huge amount of preclinical studies confirming the pleiotropic effects of curcumin due to HO modulation, the clinical evidence is not strong enough to include chronic curcumin supplementation as an effective strategy to prevent or contrast neurodegeneration. One of the reasons behind the dichotomy between preclinical and clinical results has been identified in curcumin pharmacokinetics in humans; first of all, the poor bioavailability after ingestion and the effective concentrations reached in tissues. However, several efforts have been made over recent years to overcome these limitations, with encouraging results.
The aim of this review is to summarize the preclinical and clinical outcomes which have appeared in the scientific literature, supporting or contrasting the claimed therapeutic efficacy of curcumin in neurodegeneration. The reason why the focus has been on the HO/BVR system depends on the several lines of evidence highlighting its role as a determinant of curcumin neuroprotection. Finally, some safety issues related to curcumin supplementation have been also reported.
2. The Heme Oxygenase/Biliverdin Reductase Pathway
Heme oxygenase catalyzes the oxygen- and NADPH-dependent oxidation of hemoproteins’ heme moieties at the alpha-meso carbon bridge, yielding equimolar amounts of ferrous iron, carbon monoxide (CO), and biliverdin (BV), the latter being further reduced into bilirubin (BR) by biliverdin reductase [10,11]. Heme oxygenase exists as two main isoforms, named HO-1 and HO-2. Although these isozymes share the same mechanism of action, their regulation and distribution are quite different. Heme oxygenase-1 is the inducible isoform and both its gene transcription and protein levels increase in response to free radicals, e.g., reactive oxygen species and reactive nitrogen species (ROS and RNS, respectively) [11]. Furthermore, HO-1 is the major isoform detected in both the liver and spleen, even if it is expressed, at lower levels, in some brain areas, such as the hippocampus and hypothalamus [11,12]. Conversely, the constitutive isoform HO-2 is involved in the physiological turnover of heme and is mainly detectable in neurons and testes [13,14].
The cytoprotective effects of the HO/BVR system depend on several factors: (i) the degradation of heme, which may become toxic under unbalanced redox conditions; (ii) the generation of CO, which improves mitochondrial biogenesis, counteracts NADPH oxidase-induced ROS generation, activates pro-survival systems (e.g., the protein kinase B/Akt and extracellular signal-related kinase (ERK)/p38 mitogen-activated protein kinase (MAPK) signaling pathways), modulates the release of neuroinflammatory mediators (e.g., interleukin-1β and prostaglandins), dilates cerebral and peripheral vessels, and inhibits platelet aggregation; (iii) the antioxidant and antiviral activities of BR [14,15,16,17,18,19,20]. Interestingly, the modulation of both mitochondrial respiratory chains and NADPH oxidase accounts for CO’s antiproliferative effects [21].
Under oxidative stress and inflammatory conditions, several transcription factors, including nuclear factor erythroid 2-related factor 2 (Nrf2), nuclear factor k-light-chain-enhancer of activated B cells (NF-kB), and hypoxia-inducible factor 1 (HIF1), are established as pivotal regulators of HO-1 induction in the brain [22,23]. Among these transcription factors, Nrf2 plays the conservative role of a positive regulator of HO-1 induction in the development and progression of many diseases [24]. Conversely, a few negative regulators, such as Keap-1 and Bach1, can modulate the crosstalk between the Nrf2 and HO-1 [25,26].
3. Curcumin, Neuroprotection, and the HO/BVR Pathway
Over the last 15 years, many papers have appeared in the scientific literature dealing with the cytoprotective effects of curcumin through the up-regulation of HO-1 (see Table 1).
Table 1.
Contribution of HO-1 up-regulation to the biological effects of curcumin in preclinical in vitro and in vivo models.
| Preclinical Model | Curcumin (Concentration or Dose) | Effect(s) | Reference(s) |
|---|---|---|---|
| Endothelial cells | 2–30 µM | Enhancement of cellular resistance against oxidative damage. Alleviation of vasodilator dysfunction |
[27,28,29,30] |
| Renal tubule cells | 1–50 µM | Cytoprotection. Inhibition of fibrosis. |
[31,32,33] |
| Anti-Thy 1 glomerulonephritis rats Nephrectomized rats |
100 mg/kg i.p. 75 mg/kg per os |
Reduction of renal fibrosis and proteinuria. Inhibition of lipid peroxidation, inflammation and renal fibrosis. Amelioration of renal function. |
[34,35] |
| Hepatocytes | 1–50 µM | Cytoprotection against cold/rewarming- or ethanol-induced damages. | [36,37,38] |
| Monocytes | 1–20 µM | Activation of ARE-modulated genes via PKCδ. Inhibition of inflammation. |
[39,40] |
| Macrophages | 0.5–50 µM | Inhibition of inflammation. | [41,42,43] |
| Cardiac myoblasts | 5–30 µM | Inhibition of apoptosis. Cytoprotection against cold-storage damage. |
[44,45] |
| Smooth muscle cells | 1–20 µM | Inhibition of proliferation. | [46] |
| LPS-treated mice | 30 mg/kg i.p. | Prevention of pulmonary sequestration of neutrophils. | [47] |
| Pancreatic islets | 6–10 µM | Inhibition of islet damage during cryopreservation. Improvement of insulin secretion. |
[48,49] |
| Rat testicular injury | 200 mg/kg i.v. 200 mg/kg per os for 30 days before and 45 days after injury. |
Inhibition of lipid peroxidation and increase in testicular spermatogenesis. Reduced lipid peroxidation; improvement of serum testosterone level. |
[50,51] |
| Fibroblasts | 5–25 µM | Induction of apoptosis and modulation of pathological scar formation. | [52] |
| High-fat-diet-fed mice | 50 mg/kg per os | Improvement in muscular oxidative stress and glucose tolerance. | [53] |
| Bladder cancer cells | 10 µM | Modulation of cancer cell proliferation. | [54] |
| Breast cancer cells | 5–20 µM | Inhibition of tumor invasion. | [55] |
| Hepatoma cells expressing HCV | 5–25 µM | Inhibition of HCV replication. | [56] |
| Lung cancer cells expressing influenza virus | 0.1–10 µM | Inhibition of virus-induced lung injury. | [57] |
| Keratinocytes | 1–30 µM | Anti-inflammatory activity. | [58] |
| Metabolic syndrome in rats | 5 mg/kg i.p. for 6 weeks | Prevention of hyperinsulinemia and amelioration of endothelial-dependent relaxation. | [59] |
ARE, antioxidant responsive element; HCV, hepatitis C virus; i.p., intraperitoneal route of administration; i.v., intravenous route of administration; PKC, protein kinase C.
