Short abstract
Hexavalent chromium (Cr(VI)) is a highly mutagenic and carcinogenic chemical used in many industrial processes. Occupational exposure to chromium, occurring mostly by inhalation, constitutes a major lung cancer risk affecting chromium workers. Environmental exposure, on the other hand, mainly by ingestion of contaminated drinking water, is a widespread gastrointestinal cancer risk, affecting millions of people throughout the world. One of the major mechanisms through which Cr(VI) causes carcinogenic transformation is thought to be the disruption of transcriptional regulation. Indeed, Cr(VI)-directed DNA damage and crosslinking occurs preferentially at sites where active DNA replication and transcription processes take place. Accordingly, numerous studies have shown that Cr(VI) causes gene expression changes in a wide range of cell signaling pathways, resulting from Cr(VI)-induced direct macromolecular damage, alteration in transcription factor function, and disruption of epigenetic signatures. This brief review highlights past and current information on the impact of Cr(VI) on the various mechanisms of transcriptional regulation.
Impact statement
This mini-review highlights current evidence on the mechanisms through which hexavalent chromium (Cr(VI)) disrupts transcriptional regulation, an emerging area of interest and one of the central processes by which chromium induces carcinogenesis. Several studies have shown that Cr(VI) causes widespread DNA damage and disrupts epigenetic signatures, suggesting that chromatin may be a direct Cr(VI) target. The findings discussed here suggest that Cr(VI) disrupts transcriptional regulation by causing genomic architecture changes.
Keywords: Epigenetics, chromatin, genotoxicity, mechanisms, toxicology, transcription
Introduction
Chromium (Cr) is an element abundantly available in the environment with unique chemical traits that make its various species valuable through both function and aesthetics. Of the several valence configurations that exist, the forms most commonly encountered in nature are trivalent, hexavalent, and elemental chromium (Cr(III), Cr(VI), and Cr(0), respectively). The toxicological profiles of elemental and trivalent chromium show little to no health risk; however, compounds containing hexavalent chromium possess notable toxicities and are major contributors to occupational exposure.
Although natural sources of hexavalent chromium exist, most of this compound is produced anthropogenically, with the intent to be used in a wide range of industrial applications, including stainless steel production and welding, chrome electroplating, pigments and dyes, and the manufacture of chromate compounds themselves.1 It has been estimated that 558,000 US workers and 786,000 EU workers are exposed occupationally to Cr(VI), primarily through inhalation and dermal contact.2 However, imperfect processing and disposal techniques expose a much larger proportion of local communities to chromium through airborne emissions and water contamination via industrial release and wastewater leach.1,2 A recent study investigating the sources of groundwater Cr(VI) in California found that industrial contamination was largely responsible for acute concentrations, while monitoring wells in regions considered free of industrial pollution exceeded the 10 µg L−1, possibly as a result of Cr(III) oxidation through anthropogenic and natural processes.3,4 The contamination of Cr(VI) in ground waters, despite the complexity of its originating sources, indicates that much larger populations are chronically exposed to hexavalent chromium through ingestion and highlights the need for studies assessing the potential adverse health outcomes associated with exposure.
Notably, Cr(VI) is a well-established respiratory carcinogen, though the mechanisms promoting carcinogenesis and progression remain unclear. Limited epidemiological data exist to suggest carcinogenic outcomes in humans following ingestion,1,2 although numerous animal studies have suggested that at the molecular level, Cr(VI) has carcinogenic properties regardless of the route of exposure.5,6 As a result, continued mechanistic studies of Cr(VI)’s role in genotoxicity and carcinogenesis may provide better foundations for risk assessment regarding population-level exposures. Past and current studies performed by several labs have identified that Cr(VI) may promote carcinogenesis through several different processes that include DNA damage and increased chromosome instability, protein–Cr–DNA adduct formation, silencing of tumor suppressors, and modification of epigenetic markers involved in the regulation of transcription and chromatin accessibility.
