Abstract
Background
Adjuvant radiotherapy (RT) can increase the risk of developing pain; however, the molecular mechanisms of RT-related pain remain unclear. The current study aimed to identify susceptibility loci and enriched pathways for clinically relevant acute post-RT pain, defined as having moderate to severe pain (pain score ≥ 4) at the completion of RT.
Methods
We conducted a genome-wide association study (GWAS) with 1,344,832 single-nucleotide polymorphisms (SNPs), a gene-based analysis using PLINK set-based tests of 19,621 genes, and a functional enrichment analysis of a gene list of 875 genes with p < 0.05 using NIH DAVID functional annotation module with KEGG pathways and GO terms (n = 380) among 1112 breast cancer patients.
Results
About 29% of patients reported acute post-RT pain. None of SNPs nor genes reached genome-wide significant level. Four SNPs showed suggestive associations with post-RT pain; rs16970540 in RFFL or near the LIG3 gene (p = 1.7 × 10−6), rs4584690, and rs7335912 in ABCC4/MPR4 gene (p = 5.5 × 10−6 and p = 7.8 × 10−6, respectively), and rs73633565 in EGFL6 gene (p = 8.1 × 10−6). Gene-based analysis suggested the potential involvement of neurotransmitters, olfactory receptors, and cytochrome P450 in post-RT pain, whereas functional analysis showed glucuronidation (FDR-adjusted p value = 9.46 × 10−7) and olfactory receptor activities (FDR-adjusted p value = 0.032) as the most significantly enriched biological features.
Conclusions
This is the first GWAS suggesting that post-RT pain is a complex polygenic trait influenced by many biological processes and functions such as glucuronidation and olfactory receptor activities. If validated in larger populations, the results can provide biological targets for pain management to improve cancer patients’ quality of life. Additionally, these genes can be further tested as predictive biomarkers for personalized pain management.
Electronic supplementary material
The online version of this article (10.1186/s40246-019-0212-8) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Radiotherapy, Pain, Pathway analysis, Genetic variants
Background
Breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer death in American women [1]. Early detection and improved treatment modalities have led to a remarkable reduction in the mortality rate of breast cancer patients, and currently more than 3.5 million breast cancer survivors are living in the USA [2]. Given that approximately 70% of breast cancer patients receive adjuvant radiotherapy (RT) after breast surgery to improve clinical outcomes [3], it is critical to address cancer survivorship issues relating to RT-induced symptoms which may affect the quality of life (QOL). Among many symptoms, pain occurs in up to 60% of breast cancer survivors [4, 5], where more than half of them report moderate to severe pain [4]. Unmanaged pain can interrupt planned RT schedules and impact the accurate delivery of therapeutic radiation doses to tumor tissues, which can thus diminish the potential benefits of adjuvant RT. Persistent pain after cancer treatment is also critical, affecting cancer survivor’s functional performance and productivity. Moreover, once pain develops, it may last for more than 17 years after completion of RT [6].
In addition to RT planning and treatment parameters, age, body mass index (BMI), medication, lifestyle factors such as smoking and exercise, and coexisting morbidities can contribute to pain perception during RT [7, 8]; however, inter-individual genetic variations can also influence post-RT pain severity. Several studies have reported genetic variants associated with cancer treatment-related pain among breast cancer patients. For example, genotype AA for interleukin (IL)-13 single-nucleotide polymorphism (SNP) rs1295686 was associated with both pain and lymphedema after breast cancer surgery [9]. Also, SNPs in cytokine genes IFNG1, IL, and NFKB1 have been associated with severe breast pain following breast cancer surgery [10]. Genetic variations in cytidine deaminase (CDD) contributed to chemotherapy-induced neuropathy [11]. Furthermore, variations in cytochrome P450 (CYP) and vitamin D receptor (VDR) genes have been associated with aromatase inhibitor-related arthralgia [12]. However, there is a scientific knowledge gap regarding the molecular mechanisms or the genetic variants influencing pain in patients receiving adjuvant RT.
Thus, to identify susceptibility loci for post-RT pain, we completed a genome-wide association study (GWAS) of 1,344,832 SNPs in a prospectively followed cohort of breast cancer patients undergoing adjuvant RT for breast cancer. As part of this study, we completed gene-based association analyses and functional enrichment pathway analyses to describe the biological profiles underlying genetic mechanisms of post-RT pain. Gene-based association approach considers the joint actions of multiple SNPs within a gene and assigns a representative p value for a gene. If a gene contains more than one causative SNPs with small or moderate effect, then joint effects of several SNPs within that gene may be more detectable than single SNP effect. Functional enrichment pathway analysis, using the gene list produced by gene-based association analyses, is complementary to GWAS in finding risk loci as well as interpreting GWAS results in terms of biological features or function.
Materials and methods
Study populations
This study analyzed 1112 participants from two cohort studies which employed the same protocol to evaluate the impact of molecular genomics on radiosensitivity among breast cancer patients. The first study population consisted of a cohort of 513 women with newly diagnosed, histologically confirmed breast cancer, recruited from the Department of Radiation Oncology of the University of Miami (UM) Sylvester Comprehensive Cancer Center, University of Miami Hospital, and Jackson Memorial Hospital between December 2008 and January 2014. We obtained sufficient quantity and quality of DNA for 458 patients, and among these, 377 patients with complete genotype and pain data were included in the current study. The second study population consisted of a nationwide cohort of breast cancer patients who were enrolled in the Wake Forest (WF) National Cancer Institute Community Clinical Oncology Program (CCOP) Research Base 97609 Study. This study enrolled 1000 patients between November 2011 and August 2013. Among these, 728 patients with complete genotype and pain data were included in the current analysis. Protocols were approved by each participating site’s Institutional Review Boards, and written informed consent was obtained from each study participant before entering the study.
Each patient completed a baseline questionnaire and provided blood samples (20 ml) before the initiation of RT (baseline) and immediately after completion of RT (post-RT). Blood samples from participants enrolled in the WF Research Base 97609 study were transported to the University of Miami via overnight shipping for DNA extraction and genotyping. All the DNA samples were stored at − 20 °C until assay.
Radiation treatment
Detailed information on radiation treatment was described in the previous papers [13, 14]. In brief, RT was delivered using 6 or 10 MV standard or partially wide photon tangents with a forward planned field-in-field technique to maximize dose homogeneity. In general, patients received a total dose of 42.4 to 66 Gy to their intact breast or chest wall for 3 to 7 weeks depending on both the fractionation scheme and additional boost.
Phenotype definition: post-RT pain
All women enrolled in the study filled out the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 protocol QOL questionnaire at baseline and post-RT, which contains four pain severity items (i.e., pain at its worst, least, average during the past 4 weeks, and now) from the Brief Pain Inventory (BPI). A pain score was determined as the mean of these four pain severity items (from 0 = no pain to 10 = the worst imaginable pain) as suggested by the BPI developers [15], and moderate to severe pain (pain score ≥ 4) was considered clinically relevant [16, 17]. Therefore, cases were defined as those that had a pain score ≥ 4 at post-RT (n = 326), and the reference group included those with a pain score < 4 at post-RT (n = 786).
Genotyping and quality control
Genomic DNA was extracted from frozen whole blood using the QIAamp DNA Blood Mini kit (Qiagen, Inc., Valencia, CA), and the DNA genotype was screened for ∼ 2,500,000 haplotype tagging SNPs using an Illumina HumanOmni2.5-8v1 BeadChip (Illumina, San Diego, CA) according to Illumina protocols at the University of Miami Hussman Institute for Human Genomics Genotyping Core. Both genotype clustering and calling were performed using Illumina’s GenomeStudio V2011.1 software. The genotyping quality control/assurance included (i) four internal controls in each plate, (ii) randomly assigned case and reference samples in each plate to avoid any biases between plates, and (iii) the Hardy-Weinberg equilibrium (HWE) test to identify problematic SNPs. SNPs were excluded from the analysis if they had no genotype for > 5% of individuals, were not in HWE within a reference group (using threshold p < 1.0 × 10−6) or had minor allele frequency < 5%. Subjects were also excluded if they had > 5% of all variants missing. The final dataset contained 1,344,832 SNPs with a genotype call rate of 99.8%. All the quality control procedures were conducted using PLINK (v1.09) (http://zzz.bwh.harvard.edu/plink/) [18].