The following are the main studies supporting the neuroprotective effects of curcumin via the modulation of the HO/BVR pathway.
Scapagnini et al. [60] have shown how curcumin (5–25 µM) up-regulates HO-1 in cultured rat hippocampal neurons and, thus, the polyphenol enhances the cell stress response against glucose oxidase-mediated oxidative damage. Shin et al. [61] reported that curcumin (200 mg/kg by intraperitoneal route (i.p.)) reduced kainic acid-induced seizures in mice through the increased expression of HO-1 and endothelial nitric oxide synthase (eNOS) in hippocampal astrocytes, whereas Park and Chun [62] demonstrated that curcumin (0.1–10 µM) reduces oxidative stress, apoptosis, and mitochondrial damage through the direct involvement of HO-1 in BV-2 microglial cells.
These early studies were followed by several others describing the neuroprotective effects of curcumin in neurovascular disorders. Curcumin (100 mg/kg i.p. or 5–30 µM), via HO-1 over-expression, was neuroprotective in a rat model of focal ischemia [63] and in rat cerebellar granule neurons exposed to hemin [64]. In an experimental system of rat hypoxic-ischemic brain injury, curcumin (150 mg/kg per os for three days) overexpressed HO-1 with a mechanism related to Nrf2 nuclear translocation [65]. In addition, curcumin (1–100 µM) has been shown to up-regulate HO-1 and, through this mechanism, it prevents oxygen glucose deprivation-induced damage in rat brain microvascular endothelial cells, a model mimicking the blood–brain barrier (BBB) function [66].
With regard to neurodegenerative diseases, in a rodent model of Alzheimer’s disease (AD), e.g., the SAMP8 mouse, 500 mg/kg of curcumin in a five month diet increased HO-1 gene expression, together with regulators of mitochondrial function, e.g., the translocator protein (TSPO) [67]. Similarly, by up-regulating HO-1, curcumin (1.25–20 µM) inhibited programmed cell death and prevented the loss of mitochondrial function in SH-SY5Y neuroblastoma cells transfected with appoptosin, a pro-apoptotic protein overexpressed in AD [68]. Concerning neurodegenerative diseases, curcumin (100 mg/kg twice a day for 50 days intragastrically) contrasted extrapyramidal symptoms and increased HO-1 expression, through Akt/Nrf2 phosphorylation, in the substantia nigra pars compacta of rats treated with rotenone, a pharmacological tool able to destroy dopaminergic neurons and, therefore, used to induce experimental Parkinson’s disease (PD) [69]. It is no longer a hypothesis that the cytoprotective effects of curcumin against neuroinflammation depend on the inhibition, HO-1-mediated, of cytokine release and iNOS overexpression in rat microglia [70,71].
Finally, curcumin (15 µM or 200 mg/kg for four days) has been shown to counteract both hydrogen peroxide-induced damage in human retinal pigment cells [72] and cisplatin-induced ototoxicity in outer hair cells [73].
As far as the modulation of HO-2 by curcumin and the potential neuroprotective features, only limited evidence is available. As shown by Yin et al. [74], curcumin (5 µM) up-regulated HO-1 but down-regulated HO-2 in APPswe transfected SH-SY5Y. In the same experimental system, curcumin was able to activate phosphoinositide 3-kinase (PI3K) and Akt [74]. By keeping this in mind, it is necessary to draw the conclusion that in selected experimental settings, the neuroprotective outcomes of curcumin strictly depend on the fine-tuning of the HO-1/HO-2 balance, in concert with the modulation of other pro-survival systems, such as PI3K and Akt.
An accurate analysis of both previous paragraphs and Table 1 has drawn attention to the fact that the concentrations of curcumin responsible for protective effects on various organs and tissues, primarily on the brain, were obtained with polyphenol concentrations in the micromolar size range. That said, curcumin, per os, has about a 60% bioavailability, due to a marked first-pass metabolism [9,75]. This implies a low concentration of curcumin in both blood and tissues, even at high doses. Curcumin plasma levels up to 0.16 µM have been detected in humans treated with polyphenol at supra-maximal doses (10–12 g/day), whereas at the lowest doses, curcumin (450–3600 mg/day for one week) reached the plasma concentration of about 0.003 µM [76,77]. In chronic administrations, curcumin (1–4 g/day for six months) exhibited plasma concentrations in the range of 0.06–0.27 µM [78]. With regard to tissue levels, the available data are quite limited. In patients suffering from colorectal cancer and treated with curcumin (1.8 to 3.6 g/day for seven days), concentrations of polyphenol in colorectal tumor tissue and normal tissue were about 7 nmol/g and 20 nmol/g, respectively [79]. These data lead to the conclusion that the plasma concentrations of curcumin that can be reached in the plasma, even after high dose chronic supplementation, are at least two–three orders of magnitude lower than those at which the polyphenol has shown therapeutic effects in in vitro preclinical models. The calculation of the concentrations of curcumin in the tissues is more difficult and may appear less accurate. In the brain, which is protected by BBB, the achievable curcumin concentrations are even lower than those detected in the blood and other tissues. These analytical data have important consequences also from a functional point of view. In subjects with AD, supplementation with curcumin (1–4 g/day for six months) reduced neither peripheral biomarkers of inflammation (e.g., isoprostanes) nor amyloid-β-peptide (Aβ) serum levels; importantly, curcumin did not improve cognitive functions—evaluated through the mini-mental status examination test—in AD patients [78]. Concerning the contribution of the HO/BVR system to the cytoprotective effects of curcumin, the study by Klickovic et al. [80] is significant, showing how 10 healthy male subjects treated with 12 g curcumin per os, did not have any significant induction of HO-1 gene and protein in peripheral blood mononuclear cells up to 48 h from treatment.