The intracellular fate of Cr(VI) and its toxicological outcomes
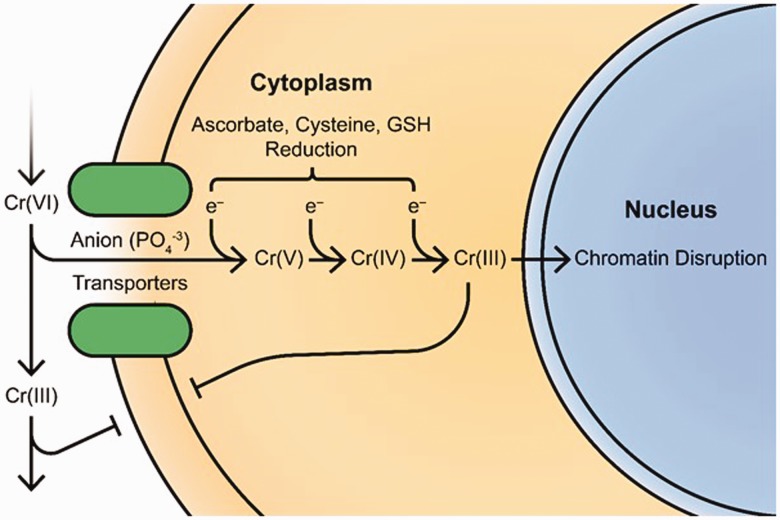
Key steps in the biotransformation and bioactivation of Cr(VI) are schematically presented in Figure 1. Following absorption, the majority of Cr(VI) is extracellularly reduced to Cr(III), which does not efficiently diffuse across cell membranes; the remaining Cr(VI) is rapidly transported across the cell membrane by the sulfate and phosphate anion transporters. Antioxidants such as glutathione (GSH), ascorbate, and cysteine reduce Cr(VI) stepwise one electron at a time to its trivalent state with ascorbate being the major reducer, forming reactive intermediates in between that include Cr(V), Cr(IV), and ultimately Cr(III). Reduction by ascorbate involves two simultaneous electrons, thus reducing the hexavalent form to Cr(IV) directly and skipping Cr(V), the first intermediate. The intermediates generated at each step are highly reactive and able to interact with DNA mainly at the phosphate backbone, generating both binary (Cr–DNA) and ternary adducts (X–Cr–DNA) with ascorbate, GSH, cysteine, and proteins. While DNA–Cr–DNA adducts have been demonstrated in vitro, they may be the result of experimental conditions in vitro that do not take place in vivo and may not be major contributors to the genotoxicity of chromium towards DNA.7 Numerous studies have shown that chromium frequently crosslinks free amino acids, antioxidants such as GSH and ascorbate, and proteins to DNA, which then becomes a target of multiple DNA repair processes, most notably the mismatch repair pathway.8–10 Of particular interest, Cr(VI)’s genotoxicity is exacerbated by the protective mechanisms used to detoxify it. Reynolds et al. have shown that the restoration of ascorbate, the main reducer of Cr(VI), in vitro prior to Cr(VI) increased chromosomal damage significantly and exhibited a 10-fold increase in mutagenicity.11
Figure 1.
Cr(VI) uptake and biotransformation. Following absorption, Cr(VI) is largely reduced in extracellular fluids to Cr(III), hindering diffusion across cell membranes and promoting excretion from the body. Remaining Cr(VI) is rapidly shuttled across the lipid bilayer through non-specific anion transporters and reduced in a step-wise manner to Cr(III) by antioxidants, forming reactive intermediates capable of interacting with several intracellular substrates. Of note, chromium forms binary and ternary adducts with chromatin resulting in the disruption of regulatory mechanisms and double-strand break formation. (A color version of this figure is available in the online journal.)