Population substructure
Population substructure was evaluated using principal component analysis (PCA). To remove outliers, we first computed the analysis with a randomly selected and pruned subset of 30,929 common SNPs (LD = 0.5 and minor allele frequency = 0.05) for the study subjects as well as four reference populations from the International HapMap/1000Genomes Project: 85 European-Americans from Utah (CEU); 88 Yorubans from Ibadan, Nigeria (YRI); 97 Han Chinese from Beijing, China (CHB); and 89 Japanese in Tokyo (JPT). Next, we computed the analysis for the study subjects only without the reference populations merged in to determine principal components (PCs) for covariates. The first three PCs were included to adjust for population substructure to minimize spurious associations and test inflation and improve power to detect true associations in subsequent analyses. PCA was performed using EIGENSTRAT v5.0 (https://reich.hms.harvard.edu/software) [19].
Statistical analysis
Single marker genome-wide association analyses
Pearson’s chi-square test or Fisher’s exact test were used to find the potential risk factors for post-RT pain, which compared proportions of patients with post-RT pain by study variables in univariate analysis. These factors were further included in the multivariable logistic regression analysis. The variables that were identified as significant in multivariable analysis were then included in subsequent analyses to adjust for potential confounding effects: surgery type (mastectomy vs lumpectomy), age (continuous), BMI (continuous), smoking (never vs. ever), the number of comorbidities (0, 1, vs 2+), pre-RT pain score (< 4 vs. ≥ 4), and population sub-stratification (PC1, PC2, PC3).
The associations between post-RT pain and genotype frequency, assuming an additive genetic model for minor allele counts of SNPs coded as 0/1/2, were assessed using multivariable logistic regression after adjusting for aforementioned potential confounders. The odds ratios (ORs) and 95% confidence intervals (95% CIs) for each SNP are reported. A quantile-quantile (Q-Q) plot of observed versus expected chi-square test statistics and estimated inflation factor confirmed the tests met the distributional assumptions. The genome-wide significance was set at the standard p < 5 × 10−8 to account for the number of tests. General data management and statistical analyses were performed using PLINK and R (http://cran.r-project.org/). A Manhattan plot for the result was generated using R package, qqman.
We estimated the statistical power using the software program, PS Power, and Sample Size Program [20]. Given 326 cases and 786 controls with minor allele frequency = 0.24 and alpha = 5 × 10−8, we had 80% power to detect an OR of 2.41 for an association between a SNP and post-RT pain.
Gene-based association analysis
First, a total of 950,621 SNPs were mapped to 19,621 genes according to genomic positions on the Ensembl/Entrez hg19/GRCh37 Consensus Genes, which were downloaded on 3 September 2016 from the Figshare, the online academic digital repository (https://figshare.com/articles/hg19_GRCh37_Consensus_Genes/103113/4) [21] using ± 20 kb gene boundaries as delimiters to include regulatory SNPs [22]. These genes are consistently annotated across Ensembl and Entrez-gene databases and have HUGO gene symbol identifiers.
Second, gene-based association analyses were performed using PLINK set-based tests, which required raw genotype data as input and aggregate p values from the set of SNPs within a gene accounting for linkage disequilibrium (LD) and gene size with phenotype permutation. Although its computational burden is high, PLINK set-based tests are more relevant in the current study where we are more interested in joint effects of multiple SNPs with moderate effects. PLINK performs a single SNP association analysis for each gene accounting for the covariates. A mean SNP statistic is calculated from the significant and independent set of SNPs under the defined p value and LD threshold setting. The empirical p value for the gene is calculated after repeated analysis in simulated datasets with permutation of the phenotype. The empirical p value indicates the number of times the test statistics of the simulated gene exceed that of the original gene. Gene with empirical p value < 2.5 × 10−6, a Bonferroni-corrected threshold (≈ 0.05/19,621), was considered significant accounting for multiple testing corrections. The parameters in PLINK set-based test for the current study were set at p (p value threshold for selection of SNPs from a single SNP association) < 0.05, LD r2 (pair-wise correlation between two SNPs) < 0.5, mperm (number of permutation) = 10,000, and set-max (max number of SNPs in a gene) = 99,999.
Pathway analysis
To identify which biological terms/functions are specifically enriched with post-RT pain, we conducted pathway analysis of the GWAS results. The Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) terms were used for functional annotation and enrichment analyses. In total, 530 pathways with minimum gene size ≥ 5 were analyzed since small pathways can exhibit spurious associations due to large single locus effects [23]. A total of 875 genes having p < 0.05 in PLINK gene-based association analyses were selected for pathway analysis. Modified Fisher’s exact tests were performed using the web-based gene-enrichment analysis tool, the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) v6.8 [24], and a pathway with the false discovery rate (FDR) < 0.05 after accounting for multiple testing was considered significant.
Results
Patient characteristics and post-RT pain
The study population across two datasets consisted of 401 Hispanic Whites (HW, 36%), 357 non-Hispanic Whites (NHW, 32%), 296 black or African Americans (AA, 27%), and 58 of other races (5%). Mean (±SD) age at the time of enrollment was 57.4 ± 10.5 years (range 23.5 – 88.9) and 77% of patients were overweight or obese. 86% of patients received post-lumpectomy RT, and 14% had post-mastectomy RT. They were treated with a mean of 58.6 ± 5.7 Gy radiation dose to either the whole breast or the chest wall.
A total of 326 (29%) patients showed clinically relevant post-RT pain. Patient-, tumor-, and treatment-related factors that may be related to post-RT pain were compared between case and reference groups (Table 1). Those who were AA or HW women, younger, obese, ever smoked, had comorbidities ≥ 2, had received mastectomy, conventionally fractionated RT, and whose pre-RT pain score ≥ 4 were more likely to report post-RT pain.