In order to overcome limitations due to the poor bioavailability after ingestion and the low plasma concentrations, new formulations of curcumin complexed with liposoluble matrices have been developed (for an extensive review on this topic see [81]) (Table 2).
Table 2.
The main pharmacokinetic parameters of curcumin and some of its novel formulations (adapted from [82]).
| Formulation | AUC | Cmax | Tmax | T1/2 |
|---|---|---|---|---|
| Curcumin | ~312 ng/mL·h a | ~ 245 nM a | 0.5 h a | ~1.0 h a |
| Curcumin-PLGA | ~3224 ng/mL·h b | ~ 710 nM b | 2.0 h b | |
| Curcumin-TMC | ~12,760 ng/mL·h c | ~3.3 μM c | 2.0 h c | ~12 h c |
| Curcumin-SLN | ~42,000 ng/mL·h d | ~38 μM d | 0.5 h d |
a Male Sprague-Dawley rats treated with 250 mg/kg curcumin per os; b male Sprague-Dawley rats treated with 100 mg/kg curcumin-PLGA per os; c Balb/c mice treated with 50 mg/kg curcumin-TMC per os; d male Wistar rats treated with 50 mg/kg curcumin-SLN per os; AUC, area under the curve; Cmax, peak plasma concentration; PLGA, poly(lactic-co-glycolic) acid; SLN, solid lipid nanoparticles; Tmax, time necessary to reach the Cmax; T1/2, half-life; TMC, N-trimethyl chitosan.
Among the matrices complexed with curcumin, the ones that are better characterized, from a pharmacokinetic viewpoint, are poly(lactic-co-glycolic) acid (PLGA) derivatives, solid lipid nanoparticles (SLN), and N-trimethyl-chitosan (TMC) [82,83]. Preclinical studies in rodents (Table 2) have shown how the complexation of curcumin with these different carriers increases the Cmax of both SLN and TMC (155 times and 13 times greater than curcumin, respectively) markedly, suggesting a more effective absorption of the active ingredient [82]. Furthermore, the increase in the area under the curve demonstrates how the presence of SLN or TMC can improve curcumin bioavailability by about 135 times and 41 times, respectively [82]. Finally, an approximately 10-fold increase in the half-life (T1/2) of curcumin in the case of formulations based on SLN and TMC implies an extension of the time of persistence of the active agent in the body and, therefore, a more prolonged pharmacological action [82]. Unfortunately, no studies are available in the literature on the interaction of such novel curcumin liposoluble formulations and HO. Indeed, few studies which have been carried out using novel gelatin-based water-soluble formulations of curcumin and remarkable results have been reported. The oral administration of water-soluble curcumin (2–10 mg/kg per os for 45 days) increased plasma insulin levels and improved glucose absorption in diabetic rats by up-regulating HO-1 expression in the pancreas and liver [84]. The same authors supported the beneficial effects of water-soluble curcumin (2–10 mg/kg per os up to one week) in an experimental model of erectile dysfunction. At a dose of 10 mg/kg, water-soluble curcumin over-expressed HO-1 and soluble guanylyl cyclase (sGC) as early as 1 h after treatment, with a concomitant increase in intracavernosal pressure. These effects were maintained over one week from treatment [85].
Although not strictly related to any modulation of the HO system, it is worth mentioning a novel formulation of curcumin complexed with exosomes; these latter are extracellular microvesicles (diameter ranging from 30 to 100 nm) able to carry several types of agents, thus enhancing their bioavailability [86]. Interestingly, curcumin-exosome has been shown to improve cognitive function in a preclinical model of AD, through the inhibition of tau hyperphosphorylation via Akt activation [87].
4. Curcumin’s Safety Profile
In any case, regardless of whether it is pure curcumin or new liposoluble or water-soluble formulations, it is worth considering the possibility that the administration of high doses of curcumin causes toxic effects. An organic extract, called turmeric oleoresin, containing a high percentage of curcumin (79–85%), at the concentration of 50,000 ppm (equivalent to 2600 mg/kg and 2800 mg/kg in male and female rats, respectively) has been shown to increase the incidence of ulcers, hyperplasia, and inflammation in the forestomach, cecum, and colon of male and female rats supplemented for two years [88]. Increased evidence of small intestine carcinomas in male mice supplemented with curcumin (0.2 mg/kg) has also been described [88]. Furthermore, curcumin (0.5–2% with the diet for either 2 or 12 weeks) exhibited iron-chelating activity in mice, thus suggesting its involvement in the onset of hypochromic anemia [88]. Finally, curcumin (1 g or 4 g per os for one or six months) modestly increased cholesterol plasma levels in Chinese subjects aged 50 years or older [89]. Regarding the interaction with drug-metabolizing enzymes, curcumin has been shown to inhibit not only several subtypes of cytochrome P450 (CYP), such as CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, and CYP3A4, but also uridine dinucleotide phosphate glucuronosyltransterases (UGT), sulfotransferase, glutathione-S-transferase, and organic anion transporting polypeptides (OATP) [9,75,90]. Among the drugs metabolized by these enzymes, whose blood levels may be altered by curcumin and for which further research is needed to assess the effects in cases of chronic supplementation, there are midazolam, talinolol, nifedipine, rosuvastatin, docetaxel, warfarin, clopidogrel, and norfloxacin ([90] and references therein).