Cr(VI) disrupts transcriptional regulation
Many studies have explored the transcriptomic changes that occur following exposure to Cr(VI), to identify the mechanisms that alter the transcriptional response. Intracellular Cr(VI) reduction generates a range of reactive oxygen species that damage key macromolecules, resulting in widespread changes in gene expression. A wide range of cell signaling pathways respond to Cr(VI) exposure including redox stress, calcium mobilization, energy metabolism, DNA repair, biosynthetic, cell proliferation, apoptosis, growth, differentiation, survival, and cell cycle regulation pathways.12,13 As an example, Cr(VI) activates the MAPK family components JNK, p-38, and ERK1/2,14 the Src family kinases,15 NF-kB, AP-1, and Akt signaling16; and downregulates the expression of several tumor suppressor genes including p16,17 hMLH1,18,19 APC, MGMT,19 PDCD4,20 p15, p18, p19, p21, and p2721 through diverse mechanisms. Direct DNA damage, bulky DNA–protein crosslinks, altered transcription factor function, epigenetic changes, and shifts in chromatin accessibility are some of the mechanisms through which Cr(VI) disrupts transcriptional programs. Figure 2 shows a summary of the sequence of molecular events leading to the disruption of transcription by chromium.
Figure 2.
Cr(VI) disrupts transcriptional regulatory mechanisms. The reduction of intracellular Cr(VI) yields reactive intermediates that damage several key macromolecules and form bulky DNA lesions, such as Cr–DNA adducts and protein–Cr–DNA crosslinks, resulting in widespread DNA double-strand breaks, arrest of RNA polymerase-II (RNAPII) transcription elongation and global chromatin disruption. Direct effects of Cr(VI) on proteins such as transcription factors lead to transcriptional disruption in pathways regulated by these proteins. The altered chromatin configuration arising from Cr(IV) bulky lesions gives way to conflicting epigenetic marks (altered DNA methylation, histone modifications, and non-coding RNA profiles) that not only inhibit transcription of inducible genes but also destabilize the global transcription machinery.
Cr(VI) alters transcription factor and epigenetic modifier functions
Cr(VI)-induced direct DNA damage accounts for major transcriptional disruption due to RNA polymerase arrest and elongation stasis. The presence of DNA double-strand breaks (DSBs), DNA–protein crosslinks, DNA–chromium, and X–DNA–chromium adducts combine to suppress mRNA transcript synthesis.22,23 The fact that chromium-induced DNA lesions and crosslinks form preferentially in the nuclear matrix DNA,24 a nuclear sub-compartment where transcription and replication occur, suggests that Cr(VI) targets transcriptional regulatory mechanisms and directly impacts transcription factor function. Accordingly, Cr(VI) has been shown to impair the expression of highly inducible genes that bear active promoters, without blocking the transcription of constitutively expressed genes. Bulky DNA–protein lesions arising from Cr(VI)-mediated crosslinks are thought to block the expression of several inducible genes.25–28 Evidence from our laboratory indicates that Cr(VI) crosslinks the repressive DNMT1-HDAC1/2 complex to the Cyp1a1 promoter, preventing RNAPII recruitment and induction by the aryl hydrocarbon receptor ligand, benzo[a]pyrene.25 It is thus plausible that global changes in the transcriptome arise partly from Cr(VI)-induced DNA–protein crosslinks involving epigenetic writer/reader molecules that cause extensive epigenomic alterations and shifts in chromatin accessibility. Context-dependent changes in transcription can be anticipated, depending on the local chromatin configuration, the specific epigenetic factor that is crosslinked, and the affected genomic locus.
Cr(VI) selectively hinders heterodimerization of coactivator molecules with transcription factors, therefore curtailing the expression of target genes. Pretreatment with Cr(VI) prevented dimerization between the p65 subunit of NF-κB and the transcriptional coactivator CBP, inhibiting IL-8 induction.29 In addition, Cr(VI) inhibited the transactivation domains of the MTF1 transcription factor,30 preventing heterodimerization with the histone acetylase complex p300 and RNAPII recruitment to the metallothionein-1 gene promoter.26 Cr(VI)-generated ROS and alterations in kinase activity are thought to alter the transactivation activity of both p300 and CBP, and to limit their interaction with transcriptional factors.
While these and other studies31 have shown that Cr(VI) does not affect the binding of transcription factors to DNA, Cr(III) complexes were shown to alter Sp1 and TFIID binding to cognate DNA sequences, thereby blocking active transcription of reporter genes.32 Furthermore, work using a thiolate complex modeling a 2-cysteine, 2-histidine zinc finger (similar to the zinc fingers found in CCCTC Binding Factor (CTCF)) indicates that Cr(V/IV) derivatives cause release of Zn(II) from the tetrahedral zinc finger.33 Zinc finger proteins are a large and widely diverse family of DNA, RNA, and protein-binding proteins involved in transcription, epigenetic, and chromatin regulation. This raises the important question whether Cr(VI) destabilizes the DNA binding ability of zinc finger proteins and if this mechanism drives transcriptional and epigenetic disruption after Cr(VI) exposure.