Table 1.
Characteristics of study populations by post-RT pain
| Variable | Categories | Post-RT pain | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No (score < 4) | Yes (score ≥ 4) | |||||||
| N | % | N | % | N | % | p 1 | p 2 | ||
| Study population | 1112 | 100 | 786 | 71 | 326 | 29 | |||
| Study site | UM | 377 | 34 | 243 | 64 | 134 | 36 | 0.001 | 0.340 |
| WFU | 735 | 66 | 543 | 74 | 192 | 26 | |||
| Race/ethnicity | AA | 296 | 27 | 194 | 66 | 102 | 34 | < 0.001 | 0.315 |
| HW | 401 | 36 | 265 | 66 | 136 | 34 | |||
| NHW | 357 | 32 | 282 | 79 | 75 | 21 | |||
| Other | 58 | 5 | 45 | 78 | 13 | 22 | |||
| Age at consent (years) | < 50 | 283 | 25 | 175 | 62 | 108 | 38 | <.0001 | <.0001 |
| 50–59 | 372 | 34 | 247 | 66 | 125 | 34 | |||
| ≥ 60 | 457 | 41 | 364 | 80 | 93 | 20 | |||
| BMI (kg/m2) | < 25 | 257 | 23 | 208 | 81 | 49 | 19 | < .0001 | 0.002 |
| 25–29.99 | 357 | 32 | 263 | 74 | 94 | 26 | |||
| ≥ 30 | 498 | 45 | 315 | 63 | 183 | 37 | |||
| Smoking history | Never | 706 | 64 | 514 | 73 | 192 | 27 | 0.033 | 0.026 |
| Ever | 390 | 35 | 260 | 67 | 130 | 33 | |||
| No. of comorbidity3 | 0 | 464 | 42 | 337 | 73 | 127 | 27 | 0.035 | 0.001 |
| 1 | 378 | 34 | 275 | 73 | 103 | 27 | |||
| ≥ 2 | 270 | 24 | 174 | 64 | 96 | 36 | |||
| Tumor stage | 0 | 217 | 20 | 159 | 73 | 58 | 27 | 0.077 | – |
| I | 528 | 47 | 384 | 73 | 144 | 27 | |||
| II | 234 | 21 | 161 | 69 | 73 | 31 | |||
| III–IV | 132 | 12 | 82 | 62 | 50 | 38 | |||
| ER | Positive | 894 | 80 | 632 | 71 | 262 | 29 | 0.957 | – |
| Negative | 217 | 20 | 153 | 71 | 64 | 29 | |||
| PR | Positive | 767 | 69 | 533 | 69 | 234 | 31 | 0.178 | – |
| Negative | 343 | 31 | 252 | 73 | 91 | 27 | |||
| HER2 | Positive | 143 | 13 | 106 | 74 | 37 | 26 | 0.283 | – |
| Negative | 798 | 72 | 556 | 70 | 242 | 30 | |||
| Triple negative | No | 964 | 87 | 687 | 71 | 277 | 29 | 0.248 | – |
| Yes | 134 | 12 | 89 | 66 | 45 | 34 | |||
| Surgery | Lumpectomy | 959 | 86 | 698 | 73 | 261 | 27 | < .0.001 | 0.023 |
| Mastectomy | 153 | 14 | 88 | 58 | 65 | 42 | |||
| Chemotherapy | No | 610 | 55 | 441 | 72 | 169 | 28 | 0.193 | – |
| Yes | 502 | 45 | 345 | 69 | 157 | 31 | |||
| Hormonal therapy | No | 785 | 71 | 570 | 73 | 215 | 27 | 0.029 | 0.587 |
| Yes | 327 | 29 | 216 | 66 | 111 | 34 | |||
| RT fractionation | Conventional | 972 | 87 | 670 | 69 | 302 | 31 | 0.003 | 0.414 |
| Hypo | 138 | 13 | 114 | 83 | 24 | 17 | |||
| Partial | 2 | 0.1 | 2 | 100 | . | . | |||
| RT dose (Gy) | < 60 | 266 | 24 | 205 | 77 | 61 | 23 | 0.010 | – |
| ≥ 60 | 834 | 75 | 574 | 69 | 260 | 31 | |||
| Boost | No | 121 | 11 | 94 | 78 | 27 | 22 | 0.078 | – |
| Yes | 979 | 88 | 685 | 70 | 294 | 30 | |||
| Pre-RT pain score | < 4 | 936 | 84 | 715 | 76 | 221 | 24 | < .0001 | < .0001 |
| ≥ 4 | 151 | 14 | 57 | 38 | 94 | 62 | |||
1p values from chi-square or Fisher’s exact test. Significant findings are in italics
2p values from multivariable logistic regression adjusting for other variables in tables
3The number of comorbidities, sum of 12 patient-reported comorbid conditions: diabetes, hypertension, heart disease, lung disease, thyroid condition, cirrhosis liver, stroke, chronic bronchitis, hepatitis, tuberculosis, and 2 others. AA African American; HW Hispanic Whites; NHW = non-Hispanic Whites; BMI body mass index
Genome-wide single-marker association analyses
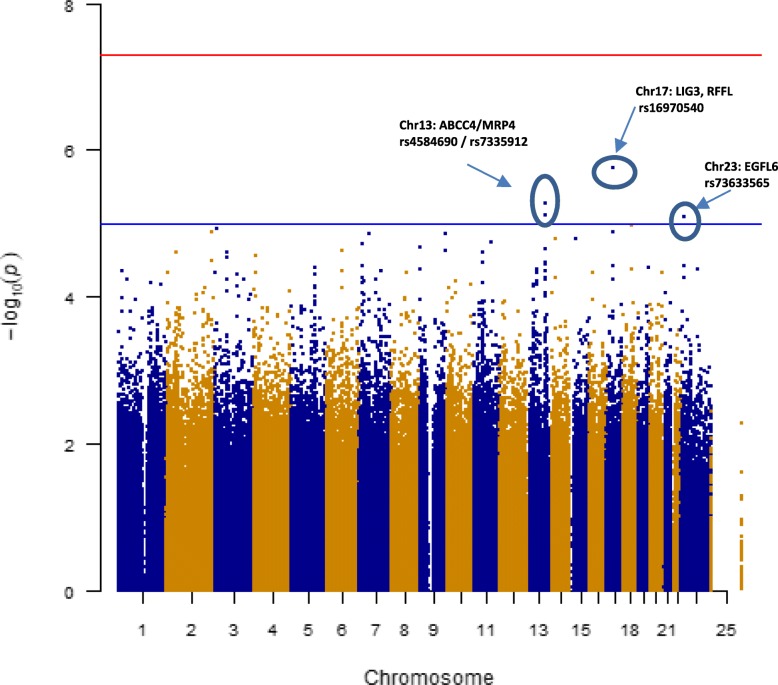
Genome-wide single SNP associations were conducted with 1,344,832 SNPs that passed quality control. The Q-Q plot (Additional File 1: Fig. S1) showed no evidence for test statistic inflation due to population substructure (inflation factor 1.016). None of SNPs achieved a genome-wide significance level of p < 5 × 10−8 (Fig. 1). Four SNPs showed associations with post-RT pain at the marginal significance level of p < 1 × 10−5; rs16970540 in ring finger and FYVE-like domain containing E3 ubiquitin protein ligase (RFFL) or near to DNA ligase 3 (LIG3) gene (p = 1.7 × 10−6), rs4584690, and rs7335912 in ATP-binding cassette, sub-family A, member 4 (ABCC4)/multidrug resistance protein 4 (MRP4) gene (p = 5.5 × 10−6 and p = 7.8 × 10−6, respectively), and rs73633565 in epidermal growth factor-like protein 6 (EGFL6) gene (p = 8.1 × 10−6). The top 30 significant SNPs are summarized in Table 2. For rs16970540, those who had at least one minor T allele were 2.2 times more likely to have post-RT pain compared to those who had C allele (95% CI = 1.59 – 3.04).
Fig. 1.
Manhattan plot for post-RT pain among breast cancer patients. This figure shows the p values of the SNPs after applying the additive genetic model in the multivariable logistic regression model by genomic location. No region exceeded genome-wide significance in the sample. Red line indicates genome-wide significance level of 5 × 10−8 and blue line indicates suggestive level of significance of 1 × 10−5
Table 2.
Top 30 SNPs from genome-wide association study of post-RT pain among breast cancer patients
| Allele | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | SNP | BP | Minor | Major | MAF | OR1 | 95% CI | Pvalue1 | Nearest gene | |
| 17 | rs16970540 | 33,338,447 | T | C | 0.10 | 2.20 | 1.59 | 3.04 | 1.73E-06 | RFFL, LIG3 |
| 13 | rs4584690 | 95,680,132 | T | C | 0.18 | 1.85 | 1.42 | 2.42 | 5.46E-06 | ABCC4(MRP4) |
| 13 | rs7335912 | 95,667,680 | G | A | 0.19 | 1.80 | 1.39 | 2.33 | 7.81E-06 | ABCC4(MRP4) |
| 23 | rs73633565 | 13,477,311 | G | A | 0.16 | 1.90 | 1.43 | 2.52 | 8.06E-06 | EGFL6 |
| 18 | rs986117 | 44,128,503 | C | A | 0.36 | 1.62 | 1.31 | 2.01 | 1.04E-05 | LOXHD1 |
| 3 | rs6800849 | 11,790,188 | A | C | 0.17 | 1.84 | 1.40 | 2.41 | 1.15E-05 | VGLL4 |
| 2 | rs13031207 | 226,076,914 | G | A | 0.23 | 1.72 | 1.35 | 2.20 | 1.30E-05 | DOCK10 |
| 17 | rs10083888 | 33,402,980 | T | C | 0.11 | 2.00 | 1.47 | 2.73 | 1.30E-05 | RFFL |
| 7 | rs1108228 | 51,787,366 | A | G | 0.46 | 1.61 | 1.30 | 1.99 | 1.40E-05 | COBL |
| 9 | rs10901329 | 133,924,008 | G | A | 0.43 | 0.61 | 0.49 | 0.76 | 1.41E-05 | LAMC3 |
| 15 | rs4497630 | 30,276,341 | A | G | 0.26 | 0.57 | 0.45 | 0.74 | 1.62E-05 | FAM7A3 |
| 14 | rs11849204 | 34,332,971 | G | A | 0.11 | 1.98 | 1.45 | 2.70 | 1.62E-05 | NPAS3 |
| 11 | rs76647546 | 88,929,531 | G | A | 0.06 | 2.57 | 1.67 | 3.97 | 1.84E-05 | TYR |