In April 2017, the European Food Scientific Agency (EFSA) pointed out that there is no scientific evidence strong enough to justify the use of curcumin in inflammatory diseases, such as osteoarthritis and rheumatoid arthritis [91].
5. Conclusions
In this review, we have summarized the conflicting preclinical and clinical results on the neuroprotective effects of curcumin. Furthermore, we have made our best efforts to provide a critical analysis of the pharmacological issues responsible for this divergence, which have precluded the full development of curcumin supplementation as a useful strategy in neurodegenerative diseases. The intriguing results, in terms of improved absorption and bioavailability, obtained with lipid- and water-soluble curcumin formulations, should prompt researchers to transfer this technology to clinical studies, with the hope of overwhelming the pharmacokinetic limitations experienced with standard curcumin. The contribution of pharmaceutical companies to scale up and transpose into clinics these encouraging preclinical results is more than welcome.
Author Contributions
Conceptualization, C.M., A.T., L.T.; Data Curation, E.M., L.P.; Writing—Original Draft Preparation, C.M., E.M., A.T.; Writing—Review & Editing, E.M., A.T., L.P., V.C., L.T., C.M.; Supervision, C.M., L.T., V.C.
Funding
This work was supported by Catholic University grant “Fondi Ateneo” to C.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Den Haan J., Morrema T.H.J., Rozemuller A.J., Bouwman F.H., Hoozemans J.J.M. Different Curcumin Forms Selectively Bind Fibrillar Amyloid Beta In Post Mortem Alzheimer’s Disease Brains: Implications for In-Vivo Diagnostics. Acta Neuropathol. Commun. 2018;6:75. doi: 10.1186/s40478-018-0577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lestari M.L., Indrayanto G. Profiles Drug Subst. Excip. Relat. Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- 3.Jitoe-Masuda A., Fujimoto A., Masuda T. Curcumin: From Chemistry to Chemistry-Based Functions. Curr. Pharm. Des. 2013;19:2084–2092. [PubMed] [Google Scholar]
- 4.Mantzorou M., Pavlidou E., Vasios G., Tsagalioti E., Giaginis C. Effects of Curcumin Consumption on Human Chronic Diseases: A Narrative Review of The Most Recent Clinical Data. Phytother. Res. 2018;32:957–975. doi: 10.1002/ptr.6037. [DOI] [PubMed] [Google Scholar]
- 5.Marchiani A., Rozzo C., Fadda A., Delogu G., Ruzza P. Curcumin and Curcumin-Like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014;21:204–222. doi: 10.2174/092986732102131206115810. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese V., Bates T.E., Mancuso C., Cornelius C., Ventimiglia B., Cambria M.T., Di Renzo L., de Lorenzo A., Dinkova-Kostova A.T. Curcumin and The Cellular Stress Response in Free Radical-Related Diseases. Mol. Nutr. Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 7.Priyadarsini K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Prado-Audelo M.L., Caballero-Floran I.H., Meza-Toledo J.A., Mendoza-Munoz N., Gonzalez-Torres M., Floran B., Cortes H., Leyva-Gomez G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules. 2019;9:56. doi: 10.3390/biom9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancuso C., Siciliano R., Barone E., Preziosi P. Natural Substances and Alzheimer’s Disease: From Preclinical Studies to Evidence Based Medicine. Biochim. Biophys. Acta. 2012;1822:616–624. doi: 10.1016/j.bbadis.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso C. Bilirubin and Brain: A Pharmacological Approach. Neuropharmacology. 2017;118:113–123. doi: 10.1016/j.neuropharm.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Maines M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso C. Heme Oxygenase and Its Products in The Nervous System. Antioxid. Redox Signal. 2004;6:878–887. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- 13.Maines M.D. The Heme Oxygenase System: Update 2005. Antioxid. Redox Signal. 2005;7:1761–1766. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso C., Santangelo R., Calabrese V. The Heme Oxygenase/Biliverdin Reductase System: A Potential Drug Target in Alzheimers Disease. J. Biol. Regul. Homeost. Agents. 2013;27:75–87. [PubMed] [Google Scholar]
- 15.Mancuso C., Barone E., Guido P., Miceli F., Di Domenico F., Perluigi M., Santangelo R., Preziosi P. Inhibition of Lipid Peroxidation and Protein Oxidation By Endogenous and Exogenous Antioxidants in Rat Brain Microsomes In Vitro. Neurosci. Lett. 2012;518:101–105. doi: 10.1016/j.neulet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso C., Tringali G., Grossman A., Preziosi P., Navarra P. The Generation of Nitric Oxide and Carbon Monoxide Produces Opposite Effects on The Release of Immunoreactive Interleukin-1β From the Rat Hypothalamus In Vitro: Evidence for The Involvement of Different Signaling Pathways. Endocrinology. 1998;139:1031–1037. doi: 10.1210/endo.139.3.5822. [DOI] [PubMed] [Google Scholar]