The epigenome as a target of chromium
DNA methylation
DNA methylation is a well-studied epigenetic mark that responds dynamically to diverse environmental factors and is important to modulate gene expression and maintain genomic stability.34 DNA methylation state directs genomic binding of several classes of transcription factors and regulatory proteins.35 For example, CTCF, a protein that controls genome architecture and transcription, has been shown to have varying genomic occupancy that is tightly linked with the DNA methylation status; regions with high methylation having low CTCF binding.36 With Cr(VI)-induced widespread methylome changes, it can be anticipated that there is global disruption in transcriptional regulation arising from diverse changes in transcription factor and co-regulator protein binding.
Cr(VI) upregulates the stress protein NUPR1 via promoter demethylation37 and Cr(III) treatment hypomethylates the 45S ribosomal RNA gene in mouse sperm.38 Studies in human lung cancer samples indicate that chromium specifically targets and silences the tumor suppressor genes p16INK4a and Human MUTL Homolog 1 (hMLH1) through promoter hypermethylation, though the question remains whether this is a targeted response or the selection of cell populations with deficient repair systems.17,19 Global DNA hypomethylation induced by chromium has been shown to contribute to genomic instability and cell cycle arrest39 in vitro, in vivo, and in exposed chromate manufacturing workers.39–41 Cr(VI) also causes a general decline in the DNA demethylation intermediates 5-hydroxylmethylcytosine, 5-formylcytosine, and 5-carboxycytosine, and inhibits the TET demethylation proteins,42 likely through depletion of ascorbate which is a cofactor of the TET proteins alongside α-ketoglutarate and iron. Furthermore, the resultant massive DNA damage after Cr(VI) exposure may recruit most of the repair proteins that are also required for base excision repair that occurs during active DNA demethylation. Overall, genome-wide studies to characterize the methylome response to Cr(VI) have yet to be carried out. These will detail the genomic sites of methylation changes, and whether these correlate with Cr(IV) changes in other epigenetic marks and alterations in gene transcription.
Post-translational histone modifications
Post-translational histone modifications play a crucial role in regulating chromatin accessibility and transcription. While histone acetylation is generally associated with open and transcriptionally active chromatin, the role of histone methylation in regulating gene expression is context-dependent. For example, whereas tri-methylated lysine-4 and lysine-6 in histone H3 (H3K4me3 and H3K6me3) occur in actively transcribing genes, tri-methylated lysine-9 and lysine-27 in the same histone (H3K9me3 and H3K27me3) are associated with gene silencing.43
Cr(VI) treatment reduces the global H3 and H4K16 acetylation levels, an effect attributed to the direct downregulation of the histone acetyltransferase, MOF, by NUPR1 which are induced upon Cr(VI) exposure.37 Chromium was also found to increase the levels of H3K4me3, and the repressive H3K9me2 and H3K9me3 marks by upregulating expression of the histone methyltransferases G9a, GLP, and SUV39H1, but decreased the global presence of H3K27me3 and H3R2me2.18,44,45 Wang et al., however, reported an increase in H3K27me3 that was attributed to Cr(VI) upregulation of EZH1.45 Cr(VI) depletes cellular ascorbate required as a cofactor of the H3K9me2 demethylase JHDM2A, and supplementation of Cr(VI)-treated cells with ascorbate led to H3K9me2 demethylation.37 Thus, the increase in H3K9me2 corresponds to depletion of cellular ascorbate stores. At specific gene targets, chromium upregulates the expression of NUPR1 by increasing promoter H3K9 and H3K14 acetylation,37 while silencing hMLH1 by increasing the promoter presence of H3K9me2.18
A hallmark of cancer, loss of global H4K16 acetylation co-occurs with loss of H4K20me3 and DNA hypomethylation of repetitive sequences.46 Additionally, a global increase in H3K9me3 is associated with tumor progression in multiple cancers.47 These chromium-induced alterations in local and global histone marks indicate widespread changes in chromatin accessibility and transcription, and a collapse of epigenetic homeostasis. Furthermore, the altered chromatin environment may impact the processing and repair of DSBs induced by Cr(VI), since for DSB repair to proceed, it is necessary for the local chromatin to undergo nucleosomal displacement and relaxation by histone acetylation and chromatin remodeling.48 Altogether, whether the DNA methylation and histone modification changes arising from chromium exposure may directly influence chromatin reorganization remains a question to be investigated. Alternatively, these epigenetic changes may arise from direct effects of Cr(VI) on the chromatin landscape.