| 7 | rs12374901 | 15,024,954 | T | C | 0.43 | 0.62 | 0.50 | 0.77 | 1.88E-05 | DGKB |
| 9 | rs10973146 | 3,706,109 | G | A | 0.06 | 2.42 | 1.61 | 3.65 | 2.13E-05 | GLIS3 |
| 13 | rs17189292 | 95,685,572 | G | A | 0.13 | 1.96 | 1.44 | 2.68 | 2.21E-05 | ABCC4(MRP4) |
| 6 | rs72904381 | 83,130,109 | C | T | 0.45 | 0.63 | 0.51 | 0.78 | 2.30E-05 | TPBG |
| 9 | rs2275139 | 133,924,072 | C | T | 0.43 | 0.62 | 0.50 | 0.78 | 2.35E-05 | LAMC3 |
| 11 | rs10901897 | 50,199,387 | C | T | 0.34 | 0.61 | 0.48 | 0.76 | 2.45E-05 | LOC441601 |
| 2 | rs2278358* | 43,968,167 | A | G | 0.12 | 1.90 | 1.412 | 2.569 | 2.49E-05 | PLEKHH2 |
| 3 | rs717228 | 60,602,895 | C | T | 0.17 | 0.52 | 0.38 | 0.70 | 2.55E-05 | FHIT |
| 4 | rs10023531 | 12,357,990 | A | G | 0.43 | 1.56 | 1.27 | 1.92 | 2.70E-05 | HS3ST1 |
| 3 | rs79082706 | 60,614,582 | G | A | 0.16 | 0.51 | 0.38 | 0.70 | 2.86E-05 | FHIT |
| 2 | rs1443663 | 226,074,083 | A | C | 0.25 | 1.65 | 1.30 | 2.10 | 3.25E-05 | DOCK10 |
| 13 | rs4148524 | 95,767,333 | C | T | 0.14 | 1.84 | 1.38 | 2.45 | 3.45E-05 | ABCC4(MRP4) |
| 11 | rs10082560 | 50,276,354 | C | G | 0.35 | 0.61 | 0.48 | 0.77 | 3.49E-05 | LOC441601 |
| 13 | rs12853814 | 95,720,188 | C | T | 0.13 | 1.93 | 1.41 | 2.64 | 3.55E-05 | ABCC4(MRP4) |
| 17 | rs7214759 | 35,261,346 | G | C | 0.20 | 1.68 | 1.31 | 2.15 | 3.71E-05 | LHX1 |
| 7 | rs2731551 | 18,277,585 | T | C | 0.33 | 1.58 | 1.27 | 1.95 | 3.77E-05 | HDAC9 |
| 7 | rs596699 | 18,351,391 | A | C | 0.34 | 1.57 | 1.26 | 1.94 | 3.82E-05 | HDAC9 |
OR and p values from multivariable logistic regression analysis assuming additive genetic model
Chr = chromosome, SNP = single-nucleotide polymorphism, BP = base position based on hg19/GRCh37, MAF = minor allele frequency, OR = odds ratio, CI = confidence intervals
Gene-level association analyses
To identify potential risk genes consisting of multiple SNPs with a modest functional effect, we performed gene-based association analyses using PLINK set-based tests, and the results are listed in Table 3. None of them reached our Bonferroni significance threshold of p < 2.5 × 10−6. However, seven genes showed suggestive evidence of association with p < 5.0 × 10−4: EIF4G1, FAM131A, GRID2IP, NMUR2, OR10V1, CYP4F22, and LECT1.
Table 3.
Top 30 genes from PLINK-set based test of post-RT pain among breast cancer patients
| Gene symbol | Chr | Entrez ID | Gene name | P (gene) | No. SNPs within a gene | No. SNPs with p < 0.05 | No. SNPs with p < 0.05 and R2 < 0.5 | Top SNP |
|---|---|---|---|---|---|---|---|---|
| EIF4G1 | 3q27.1 | 1981 | Eukaryotic translation initiation factor 4 Gamma 1 | 2.00E-04 | 28 | 1 | 1 | rs4912540 |
| FAM131A | 3q27.1 | 131,408 | Family with sequence similarity 131 member A | 2.00E-04 | 22 | 1 | 1 | rs4912540 |
| GRID2IP | 7p22.1 | 392,862 | Glutamate receptor, ionotropic, delta 2 (Grid2) interacting protein 1, delphilin | 2.00E-04 | 31 | 1 | 1 | rs73674133 |
| NMUR2 | 5q33.1 | 56,923 | Neuromedin U receptor 2 | 2.00E-04 | 22 | 1 | 1 | rs11739168 |
| OR10V1 | 11q12.1 | 390,201 | Olfactory receptor family 10 subfamily V member 1 | 2.00E-04 | 13 | 2 | 1 | rs7937162 |
| CYP4F22 | 19p13.12 | 126,410 | Cytochrome P450 family 4 subfamily F member 22 | 3.00E-04 | 54 | 1 | 1 | rs73514704 |
| LECT1 | 13q14.3 | 11,061 | Leukocyte cell-derived chemotaxin 1, chondromodulin-1 | 4.00E-04 | 23 | 9 | 4 | rs3759509 |
| LDHAL6B | 15q22.2 | 92,483 | Lactate dehydrogenase A like 6B | 5.00E-04 | 38 | 7 | 1 | rs11852359 |
| PPP2R3B | Xp22.33 | 28,227 | Protein phosphatase 2 regulatory subunit B″beta | 0.0007 | 38 | 1 | 1 | rs28485241 |
| PRKCDBP | 11p15.4 | 112,464 | Protein kinase C delta-binding protein | 0.0007 | 38 | 1 | 1 | rs4604857 |
| TRIM64C | 11p11.12 | 646,754 | Tripartite motif containing 64C | 0.0007 | 2 | 1 | 1 | rs1819409 |
| OR52N1 | 11p15.4 | 79,473 | Olfactory receptor family 52 subfamily N member 1 | 0.0008 | 14 | 10 | 1 | rs11607346 |
| PDE4D | 5q12.1 | 5144 | Phosphodiesterase 4D | 0.0008 | 719 | 41 | 18 | rs1498599 |
| PRPH2 | 6p21.1 | 5961 | Peripherin 2 | 0.0008 | 28 | 1 | 1 | rs200618579 |
| RFFL | 17q12 | 117,584 | Ring finger and FYVE like domain containing E3 ubiquitin protein ligase | 0.0008 | 27 | 10 | 4 | rs16970540 |
| OR4C12 | 11p11.12 | 283,093 | Olfactory receptor family 4 subfamily C member 12 | 0.0010 | 1 | 1 | 1 | rs4242812 |
| MMADHC | 2q23.2 | 27,249 | Methylmalonic aciduria and homocystinuria, CblD type | 0.0011 | 17 | 1 | 1 | rs13027589 |
| OR4A47 | 11p11.2 | 403,253 | Olfactory receptor family 4 subfamily A member 47 | 0.0011 | 6 | 3 | 2 | rs7103557 |
| MPO | 17q22 | 4353 | Myeloperoxidase | 0.0012 | 19 | 1 | 1 | rs8178409 |
| OSBP | 20q13.33 | 9885 | Oxysterol-binding protein | 0.0012 | 9 | 1 | 1 | rs4938923 |
| ANAPC13 | 3q22.2 | 25,847 | Anaphase promoting complex subunit 13 | 0.0012 | 18 | 1 | 1 | rs75858178 |
| CEP63 | 3q22.2 | 80,254 | Centrosomal protein 63 | 0.0013 | 29 | 1 | 1 | rs75858178 |
| ROD1 | 9q32 | 9991 | Regulator of differentiation 1 | 0.0014 | 33 | 20 | 2 | rs10817314 |
| SLC20A2 | 8p11.21 | 6575 | Solute carrier family 20 member 2 | 0.0015 | 33 | 2 | 1 | rs7845666 |
| MAP4K3 | 2p22.1 | 8491 | Mitogen-activated protein kinase kinase kinase kinase 3 | 0.0016 | 52 | 1 | 1 | rs17508058 |
| MGST3 | 1q24.1 | 4259 | Microsomal glutathione S-transferase 3 | 0.0016 | 60 | 3 | 1 | rs55977919 |
| CLRN2 | 4p15.32 | 645,104 | Clarin 2 | 0.0017 | 719 | 41 | 18 | rs1498599 |
| FOLH1 | 11q14.3 | 219,595 | Folate hydrolase 1 | 0.0017 | 11 | 5 | 3 | rs679470 |
| GUCY1A3 | 4q32.1 | 2982 | Guanylate cyclase 1 soluble subunit alpha | 0.0018 | 77 | 1 | 1 | rs62327005 |
| UNC119B | 12q24.31 | 84,747 | Unc-119 lipid-binding chaperone B | 0.0018 | 27 | 1 | 1 | rs12825376 |
Chr = chromosome
Pathway analysis
To interpret a gene list derived from gene-based analysis, functional enrichment analysis was performed using bioinformatics tool, DAVID, and results are shown in Table 4. Thirteen biological pathways were enriched with post-RT pain in breast cancer patients (FDR-adjusted p value < 0.05). These 13 biological pathways were then clustered into two groups: glucuronidation activity and olfactory receptor activity (enrichment score 4.60 and 3.41, respectively). These biological activities included xenobiotic and drug metabolism, ascorbate and aldarate metabolism, and olfactory signal transduction, suggesting their roles in underlying mechanisms of post-RT pain.