- 17.Mancuso C., Perluigi M., Cini C., de Marco C., Giuffrida Stella A.M., Calabrese V. Heme Oxygenase and Cyclooxygenase in The Central Nervous System: A Functional Interplay. J. Neurosci. Res. 2006;84:1385–1391. doi: 10.1002/jnr.21049. [DOI] [PubMed] [Google Scholar]
- 18.Suliman H.B., Piantadosi C.A. Mitochondrial Biogenesis: Regulation by Endogenous Gases during Inflammation and Organ Stress. Curr. Pharm. Des. 2014;20:5653–5662. doi: 10.2174/1381612820666140306095717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basuroy S., Tcheranova D., Bhattacharya S., Leffler C.W., Parfenova H. Nox4 Nadph Oxidase-Derived Reactive Oxygen Species, Via Endogenous Carbon Monoxide, Promote Survival of Brain Endothelial Cells During Tnf-α-Induced Apoptosis. Am. J. Physiol. Cell Physiol. 2011;300:C256–C265. doi: 10.1152/ajpcell.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangelo R., Mancuso C., Marchetti S., di Stasio E., Pani G., Fadda G. Bilirubin: An Endogenous Molecule with Antiviral Activity In Vitro. Front. Pharmacol. 2012;3:36. doi: 10.3389/fphar.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taille C., El-Benna J., Lanone S., Boczkowski J., Motterlini R. Mitochondrial Respiratory Chain and Nad(P)H Oxidase Are Targets for The Antiproliferative Effect of Carbon Monoxide in Human Airway Smooth Muscle. J. Biol. Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 22.Jazwa A., Cuadrado A. Targeting Heme Oxygenase-1 for Neuroprotection and Neuroinflammation in Neurodegenerative Diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 23.Wardyn J.D., Ponsford A.H., Sanderson C.M. Dissecting Molecular Cross-Talk Between Nrf2 And Nf-κb Response Pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/Ho-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J., Hoshino H., Takaku K., Nakajima O., Muto A., Suzuki H., Tashiro S., Takahashi S., Shibahara S., Alam J., et al. Hemoprotein Bach1 Regulates Enhancer Availability of Heme Oxygenase-1 Gene. Embo. J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamuro T., Takahashi K., Ogawa K., Udono-Fujimori R., Takeda K., Furuyama K., Nakayama M., Sun J., Fujita H., Hida W., et al. Bach1 Functions as A Hypoxia-Inducible Repressor for The Heme Oxygenase-1 Gene in Human Cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 27.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, An Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 And Protects Endothelial Cells Against Oxidative Stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 28.Scapagnini G., Foresti R., Calabrese V., Giuffrida Stella A.M., Green C.J., Motterlini R. Caffeic Acid Phenethyl Ester and Curcumin: A Novel Class of Heme Oxygenase-1 Inducers. Mol. Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 29.El-Bassossy H.M., El-Maraghy N.N., El-Fayoumi H.M., Watson M.L. Haem Oxygenase-1 Induction Protects Against Tumour Necrosis Factor Alpha Impairment of Endothelial-Dependent Relaxation in Rat Isolated Pulmonary Artery. Br. J. Pharmacol. 2009;158:1527–1535. doi: 10.1111/j.1476-5381.2009.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang X.D., Yang F., Zhu L., Shen Y.L., Wang L.L., Chen Y.Y. Curcumin Ameliorates High Glucose-Induced Acute Vascular Endothelial Dysfunction in Rat Thoracic Aorta. Clin. Exp. Pharmacol. Physiol. 2009;36:1177–1182. doi: 10.1111/j.1440-1681.2009.05210.x. [DOI] [PubMed] [Google Scholar]
- 31.Hill-Kapturczak N., Thamilselvan V., Liu F., Nick H.S., Agarwal A. Mechanism of Heme Oxygenase-1 Gene Induction by Curcumin In Human Renal Proximal Tubule Cells. Am. J. Physiol. Renal. Physiol. 2001;281:F851–F859. doi: 10.1152/ajprenal.0140.2001. [DOI] [PubMed] [Google Scholar]
- 32.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin Activates the Haem Oxygenase-1 Gene Via Regulation of Nrf2 and The Antioxidant-Responsive Element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Fang Y., Xu Y., Lian Y., Xie N., Wu T., Zhang H., Sun L., Zhang R., Wang Z. Curcumin Improves Amyloid β-Peptide (1-42) Induced Spatial Memory Deficits Through Bdnf-Erk Signaling Pathway. PLoS ONE. 2015;10:E0131525. doi: 10.1371/journal.pone.0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaedeke J., Noble N.A., Border W.A. Curcumin Blocks Fibrosis in Anti-Thy 1 Glomerulonephritis Through Up-Regulation of Heme Oxygenase 1. Kidney Int. 2005;68:2042–2049. doi: 10.1111/j.1523-1755.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 35.Soetikno V., Sari F.R., Lakshmanan A.P., Arumugam S., Harima M., Suzuki K., Kawachi H., Watanabe K. Curcumin Alleviates Oxidative Stress, Inflammation, And Renal Fibrosis in Remnant Kidney Through the Nrf2-Keap1 Pathway. Mol. Nutr. Food Res. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 36.Mcnally S.J., Harrison E.M., Ross J.A., Garden O.J., Wigmore S.J. Curcumin Induces Heme Oxygenase 1 Through Generation of Reactive Oxygen Species, P38 Activation and Phosphatase Inhibition. Int. J. Mol. Med. 2007;19:165–172. doi: 10.3892/ijmm.19.1.165. [DOI] [PubMed] [Google Scholar]