MicroRNAs
MicroRNAs are a large family of short RNA oligonucleotides that silence target gene expression by binding mRNA and leading to its degradation. Since miRNA regulate broad transcriptional networks, they are involved in several important biological processes.49 In steel and chromate production workers, chromium exposure was found to increase the expression of miR-22250 and decrease the expression of miR-3940-5p, which was associated with DNA damage.51 These results were replicated in vitro, where Cr(VI) downregulated the expression of miR-3940-5p allowing for DNA repair to proceed.52 In a recent study, He et al. showed that miR-143 was significantly suppressed in Cr(VI)-transformed cells, which exhibited a malignant phenotype attributed to miR-143 downregulation.53 Chromium-induced upregulation of miR-21 and the resultant downregulation of the tumor suppressor gene PDCD4 have been implicated in the malignant transformation of cells by Cr(VI).20 It is thus plausible that Cr(VI) disrupts multiple transcription pathways within the transcriptome through the direct dysregulation of miRNA expression profiles.
Chromatin structure and function
Transcriptional regulation hinges on the coordinated binding of transcription factors to canonical DNA motifs, a configuration that arises from local nucleosomal displacement and chromatin remodeling events. On a higher level, genomic regions with similar transcriptional activity and chromatin configuration are organized into chromatin loops and insulated topologically associating domains by CTCF and the cohesin complex. Previous work in our laboratory indicates that Cr(VI) alters chromatin accessibility by causing local and genome-wide nucleosomal position shifts and occupancy changes.54 These changes disrupt the accessibility of transcription factor binding motifs of CTCF, AP1, BORIS, and BACH2 proteins.54,55 Cr(VI) opens the chromatin surrounding AP1 sites, while the BACH2 and CTCF binding sites are opened in a manner dependent on Cr(VI) concentration.55 This chromatin changes may not only impact CTCF and AP1 occupancy of their cogent DNA motifs, but it may also predispose these sites to direct damage by Cr(VI). Furthermore, direct transcriptional changes of genes within the AP1 and BACH2 signaling pathways are affected, and it is likely that Cr(VI)-induced alterations in CTCF function may give rise to global changes in three dimensional (3D) chromatin organization and widespread disruption in the transcriptome.
A hallmark of Cr(VI) exposure as it pertains to DNA damage is the formation of phosphorylated γ-H2AX foci, a well-established marker for DSBs. Interestingly, DeLoughery et al. have found that Cr–DNA adducts form non-preferentially across the genome; however, formation of DSBs was localized to euchromatin, or active regions of the genome, and were likely initiated as single-stranded as a result of ATR activation.56 Recent advances have demonstrated that DSBs occurring within a transcribed gene can recruit factors that suppress expression until the damage is resolved. However, as noted by DeLoughery, genes that are initially activated may become suppressed over time due to localized DSB formation. Chronic, low-dose exposure to Cr(VI) in Hepa-1c1c7 cells resulted in the gradual accumulation of y-H2AX foci of which many failed to resolve following removal of the challenge,57 suggesting that DNA repair might be increasingly deficient over time. It is plausible that regions involved in the early response to Cr(VI) exposure are highly susceptible to damage and suppression with time. Notwithstanding, the broad expansion of γ-H2AX domains require further study to determine the positioning of the break relative to any affected gene.