Table 4.
Top pathways enriched in patients with post-RT pain in breast cancer patients from DAVID functional annotation module analysis
| Cluster | Category | Term | No. genes in a term | Fold enrichment1 | p value2 | FDR3 |
|---|---|---|---|---|---|---|
| 1 | GOTERM_BP_DIRECT | GO:0052697~xenobiotic glucuronidation | 9 | 19.81952 | 3.45E-10 | 9.46E-07 |
| GOTERM_BP_DIRECT | GO:2001030~negative regulation of cellular glucuronidation | 8 | 19.81952 | 6.14E-09 | 8.43E-06 | |
| GOTERM_BP_DIRECT | GO:1904224~negative regulation of glucuronosyltransferase activity | 8 | 19.81952 | 6.14E-09 | 8.43E-06 | |
| GOTERM_BP_DIRECT | GO:0045922~negative regulation of fatty acid metabolic process | 8 | 17.61735 | 2.64E-08 | 2.42E-05 | |
| GOTERM_BP_DIRECT | GO:0052695~cellular glucuronidation | 8 | 10.57041 | 3.62E-06 | 0.001983 | |
| GOTERM_BP_DIRECT | GO:0052696~flavonoid glucuronidation | 9 | 8.494079 | 4.53E-06 | 0.002071 | |
| GOTERM_MF_DIRECT | GO:0015020~glucuronosyltransferase activity | 9 | 7.057233 | 2.18E-05 | 0.019001 | |
| GOTERM_MF_DIRECT | GO:0001972~retinoic acid binding | 8 | 6.818583 | 1.04E-04 | 0.04456 | |
| KEGG_PATHWAY | hsa00053:Ascorbate and aldarate metabolism | 9 | 6.071802 | 6.39E-05 | 0.016351 | |
| KEGG_PATHWAY | hsa00040:Pentose and glucuronate interconversions | 10 | 5.159047 | 8.29E-05 | 0.007101 | |
| KEGG_PATHWAY | hsa00860:Porphyrin and chlorophyll metabolism | 11 | 4.706058 | 7.16E-05 | 0.009189 | |
| KEGG_PATHWAY | hsa05204:Chemical carcinogenesis | 13 | 3.081485 | 8.48E-04 | 0.053271 | |
| KEGG_PATHWAY | hsa00982:Drug metabolism - cytochrome P450 | 12 | 3.189229 | 0.001097 | 0.055041 | |
| 2 | GOTERM_BP_DIRECT | GO:0050911~detection of chemical stimulus involved in sensory perception of smell | 37 | 2.020171 | 8.21E-05 | 0.031689 |
| GOTERM_MF_DIRECT | GO:0004984~olfactory receptor activity | 37 | 1.992656 | 1.09E-04 | 0.03161 | |
| KEGG_PATHWAY | hsa04740:Olfactory transduction | 36 | 1.730048 | 0.001446 | 0.060346 | |
| GOTERM_MF_DIRECT | GO:0004930~G-protein coupled receptor activity | 48 | 1.589467 | 0.0017 | 0.312315 |
1The fold enrichment is defined as the ratio of the two proportions; one is the proportion of genes in your list belong to certain pathway, and the other is the proportion of genes in the background information (i.e., universe genes) that belong to that pathway
2p values from modified Fisher’s exact test
3FDR, false discovery rate from Benjamini and Hochberg
DAVID = Database for Annotation, Visualization and Integrated Discovery, GO = Gene Ontology, KEGG = The Kyoto Encyclopedia of Genes and Genomes
Discussion
This study reported results of the first GWAS of acute post-RT pain in breast cancer patients who had undergone adjuvant RT after surgery. Although no individual association reached genome-wide significance, collectively our results suggest genetic involvement in acute post-RT pain. These results, like all large-scale agnostic search for genetic associations, need validation. At the completion of RT, about 29% of patients reported having clinically relevant pain; of this subset, 30% reported moderate or severe levels of pain at pre-RT, while 70% had no or mild pain at pre-RT. The most significant factor associated with post-RT pain was the presence of pre-RT pain, which is in line with literature reporting that prior pain is the most significant prognostic factor for pain persistence [8, 25]. Besides pre-RT pain, other potential risk factors identified from multivariable regression analyses were included as covariates in the subsequent genetic association analyses to control for confounding effects. We conducted gene-based association analyses and functional enrichment analyses to identify additional loci complementary to GWAS. We identified four suggestive susceptibility loci from GWAS, seven suggestive genes from gene-level analysis, and two significantly enriched functional pathways associated with post-RT pain.
First, we reported four suggestive susceptibility loci for post-RT pain, rs16970540 (17q12), rs4584690 (13q32.1), rs7335912 (13q32.1), and rs73633565 (Xp22.2) proximal to three genes. The most significant marker, rs16970540, is mapped to the 3′-untranslated region (UTR) of RFFL gene or close to LIG3 in chromosome 17. RFFL encodes a protein that regulates several biological processes through the ubiquitin-mediated proteasomal degradation of various target proteins. In the context of irradiation, RFFL negatively regulates p53/tumor protein 53 (TP53), the expression of which can be activated by radiation, directly, or indirectly through its ubiquitination [26]. The loss of TP53 function was related to sensitivity to ionizing radiation. The fraction of p53-positive fibroblasts was significantly higher in cultures from RT-sensitive patients compared to RT-resistant patients after in vitro irradiation [27]. Thus, RFFL can mediate radiation sensitivity via regulation of TP53. On the other hand, LIG3 encodes a protein that catalyzes the joining of DNA ends and is involved in DNA replication, recombination, and repair. LIG3 corrects defective DNA strand-break repair and sister chromatid exchange following RT through base excision repair and alternative non-homologous end-joining pathways. Polymorphisms near the LIG3 gene (rs3744355, rs2074518, and rs3744357) have been reported to be associated with acute breast skin toxicity following RT both in a Japanese cohort (n = 399) and a European Caucasian cohort (n = 480) [28, 29]. It is possible that acute skin toxicity may lead to acute post-RT pain [30]. Thus, LIG3 gene may not be specific to pain, and they can rather be applied to a more common genetic susceptibility to acute RT-induced normal tissue toxicities.
The next significant markers, rs4584690 and rs7335912, were mapped to ABCC4/MRP4 gene, and three additional signals from the list of top 30 SNPs were also mapped to this gene. The Manhattan plot shows a stack of points in chromosome 13 (Fig. 1), which implies a possible haploblock structure and suggests a potential strong association of ABCC4/MRP4 with post-RT pain. The range of pair-wise LD among five SNPs was 0.89–1.00 in CEU population according to the SNAP (https://data.broadinstitute.org/mpg/snpsnap/match_snps.html) (Additional File 2: Fig. S2). ABCC4/MRP4 encodes a protein that is a member of ATP-binding cassette (ABC) transporter superfamily as well as a member of multidrug resistance-associated proteins (MRPs). ABCC4/MPR4 transports most prostaglandins (PGs), which can sensitize spinal neurons to pain. In an animal study with mrp4-knockout mice, Lin et al. showed that a deficiency of mrp4 function led to a significant reduction of extracellular PG levels and consequent altered inflammatory nociceptive responses via modulating cAMP-mediated signaling pathway [31]. In a human candidate gene approach study, ABCC4 rs9524885 has been associated with reduced pain among patients with non-small cell lung cancer [32].