- 37.Mcnally S.J., Harrison E.M., Ross J.A., Garden O.J., Wigmore S.J. Curcumin Induces Heme Oxygenase-1 In Hepatocytes and Is Protective in Simulated Cold Preservation and Warm Reperfusion Injury. Transplantation. 2006;81:623–626. doi: 10.1097/01.tp.0000184635.62570.13. [DOI] [PubMed] [Google Scholar]
- 38.Bao W., Li K., Rong S., Yao P., Hao L., Ying C., Zhang X., Nussler A., Liu L. Curcumin Alleviates Ethanol-Induced Hepatocytes Oxidative Damage Involving Heme Oxygenase-1 Induction. J. Ethnopharmacol. 2010;128:549–553. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Rushworth S.A., Ogborne R.M., Charalambos C.A., O’connell M.A. Role of Protein Kinase C Delta in Curcumin-Induced Antioxidant Response Element-Mediated Gene Expression in Human Monocytes. Biochem. Biophys. Res. Commun. 2006;341:1007–1016. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 40.Hsu H.Y., Chu L.C., Hua K.F., Chao L.K. Heme Oxygenase-1 Mediates the Anti-Inflammatory Effect of Curcumin Within Lps-Stimulated Human Monocytes. J. Cell Physiol. 2008;215:603–612. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 41.Kim K.M., Pae H.O., Zhung M., Ha H.Y., Ha Y.A., Chai K.Y., Cheong Y.K., Kim J.M., Chung H.T. Involvement of Anti-Inflammatory Heme Oxygenase-1 In the Inhibitory Effect of Curcumin on The Expression of Pro-Inflammatory Inducible Nitric Oxide Synthase in Raw264.7 Macrophages. Biomed. Pharmacother. 2008;62:630–636. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Y., Liu T., Lai W., Tan Y., Tian D., Guo Z. Heme Oxygenase-1-Mediated Reactive Oxygen Species Reduction Is Involved in The Inhibitory Effect of Curcumin on Lipopolysaccharide-Induced Monocyte Chemoattractant Protein-1 Production in Raw264.7 Macrophages. Mol. Med. Rep. 2013;7:242–246. doi: 10.3892/mmr.2012.1138. [DOI] [PubMed] [Google Scholar]
- 43.Liu L., Shang Y., Li M., Han X., Wang J., Wang J. Curcumin Ameliorates Asthmatic Airway Inflammation by Activating Nuclear Factor-E2-Related Factor 2/Haem Oxygenase (Ho)-1 Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2015;42:520–529. doi: 10.1111/1440-1681.12384. [DOI] [PubMed] [Google Scholar]
- 44.Abuarqoub H., Green C.J., Foresti R., Motterlini R. Curcumin Reduces Cold Storage-Induced Damage in Human Cardiac Myoblasts. Exp. Mol. Med. 2007;39:139–148. doi: 10.1038/emm.2007.16. [DOI] [PubMed] [Google Scholar]
- 45.Yang X., Jiang H., Shi Y. Upregulation of Heme Oxygenase-1 Expression by Curcumin Conferring Protection from Hydrogen Peroxide-Induced Apoptosis in H9c2 Cardiomyoblasts. Cell Biosci. 2017;7:20. doi: 10.1186/s13578-017-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pae H.O., Jeong G.S., Jeong S.O., Kim H.S., Kim S.A., Kim Y.C., Yoo S.J., Kim H.D., Chung H.T. Roles of Heme Oxygenase-1 In Curcumin-Induced Growth Inhibition in Rat Smooth Muscle Cells. Exp. Mol. Med. 2007;39:267–277. doi: 10.1038/emm.2007.30. [DOI] [PubMed] [Google Scholar]
- 47.Olszanecki R., Gebska A., Korbut R. The Role of Haem Oxygenase-1 in The Decrease of Endothelial Intercellular Adhesion Molecule-1 Expression by Curcumin. Basic Clin. Pharmacol. Toxicol. 2007;101:411–415. doi: 10.1111/j.1742-7843.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 48.Kanitkar M., Bhonde R.R. Curcumin Treatment Enhances Islet Recovery by Induction of Heat Shock Response Proteins, Hsp70 And Heme Oxygenase-1, During Cryopreservation. Life Sci. 2008;82:182–189. doi: 10.1016/j.lfs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Abdel Aziz M.T., El-Asmar M.F., El Nadi E.G., Wassef M.A., Ahmed H.H., Rashed L.A., Obaia E.M., Sabry D., Hassouna A.A., Abdel Aziz A.T. The Effect of Curcumin on Insulin Release in Rat-Isolated Pancreatic Islets. Angiology. 2010;61:557–566. doi: 10.1177/0003319709356424. [DOI] [PubMed] [Google Scholar]
- 50.Wei S.M., Yan Z.Z., Zhou J. Curcumin Attenuates Ischemia-Reperfusion Injury in Rat Testis. Fertil. Steril. 2009;91:271–277. doi: 10.1016/j.fertnstert.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 51.Abd El-Fattah A.A., Fahim A.T., Sadik N.A.H., Ali B.M. Resveratrol and Curcumin Ameliorate Di-(2-Ethylhexyl) Phthalate Induced Testicular Injury in Rats. Gen. Comp. Endocrinol. 2016;225:45–54. doi: 10.1016/j.ygcen.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Scharstuhl A., Mutsaers H.A., Pennings S.W., Szarek W.A., Russel F.G., Wagener F.A. Curcumin-Induced Fibroblast Apoptosis And In Vitro Wound Contraction Are Regulated by Antioxidants and Heme Oxygenase: Implications for Scar Formation. J. Cell Mol. Med. 2009;13:712–725. doi: 10.1111/j.1582-4934.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He H.J., Wang G.Y., Gao Y., Ling W.H., Yu Z.W., Jin T.R. Curcumin Attenuates Nrf2 Signaling Defect, Oxidative Stress in Muscle and Glucose Intolerance in High Fat Diet-Fed Mice. World J. Diabetes. 