Current initiatives and future directions
Numerous studies investigating the mechanisms of Cr(VI) toxicity suggest that the hexavalent species functions as a carcinogen at the molecular level, though the mechanisms are not fully understood. Promising studies have highlighted the dual-role that antioxidants play, both by helping to detoxify Cr(VI) as well as by enhancing its genotoxicity through ternary adduct formation with DNA. The capacity to directly interact with DNA and tether protein complexes highlights the role that Cr(VI) may play in reshaping the chromatin landscape. To this end, there is a need for deep genome-wide studies to outline the dynamics of epigenetic changes that arise from Cr(VI) exposure. Current work in our laboratory is geared towards addressing these questions and to further understand the impact of Cr(VI) on 3D chromatin architecture, focusing on CTCF as a direct target of chromium. Ultimately, there is need to integrate epigenomic, transcriptomic and chromatin architecture data arising from Cr(VI) studies in order to build a comprehensive model and an understanding of the impact of chromium on transcriptional regulatory mechanisms.
Authors’ contributions
AVH, HZ, and AP contributed equally to this manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The work in our laboratory is supported by the NIEHS grant R01 ES010807 and by the NIEHS Center for Environmental Genetics grant P30 ES06096. AVH is supported by the NIEHS Training Grant T32 ES007250.
References
- 1.Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S. Toxicological profile for chromium. USA: Agency for Toxic Substances and Disease Registry, 2012 [PubMed] [Google Scholar]
- 2.Mitsunobu S, Nagai H. Heat Treatment in Molecular Precursor Method for Fabricating Metal Oxide Thin Films. 2012. DOI:10.5772/50676
- 3.Hausladen DM, Alexander-Ozinskas A, McClain C, Fendorf S. Hexavalent chromium sources and distribution in California groundwater. Environ Sci Technol 2018; 52:8242–51 [DOI] [PubMed] [Google Scholar]
- 4.Oze C, Bird DK, Fendorf S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc Natl Acad Sci 2007; 104:6544–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Brocato J, Costa M. Oral chromium exposure and toxicity. Curr Envir Health Rpt 2015; 2:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Toxicology Program. Chromium hexavalent compounds. Rep Carcinog. Available at: https://ntp.niehs.nih.gov/ntp/roc/content/profiles/chromiumhexavalentcompounds.pdf [Google Scholar]
- 7.Morse JL, Luczak MW, Zhitkovich A. Chromium(VI) causes interstrand dna cross-linking in vitro but shows no hypersensitivity in cross-link repair-deficient human cells. Chem Res Toxicol 2013; 26:1591–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhitkovich A, Voitkun V, Costa M. Formation of the amino acid−DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biochemistry 1996; 35:7275–82 [DOI] [PubMed] [Google Scholar]
- 9.Zhitkovich A, Voitkun V, Costa M. Glutathione and free amino acids form stable complexes with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis 1995; 16:907–13 [DOI] [PubMed] [Google Scholar]
- 10.Nickens KP, Patierno SR, Ceryak S. Chromium genotoxicity: a double-edged sword. Chem Biol Interact 2010; 188:276–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res 2006; 35:465–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye J, Shi X. Gene expression profile in response to chromium-induced cell stress in A549 cells. Mol Cell Biochem 2001; 222:189–97 [PubMed] [Google Scholar]
- 13.Pritchard DE, Ceryak S, Ramsey KE, O’Brien TJ, Ha L, Fornsaglio JL, Stephan DA, Patierno SR. Resistance to apoptosis, increased growth potential, and altered gene expression in cells that survived genotoxic hexavalent chromium [Cr(VI)] exposure. Mol Cell Biochem 2005; 279:169–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis 2000; 21:1491–500 [PubMed] [Google Scholar]
- 15.O’Hara KA, Klei LR, Barchowsky A. Selective activation of Src family kinases and JNK by low levels of chromium(VI). Toxicol Appl Pharmacol 2003; 190:214–23 [DOI] [PubMed] [Google Scholar]