Additionally, we searched gene regulation databases using HaploReg v4.1 (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php) to explore the potential roles of SNPs rs16970540, rs4584690, rs7335912, and rs73633565 as expression quantitative trait loci (eQTLs); rs16970540 exhibited direct eQTL effects (in total 19 hits) in regulating expressions of LIG3 in 12 tissues including blood, skin, nerve, and breast mammary tissues. According to GTEx Portal (https://www.gtexportal.org/home/), for instance, those who were heterozygous (CT) or homozygous (TT) for the minor allele of rs16970540 showed higher expression of LIG3 in breast tissue compared to those homozygous (CC) for the reference allele (OR = 2.63 per allele, p = 5.1 × 10−8).
In gene-based association analyses, we found seven susceptibility genes for post-RT pain: EIF4G1, FAM131A, GRID2IP, NMUR2, OR10V1, CYP4F22, and LECT1. This suggests the involvement of neurotransmitters, olfactory receptor genes, and cytochrome P450 in post-RT pain. Among these genes, Neuromedin U Receptor 2 (NMUR2) has been found to have a role in nociception and inflammation. NMUR2 encodes a receptor protein for Neuromedin, which is a neuropeptide that is widely distributed in the central nervous system. Neuromedin U receptors are a group of Gq/11-protein-coupled receptors. In animal studies, NMUR2-null mice showed a reduced thermal nociceptive response in the hot plate tests and a significant reduction in acute chemo-nociception following capsaicin or formalin injection [33], by inhibiting T-type Ca2+ channel currents via pertussis toxin-sensitive protein kinase A pathway in a dose-dependent manner in mouse small dorsal root ganglion neurons [34]. However, one recent study reported that NMUR2 did not play a role in the development of mechanical hypersensitivity following nerve injury by showing that there were no significant differences in heat hyperalgesia between wild-type and NMUR2-null mice [35]. Further studies are needed to confirm the involvement of NMUR2 in mechanical hypersensitivity in humans, including patients with cancer.
To date, several genome-wide association studies of pain have been reported. According to the NHGRI-EBI GWAS Catalog [36, 37], a total of eight studies reported 30 SNPs associated with any pain. Among these eight studies, four studies reported nine SNPs reaching a genome-wide significance (p values ≤ 5 × 10−8) [38–41], while the other four studies identified suggestive susceptibility loci with p values ≤ 5 × 10−6 [42–45]. In 2013, Kim et al. reported the first GWAS of pain that identified rs2562456 in ZNF429 gene to be significantly associated with acute post-surgery pain (N = 112; p = 2.0 × 10−10) [38]. In 2013, a large study with 7099 Europeans reported another genome-wide significant SNP, rs13361160 in CCT5 gene for widespread pain (p = 1.2 × 10−8). However, this significance was attenuated when it was combined with the replication sample of 9469 Europeans (p = 4.7 × 10−7) [44].
In 2016, Reyes-Gibby et al. reported another genome-wide significant SNP, rs3862188 in RP11-634B7.4 gene for severe pre-treatment pain in head and neck cancer patients (N = 1368; p = 3 × 10−8) [39]. Reyes-Gibby et al. identified 2 years later additional four SNPs which had statistically significant associations with neuropathic pain in head and neck cancer patients (N = 1043; rs10950641, p = 3 × 10−14; rs4804217, p = 3 × 10−9; rs6796803, p = 6 × 10−9; rs4775319, p = 1 × 10−8) [40]. These findings suggest that statistical power may increase when GWAS targets specific types of pain such as neuropathic pain. Another approach to increase statistical power would be a meta-analysis. A recent study of meta-analysis of individual data from 15 cohorts (N = 158,000) reported three SNPs significantly associated with chronic back pain (rs12310519, p = 5 × 10−19; rs7833174, p = 4 × 10−13; rs4384683, p = 2 × 10−10) [41]. Susceptibility genes identified previously [36, 37] for any type of pain traits included CD3E, HMGB1P46, C5, DDC, DIS3L2, ESRRG, GFRA2, DOK2, GPD2, IL1R1, LCLAT1, MCM3, PRKCA, RORA, SNX8, SOX5, TESC, and ZSCAN20 [36]. None of these genes reached the pre-defined significance level of p < 2.5 × 10−6 in our study.
We identified 13 enriched biological pathways for post-RT pain, which were clustered into two groups by DAVID functional annotation module: glucuronidation and olfactory receptor activities. Glucuronidation activity is involved in detoxification and xenobiotic metabolism of substances such as drugs, bilirubin, and fatty-acid derivatives. Glucuronidation transfers glucuronic acid component of uridine diphosphate (UDP)-glucuronic acid to a substrate by UDP-glucuronosyltransferase to make substances more water-soluble, so they can be excreted from body or less toxic. The ascorbate and aldarate metabolism pathway include glucuronidation in the upstream processes of ascorbate synthesis. Ascorbate, which is well known as vitamin C, plays a critical role as an antioxidant in many biological processes such as detoxification of exogenous compounds. Vitamin C has a beneficial effect on pain relief in different pain conditions including cancer pain by decreasing oxidative stress and/or inflammation, which can both be caused by anti-cancer treatments [46, 47]. Ascorbate also functions as a cofactor for a family of enzymes involved in the biosynthesis of neurotransmitters and neuropeptide hormones that can modulate pain transmission.
Olfactory receptor activity can be aligned with our findings of OR10V1 as one of the genes associated with post-RT pain. We also found three additional olfactory receptor genes (OR52N1, OR4C12, OR4A47) included in the top 30 genes. Recently, Reyes-Gibby et al. have reported that genetic variants in RP11-634B7.4 gene, which is annotated as antisense to the three olfactory receptor genes, OR13G1, OR6F1, and OR14A2, were significantly associated with severe pre-treatment pain among patients with head and neck cancer at genome-wide significance levels [39]. The olfactory receptors are members of G-protein-coupled receptors, which are involved in signal transduction and play important roles in many physiological processes including sensory perception, regulation of behavior and mood, regulation of immune system activity and inflammation, and tumor growth and metastasis. The authors speculated that olfactory receptor genes may be involved in pain pathway via activating downstream mitogen-activated kinases (MAPK) signaling pathway [48], by linking to their previous finding of MAPK1/ERK2 as a novel target gene for cancer pain [49]. In fact, there have been many animal experiments to modulate neuropathic cancer pain by inhibiting MAPK signaling pathway using upstream effectors, such as R419, adenosine monophosphate-activated protein kinase activator [50], and bisphosphonates [51]. Considering that majority of breast cancer pain is neuropathic in nature [52, 53], the investigation of a functional mechanism which connects olfactory receptors, MAPK pathway, and pain perception in breast cancer patients may seem worthwhile. More studies in larger populations are needed to validate our findings.
This study has several strengths and limitations. To the best of our knowledge, this is the first report of GWAS of post-RT pain among breast cancer patients of different race and ethnicity. Considering that majority of GWAS data currently available are for NHW, the results from diverse race/ethnic background have more potential for generalizability. Second, the ascertainment of outcome variables was relatively homogeneous compared to large consortium-based studies because we obtained self-reported pain severity data using the same questionnaires from all participating centers. The first limitation of this study is the relatively small sample size, which might have limited the statistical power of the analysis. Based on our findings of rs16970540, with minor allele frequency of 0.1, OR of 2.2, and type 1 error rate of 5 × 10−8, we had only 17% statistical power to be able to reject the null hypothesis. We will need 694 cases and 1673 controls to have at least 80% of statistical power. So, a larger joint GWAS with multiple cohorts is warranted to validate our findings. In addition to limited statistical power, the failure of GWAS may be attributed to the complex nature of the phenotype, post-RT pain, we evaluated. Pain is a more complex functional end-point, which is affected by multiple genes within a pathway rather than a simple Mendelian disease. We employed gene-based association analyses and pathway-based analyses to increase statistical power as well as to find additional genetic loci underlying molecular mechanisms of post-RT pain. Another limitation would be the lack of replication with an independent dataset.