2012;3:94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu S.Y., Lee Y.R., Huang C.C., Li Y.Z., Chang Y.S., Yang C.Y., Wu J.D., Liu Y.W. Curcumin-Induced Heme Oxygenase-1 Expression Plays A Negative Role for Its Anti-Cancer Effect in Bladder Cancers. Food Chem. Toxicol. 2012;50:3530–3536. doi: 10.1016/j.fct.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 55.Lee W.Y., Chen Y.C., Shih C.M., Lin C.M., Cheng C.H., Chen K.C., Lin C.W. The Induction of Heme Oxygenase-1 Suppresses Heat Shock Protein 90 And the Proliferation of Human Breast Cancer Cells Through Its Byproduct Carbon Monoxide. Toxicol. Appl. Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 56.Chen M.H., Lee M.Y., Chuang J.J., Li Y.Z., Ning S.T., Chen J.C., Liu Y.W. Curcumin Inhibits HCV Replication by Induction of Heme Oxygenase-1 And Suppression of Akt. Int. J. Mol. Med. 2012;30:1021–1028. doi: 10.3892/ijmm.2012.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han S., Xu J., Guo X., Huang M. Curcumin Ameliorates Severe Influenza Pneumonia via Attenuating Lung Injury and Regulating Macrophage Cytokines Production. Clin. Exp. Pharmacol. Physiol. 2018;45:84–93. doi: 10.1111/1440-1681.12848. [DOI] [PubMed] [Google Scholar]
- 58.Youn G.S., Kwon D.J., Ju S.M., Choi S.Y., Park J. Curcumin Ameliorates TNF-α-Induced Icam-1 Expression and Subsequent Thp-1 Adhesiveness via The Induction of Heme Oxygenase-1 in The Hacat Cells. BMB Rep. 2013;46:410–415. doi: 10.5483/BMBRep.2013.46.8.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Bassossy H.M., Hassan N., Zakaria M.N. Heme Oxygenase-1 Alleviates Vascular Complications Associated with Metabolic Syndrome: Effect on Endothelial Dependent Relaxation and No Production. Chem. Biol. Interact. 2014;223:109–115. doi: 10.1016/j.cbi.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Scapagnini G., Colombrita C., Amadio M., D’agata V., Arcelli E., Sapienza M., Quattrone A., Calabrese V. Curcumin Activates Defensive Genes and Protects Neurons Against Oxidative Stress. Antioxid. Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 61.Shin H.J., Lee J.Y., Son E., Lee D.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Curcumin Attenuates the Kainic Acid-Induced Hippocampal Cell Death in the Mice. Neurosci. Lett. 2007;416:49–54. doi: 10.1016/j.neulet.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 62.Park E., Chun H.S. Protective Effects of Curcumin on Manganese-Induced Bv-2 Microglial Cell Death. Biol. Pharm. Bull. 2017;40:1275–1281. doi: 10.1248/bpb.b17-00160. [DOI] [PubMed] [Google Scholar]
- 63.Yang C., Zhang X., Fan H., Liu Y. Curcumin Upregulates Transcription Factor Nrf2, Ho-1 Expression and Protects Rat Brains Against Focal Ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Reyes S., Guzman-Beltran S., Medina-Campos O.N., Pedraza-Chaverri J. Curcumin Pretreatment Induces Nrf2 And an Antioxidant Response and Prevents Hemin-Induced Toxicity in Primary Cultures of Cerebellar Granule Neurons Of Rats. Oxid. Med. Cell. Longev. 2013;2013:801418. doi: 10.1155/2013/801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui X., Song H., Su J. Curcumin Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats Through Induction of Nuclear Factor Erythroid-2-Related Factor 2 And Heme Oxygenase-1. Exp. Ther. Med. 2017;14:1512–1518. doi: 10.3892/etm.2017.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y.F., Gu Y.T., Qin G.H., Zhong L., Meng Y.N. Curcumin Ameliorates the Permeability of The Blood-Brain Barrier During Hypoxia by Upregulating Heme Oxygenase-1 Expression in Brain Microvascular Endothelial Cells. J. Mol. Neurosci. 2013;51:344–351. doi: 10.1007/s12031-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 67.Eckert G.P., Schiborr C., Hagl S., Abdel-Kader R., Muller W.E., Rimbach G., Frank J. Curcumin Prevents Mitochondrial Dysfunction in the Brain of the Senescence-Accelerated Mouse-Prone 8. Neurochem. Int. 2013;62:595–602. doi: 10.1016/j.neuint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Zheng K.M., Zhang J., Zhang C.L., Zhang Y.W., Chen X.C. Curcumin Inhibits Appoptosin-Induced Apoptosis via Upregulating Heme Oxygenase-1 Expression in Sh-Sy5y Cells. Acta Pharmacol. Sin. 2015;36:544–552. doi: 10.1038/aps.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui Q., Li X., Zhu H. Curcumin Ameliorates Dopaminergic Neuronal Oxidative Damage via Activation of The Akt/Nrf2 Pathway. Mol. Med. Rep. 2016;13:1381–1388. doi: 10.3892/mmr.2015.4657. [DOI] [PubMed] [Google Scholar]
- 70.Jin M., Park S.Y., Shen Q., Lai Y., Ou X., Mao Z., Lin D., Yu Y., Zhang W. Anti-Neuroinflammatory Effect of Curcumin on Pam3csk4-Stimulated Microglial Cells. Int. J. Mol. Med. 2018;41:521–530. doi: 10.3892/ijmm.2017.3217. [DOI] [PubMed] [Google Scholar]
- 71.Parada E., Buendia I., Navarro E., Avendano C., Egea J., Lopez M.G. Microglial Ho-1 Induction by Curcumin Provides Antioxidant, Antineuroinflammatory, And Glioprotective Effects. Mol. Nutr. Food Res. 2015;59:1690–1700. doi: 10.1002/mnfr.201500279. [DOI] [PubMed] [Google Scholar]