Conclusion
In the current study, we conducted GWAS, gene-based association analyses, and pathway-based functional enrichment analyses to evaluate the genetic risk loci for acute post-RT pain among breast cancer patients. We identified two biological processes, glucuronidation activity and olfactory receptor activity, in addition to the potential role of LIG3, ABCC4/MPR4, and EGFL6 from GWAS, were involved in post-RT pain, which showed that post-RT pain is a polygenic trait. Post-RT pain can be affected by DNA damage/repair, transporter and receptor activity in signal transduction, and cellular detoxification via glucuronidation activity. Larger studies are warranted to validate our findings to facilitate the discovery of underlying genetic/molecular mechanisms of pain related to cancer treatments. The results can ultimately contribute to the development of prevention and/or intervention strategies to improve cancer pain management and QOL in cancer patients.
Additional files
Figure S1. Q-Q plots for post-RT pain. This figure shows the quantile-quantile plots of observed versus expected p values on the −log10 scale, showing the conformity of the observed results to expectations under the null. Black lines indicate the distribution of observed p value versus expected p value, and red lines indicate the null distribution. Lambda confirms appropriate control of population substructure; (a) 1.649 before adjustment, (b) 1.017 after adjusting for population substructure with the first 3 PCs, and (c) 1.016 after further adjusting for all potential confounders identified in Table 1. PCs: principal components. (DOCX 32 kb)
Figure S2. Regional association plot for rs4584690 on chromosome 13 located nearby ABCC4/MRP4 gene. The y axis is −log10 of p values and x axis is the genomic location of each SNP. Linkage disequilibrium coefficients were derived from hg19 (1000 Genomes March 2012, European population) and local estimates of recombination rates are from HapMap samples (2008–03_rel22_B36; ftp://ftp.ncbi.nlm.nih.gov/hapmap/). The plot was generated using LocusZoom (http://locuszoom.org/). (DOCX 123 kb)
Acknowledgements
The authors are thankful to all women who participated in the study and the clinical staff at the radiation oncology clinics for their support. The authors would like to thank Martine Poitevien, Omar Nelson, Gulya Kourman, L. Doug Case, Mark O. Lively, and Mark Morris for their contributions. The authors would also like to thank each of the CCOP sites and/or protocol principal investigators who participated in this trial: Southeast Cancer Control Consortium, James Atkins MD; Randolph Cancer Center Southeast Cancer Control Consortium, Sandra E. Mitchell, MD; Wichita CCOP/Cancer Center of Kansas, David Bryant, MD; St. Louis – Cape Girardeau CCOP, Bethany Sleckman MD and Kathy Baglan, MD; Upstate Carolina CCOP, James Bearden MD; Delaware/Christiana Care Health Services CCOP, Jon F. Strasser, MD; John H. Stroger, Jr. Hospital of Cook County MBCCOP, Thomas Lad MD and Anu Thakrar, MD; Hematology–Oncology Associates of Central New York CCOP, Jeffrey Kirshner MD; Delaware/Christiana Care Health Services CCOP, Stephen Grubbs MD; Wichita CCOP, Shaker Dakhil MD; San Juan MBCCOP, Luis Baez MD; North Shore University Hospital CCOP, Vincent Vinciguerra MD; Comprehensive Cancer Center Wake Forest University Research Base CCOP (CCCWFU RBCCOP), Edward Shaw MD; Colorado Cancer Research Program CCOP, Karen Sturtz MD; Northern Indiana Cancer Research Consortium CCOP, Robin Zon MD.
Abbreviations
- AAn
African American/black
- BCS
Breast-conserving surgery
- BMI
Body mass index
- CI
Confidence interval
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- eQTLs
Expression quantitative trait loci
- GO
Gene Ontology
- GWAS
Genome-wide association study
- HER2
Human epidermal growth factor receptor 2
- HW
Hispanic Whites
- HWE
Hardy-Weinberg equilibrium
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LD
Linkage disequilibrium
- NHW
Non-Hispanic Whites
- OR
Odds ratio
- PC
Principal component
- PCA
Principal component analysis
- QOL
Quality of life
- Q-Q
Quantile-quantile
- RT
Radiotherapy
- SD
Standard deviation
- SNP
Single-nucleotide polymorphism
- UDP
Uridine diphosphate
Authors’ contributions
EL, CT, JLW, ERM, JJU, CDL, GJL, EGS, and JJH designed the study. CT, JLW, JJU, and EGS developed the methodology of patient enrollment. EL, CT, JLW, JJU, EGS, and JJH collected data. EL, SHS, and ERM conducted the analysis and EL, ERM, and JJH interpreted results. EL and JJH drafted the manuscript. All authors reviewed and commented on the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the National Cancer Institute grants: R01CA135288 (to JJH) and U10CA081857 (to GJL and EGS); the University of Miami Fuente Cancer Pain Pre-doctoral fellowship (to EL); and the University of Central Florida start-up funds (to EL).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All women participated in the study provided written informed consent. The present study was approved by the Institutional Review Boards of the University of Miami, the Jackson Memorial Hospital, and the Wake Forest Research Base Community Clinical Oncology Programs.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eunkyung Lee, Email: eunkyung.lee@ucf.edu.
Jennifer J. Hu, Email: jhu@med.miami.edu
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative G. Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Gibby C, Morrow PK, Bennett MI, Jensen MP, Shete S. Neuropathic pain in breast cancer survivors: using the ID pain as a screening tool. J Pain Symptom Manag. 2010;39(5):882–889. doi: 10.1016/j.jpainsymman.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundstedt D, Gustafsson M, Malmstrom P, Johansson KA, Alsadius D, Sundberg A, et al. Symptoms 10-17 years after breast cancer radiotherapy data from the randomised SWEBCG91-RT trial. Radiother Oncol. 2010;97(2):281–287. doi: 10.1016/j.radonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Lundstedt D, Gustafsson M, Steineck G, Malmstrom P, Alsadius D, Sundberg A, et al. Risk factors of developing long-lasting breast pain after breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):71–78. doi: 10.1016/j.ijrobp.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 8.Lee E, Takita C, Wright JL, Reis IM, Zhao W, Nelson OL, et al. Characterization of risk factors for adjuvant radiotherapy-associated pain in a tri-racial/ethnic breast cancer population. Pain. 2016;157(5):1122–1131. doi: 10.1097/j.pain.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13(5):425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens K, Cooper BA, West C, Paul SM, Baggott CR, Merriman JD, et al. Associations between cytokine gene variations and severe persistent breast pain in women following breast cancer surgery. J Pain. 2014;15(2):169–180. doi: 10.1016/j.jpain.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caronia D, Martin M, Sastre J, de la Torre J, Garcia-Saenz JA, Alonso MR, et al. A polymorphism in the cytidine deaminase promoter predicts severe capecitabine-induced hand-foot syndrome. Clin Cancer Res. 2011;17(7):2006–2013. doi: 10.1158/1078-0432.CCR-10-1741. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Giralt N, Rodriguez-Sanz M, Prieto-Alhambra D, Servitja S, Torres-Del Pliego E, Balcells S, et al. Genetic determinants of aromatase inhibitor-related arthralgia: the B-ABLE cohort study. Breast Cancer Res Treat. 2013;140(2):385–395. doi: 10.1007/s10549-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 13.Hu JJ, Urbanic JJ, Case LD, Takita C, Wright JL, Brown DR, et al. Association between inflammatory biomarker C-reactive protein and radiotherapy-induced early adverse skin reactions in a multiracial/ethnic breast cancer population. J Clin Oncol. 2018;36(24):2473–2482. doi: 10.1200/JCO.2017.77.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JL, Takita C, Reis IM, Zhao W, Lee E, Nelson OL, et al. Prospective evaluation of radiation-induced skin toxicity in a race/ethnically diverse breast cancer population. Cancer Med. 2016;5(3):454–464. doi: 10.1002/cam4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 16.Butt Z, Wagner LI, Beaumont JL, Paice JA, Peterman AH, Shevrin D, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manag. 2008;35(1):20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton symptom assessment scale in cancer patients: a systematic review. J Pain Symptom Manag. 2013;45(6):1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 20.Dupont WD, Plummer WD. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 21.Turner S. Ensembl/Entrez hg19/GRCh37 Consensus Genes: figshare; 2012 [Available from: https://figshare.com/articles/hg19_GRCh37_Consensus_Genes/103113/4. Accessed 3 Sep 2016