- 72.Woo J.M., Shin D.Y., Lee S.J., Joe Y., Zheng M., Yim J.H., Callaway Z., Chung H.T. Curcumin Protects Retinal Pigment Epithelial Cells Against Oxidative Stress Via Induction of Heme Oxygenase-1 Expression and Reduction of Reactive Oxygen. Mol. Vis. 2012;18:901–908. [PMC free article] [PubMed] [Google Scholar]
- 73.Fetoni A.R., Eramo S.L., Paciello F., Rolesi R., Podda M.V., Troiani D., Paludetti G. Curcuma Longa (Curcumin) Decreases In Vivo Cisplatin-Induced Ototoxicity Through Heme Oxygenase-1 Induction. Otol. Neurotol. 2014;35:E169–E177. doi: 10.1097/MAO.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 74.Yin W., Zhang X., Li Y. Protective Effects of Curcumin in Appswe Transfected Sh-Sy5y Cells. Neural. Regen Res. 2012;7:405–412. doi: 10.3969/j.issn.1673-5374.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mancuso C., Barone E. Curcumin in Clinical Practice: Myth or Reality? Trends Pharmacol. Sci. 2009;30:333–334. doi: 10.1016/j.tips.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Lao C.D., Ruffin M.T.T., Normolle D., Heath D.D., Murray S.I., Bailey J.M., Boggs M.E., Crowell J., Rock C.L., Brenner D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcea G., Jones D.J., Singh R., Dennison A.R., Farmer P.B., Sharma R.A., Steward W.P., Gescher A.J., Berry D.P. Detection of Curcumin and Its Metabolites in Hepatic Tissue and Portal Blood of Patients Following Oral Administration. Br. J. Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baum L., Lam C.W., Cheung S.K., Kwok T., Lui V., Tsoh J., Lam L., Leung V., Hui E., Ng C., et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 79.Garcea G., Berry D.P., Jones D.J., Singh R., Dennison A.R., Farmer P.B., Sharma R.A., Steward W.P., Gescher A.J. Consumption of The Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum And Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 80.Klickovic U., Doberer D., Gouya G., Aschauer S., Weisshaar S., Storka A., Bilban M., Wolzt M. Human Pharmacokinetics of High Dose Oral Curcumin and Its Effect on Heme Oxygenase-1 Expression in Healthy Male Subjects. Biomed. Res. Int. 2014;2014:458592. doi: 10.1155/2014/458592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jamwal R. Bioavailable Curcumin Formulations: A Review of Pharmacokinetic Studies in Healthy Volunteers. J. Integr. Med. 2018;16:367–374. doi: 10.1016/j.joim.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Mancuso C. Key Factors Which Concur to the Correct Therapeutic Evaluation of Herbal Products in Free Radical-Induced Diseases. Front. Pharmacol. 2015;6:86. doi: 10.3389/fphar.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rakotoarisoa M., Angelova A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines. 2018;5:126. doi: 10.3390/medicines5040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdel Aziz M.T., El-Asmar M.F., El-Ibrashy I.N., Rezq A.M., Al-Malki A.L., Wassef M.A., Fouad H.H., Ahmed H.H., Taha F.M., Hassouna A.A., et al. Effect of Novel Water Soluble Curcumin Derivative On Experimental Type- 1 Diabetes Mellitus (Short Term Study) Diabetol. Metab. Syndr. 2012;4:30. doi: 10.1186/1758-5996-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdel Aziz M.T., El Asmer M.F., Rezq A., Kumosani T.A., Mostafa S., Mostafa T., Atta H., Abdel Aziz Wassef M., Fouad H.H., Rashed L., et al. Novel Water-Soluble Curcumin Derivative Mediating Erectile Signaling. J. Sex. Med. 2010;7:2714–2722. doi: 10.1111/j.1743-6109.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 86.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Gupta R. Exosomes for The Enhanced Tissue Bioavailability and Efficacy of Curcumin. Aaps J. 2017;19:1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 87.Wang H., Sui H., Zheng Y., Jiang Y., Shi Y., Liang J., Zhao L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in Ad Mice by Inhibiting Hyperphosphorylation of The Tau Protein Through the Akt/Gsk-3beta Pathway. Nanoscale. 2019;11:7481–7496. doi: 10.1039/C9NR01255A. [DOI] [PubMed] [Google Scholar]
- 88.Burgos-Moron E., Calderon-Montano J.M., Salvador J., Robles A., Lopez-Lazaro M. The Dark Side of Curcumin. Int. J. Cancer. 2010;126:1771–1775. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 89.Baum L., Cheung S.K., Mok V.C., Lam L.C., Leung V.P., Hui E., Ng C.C., Chow M., Ho P.C., Lam S., et al. Curcumin Effects on Blood Lipid Profile in A 6-Month Human Study. Pharmacol. Res. 2007;56:509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Bahramsoltani R., Rahimi R., Farzaei M.H. Pharmacokinetic Interactions of Curcuminoids With Conventional Drugs: A Review. J. Ethnopharmacol. 2017;209:1–12. doi: 10.1016/j.jep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 91.Turck D., Bresson J.L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., Mcardle H.J., Naska A., et al. Curcumin and Normal Functioning of Joints: Evaluation of A Health Claim Pursuant to Article 13(5) Of Regulation (Ec) No 1924/2006. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]