- 22.Veyrieras JB, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, Stephens M, et al. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;4(10):e1000214. doi: 10.1371/journal.pgen.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmans P. Statistical methods for pathway analysis of genome-wide data for association with complex genetic traits. Adv Genet. 2010;72:141–179. doi: 10.1016/B978-0-12-380862-2.00007-2. [DOI] [PubMed] [Google Scholar]
- 24.Huang d W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Zhao J, Wu CW, Zhang L, Liu X, Kang W, et al. Tumor suppressor functions of miR-133a in colorectal cancer. Mol Cancer Res. 2013;11(9):1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 27.Nuta O, Somaiah N, Boyle S, Chua ML, Gothard L, Yarnold J, et al. Correlation between the radiation responses of fibroblasts cultured from individual patients and the risk of late reaction after breast radiotherapy. Cancer Lett. 2016;374(2):324–330. doi: 10.1016/j.canlet.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Suga T, Ishikawa A, Kohda M, Otsuka Y, Yamada S, Yamamoto N, et al. Haplotype-based analysis of genes associated with risk of adverse skin reactions after radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;69(3):685–693. doi: 10.1016/j.ijrobp.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Murray RJ, Tanteles GA, Mills J, Perry A, Peat I, Osman A, et al. Association between single nucleotide polymorphisms in the DNA repair gene LIG3 and acute adverse skin reactions following radiotherapy. Radiother Oncol. 2011;99(2):231–234. doi: 10.1016/j.radonc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Pignol J-P, Thuc Vu TT, Mitera G, Bosnic S, Verkooijen HM, Truong P. Prospective evaluation of severe skin toxicity and pain during postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91(1):157–164. doi: 10.1016/j.ijrobp.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Lin ZP, Zhu YL, Johnson DR, Rice KP, Nottoli T, Hains BC, et al. Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol Pharmacol. 2008;73(1):243–251. doi: 10.1124/mol.107.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloan JA, de Andrade M, Decker P, Wampfler J, Oswold C, Clark M, et al. Genetic variations and patient-reported quality of life among patients with lung cancer. J Clin Oncol. 2012;30(14):1699–1704. doi: 10.1200/JCO.2010.34.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, et al. Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses. Pain. 2007;130(3):267–278. doi: 10.1016/j.pain.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Zhang Y, Jiang X, Zhang Y, Zhang L, Gong S, et al. Neuromedin U inhibits T-type Ca2+ channel currents and decreases membrane excitability in small dorsal root ganglia neurons in mice. Cell Calcium. 2011;49(1):12–22. doi: 10.1016/j.ceca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert AK, Puma C, Xu X, Laird JM. Neuromedin U receptor 2 does not play a role in the development of neuropathic pain following nerve injury in mice. Eur J Pain (London, England). 2013;17(8):1147–1155. doi: 10.1002/j.1532-2149.2013.00288.x. [DOI] [PubMed] [Google Scholar]
- 36.MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, et al. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS catalog) Nucleic Acids Res. 2017;45(D1):D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–D1D12. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Ramsay E, Lee H, Wahl S, Dionne RA. Genome-wide association study of acute post-surgical pain in humans. Pharmacogenomics. 2009;10(2):171–179. doi: 10.2217/14622416.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Gibby CC, Wang J, Silvas MR, Yu RK, Hanna EY, Shete S. Genome-wide association study suggests common variants within RP11-634B7.4 gene influencing severe pre-treatment pain in head and neck cancer patients. Sci Rep. 2016;6:34206. doi: 10.1038/srep34206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes-Gibby CC, Wang J, Yeung SJ, Chaftari P, Yu RK, Hanna EY, et al. Genome-wide association study identifies genes associated with neuropathy in patients with head and neck cancer. Sci Rep. 2018;8(1):8789. doi: 10.1038/s41598-018-27070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suri P, Palmer MR, Tsepilov YA, Freidin MB, Boer CG, Yau MS, et al. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet. 2018;14(9):e1007601. doi: 10.1371/journal.pgen.1007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng W, Deshmukh HA, van Zuydam NR, Liu Y, Donnelly LA, Zhou K, et al. A genome-wide association study suggests an association of Chr8p21.3 (GFRA2) with diabetic neuropathic pain. Eur J Pain (London, England) 2015;19(3):392–399. doi: 10.1002/ejp.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng W, Deshmukh HA, Donnelly LA. Wellcome Trust Case control C, surrogate markers for M, macro-vascular hard endpoints for innovative diabetes tools study g, et al. a genome-wide association study provides evidence of sex-specific involvement of Chr1p35.1 (ZSCAN20-TLR12P) and Chr8p23.1 (HMGB1P46) with diabetic neuropathic pain. EBioMedicine. 2015;2(10):1386–1393. doi: 10.1016/j.ebiom.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters MJ, Broer L, Willemen HL, Eiriksdottir G, Hocking LJ, Holliday KL, et al. Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis. 2013;72(3):427–436. doi: 10.1136/annrheumdis-2012-201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warner Sophie C, van Meurs Joyce BJ, Schiphof Dieuwke, Bierma-Zeinstra Sita M, Hofman Albert, Uitterlinden Andre G, Richardson Helen, Jenkins Wendy, Doherty Michael, Valdes Ana M. Genome-wide association scan of neuropathic pain symptoms post total joint replacement highlights a variant in the protein-kinase C gene. European Journal of Human Genetics. 2017;25(4):446–451. doi: 10.1038/ejhg.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunes-Bayir A, Kiziltan HS. Palliative vitamin C application in patients with radiotherapy-resistant bone metastases: a retrospective study. Nutr Cancer. 2015;67(6):921–925. doi: 10.1080/01635581.2015.1055366. [DOI] [PubMed] [Google Scholar]
- 47.Carr AC, Vissers MC, Cook JS. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol. 2014;4:283. doi: 10.3389/fonc.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benbernou N, Esnault S, Galibert F. Activation of SRE and AP1 by olfactory receptors via the MAPK and rho dependent pathways. Cell Signal. 2013;25(6):1486–1497. doi: 10.1016/j.cellsig.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Reyes-Gibby CC, Wang J, Silvas MR, Yu R, Yeung SC, Shete S. MAPK1/ERK2 as novel target genes for pain in head and neck cancer patients. BMC Genet. 2016;17:40. doi: 10.1186/s12863-016-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mejia Galo L., Asiedu Marina N., Hitoshi Yasumichi, Dussor Gregory, Price Theodore J. The potent, indirect adenosine monophosphate-activated protein kinase activator R419 attenuates mitogen-activated protein kinase signaling, inhibits nociceptor excitability, and reduces pain hypersensitivity in mice. PAIN Reports. 2016;1(1):e562. doi: 10.1097/PR9.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Tan YH, Light AR, Mao J, Yu AC, Fu KY. Alendronate Attenuates Spinal microglial activation and neuropathic pain. J Pain. 2016;17(8):889–903. doi: 10.1016/j.jpain.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Bokhari FN, McMillan DE, McClement S, Daeninck PJ. Pilot study of a survey to identify the prevalence of and risk factors for chronic neuropathic pain following breast cancer surgery. Oncol Nurs Forum. 2012;39(2):E141–E1E9. doi: 10.1188/12.ONF.E141-E149. [DOI] [PubMed] [Google Scholar]
- 53.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. J Am Med Assoc. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Q-Q plots for post-RT pain. This figure shows the quantile-quantile plots of observed versus expected p values on the −log10 scale, showing the conformity of the observed results to expectations under the null. Black lines indicate the distribution of observed p value versus expected p value, and red lines indicate the null distribution. Lambda confirms appropriate control of population substructure; (a) 1.649 before adjustment, (b) 1.017 after adjusting for population substructure with the first 3 PCs, and (c) 1.016 after further adjusting for all potential confounders identified in Table 1. PCs: principal components. (DOCX 32 kb)
Figure S2. Regional association plot for rs4584690 on chromosome 13 located nearby ABCC4/MRP4 gene. The y axis is −log10 of p values and x axis is the genomic location of each SNP. Linkage disequilibrium coefficients were derived from hg19 (1000 Genomes March 2012, European population) and local estimates of recombination rates are from HapMap samples (2008–03_rel22_B36; ftp://ftp.ncbi.nlm.nih.gov/hapmap/). The plot was generated using LocusZoom (http://locuszoom.org/). (DOCX 123 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.