- 16.Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol Appl Pharmacol 2009; 235:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo K, Takahashi Y, Hirose Y, Nagao T, Tsuyuguchi M, Hashimoto M, Ochiai A, Monden Y, Tangoku A. The reduced expression and aberrant methylation of p16INK4a in chromate workers with lung cancer. Lung Cancer 2006; 53:295–302 [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Zhou X, Chen H, Li Q, Costa M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol Appl Pharmacol 2009; 237:258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali AHK, Kondo K, Namura T, Senba Y, Takizawa H, Nakagawa Y, Toba H, Kenzaki K, Sakiyama S, Tangoku A. Aberrant DNA methylation of some tumor suppressor genes in lung cancers from workers with chromate exposure. Mol Carcinog 2011; 50:89–99 [DOI] [PubMed] [Google Scholar]
- 20.Pratheeshkumar P, Son Y-O, Divya SP, Turcios L, Roy RV, Hitron JA, Wang L, Kim D, Dai J, Asha P, Zhang Z, Shi X. Hexavalent chromium induces malignant transformation of human lung bronchial epithelial cells via ROS-dependent activation of miR-21-PDCD4 signaling. Oncotarget 2016; 7:51193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan Y, Ovesen JL, Puga A. Long-term exposure to hexavalent chromium inhibits expression of tumor suppressor genes in cultured cells and in mice. J Trace Elem Med Biol 2012; 26:188–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wetterhahn KE, Hamilton JW. Molecular basis of hexavalent chromium carcinogenicity: effect on gene expression. Sci Total Environ 1989; 86:113–29 [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Manning FCR, O’Brien TJ, Ceryak S, Patierno SR. Mechanisms of chromium-induced suppression of RNA synthesis in cellular and cell-free systems: relationship to RNA polymerase arrest. Mol Cell Biochem 2004; 255:151–60 [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Manning FCR, Patierno SR. Preferential formation and repair of chromium-induced DNA adducts and DNA-protein crosslinks in nuclear matrix DNA. Carcinogenesis 1994; 15:1443–50 [DOI] [PubMed] [Google Scholar]
- 25.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol 2007; 27:7089–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Li Y, Okumura F, Itoh N, Nakanishi T, Sone T, Isobe M, Andrews GK. Chromium(VI) inhibits mouse metallothionein-I gene transcription by preventing the zinc-dependent formation of an MTF-1-p300 complex. Biochem J 2008; 415:477–82 [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey J, Wolf CM, Hamilton JW. Effects of the genotoxic carcinogen chromium(VI) on basal and hormone-inducible phosphoenolpyruvate carboxykinase gene expression in vivo: correlation with glucocorticoid- and developmentally regulated expression. Mol Carcinog 1994; 10:189–98 [DOI] [PubMed] [Google Scholar]
- 28.Hamilton JW, Wetterhahn KE. Differential effects of chromium(VI) on constitutive and inducible gene expression in chick embryo liver in vivo and correlation with chromium(VI)-induced DNA damage. Mol Carcinog 1989; 2:274–86 [DOI] [PubMed] [Google Scholar]
- 29.Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor-kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J Biol Chem 1999; 274:36207–12 [DOI] [PubMed] [Google Scholar]
- 30.Majumder S, Ghoshal K, Summers D, Bai S, Datta J, Jacob ST. Chromium(VI) down-regulates heavy metal-induced metallothionein gene transcription by modifying transactivation potential of the key transcription factor, metal-responsive transcription factor 1. J Biol Chem 2003; 278:26216–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaltreider RC, Pesce CA, Ihnat MA, Lariviere JP, Hamilton JW. Differential effects of arsenic(III) and chromium(VI) on nuclear transcription factor binding. Mol Carcinog 1999; 25:219–29 [PubMed] [Google Scholar]
- 32.Raja NS, Nair BU. Chromium(III) complexes inhibit transcription factors binding to DNA and associated gene expression. Toxicology 2008; 251:61–5 [DOI] [PubMed] [Google Scholar]
- 33.Levina A, Bailey AM, Champion G, Lay PA. Reactions of chromium(VI/V/IV) with bis (O-ethyl-l-cysteinato-N, S) zinc(II): a model for the action of carcinogenic chromium on zinc-finger proteins. J Am Chem Soc 2000; 122:6208–16 [Google Scholar]
- 34.Dor Y, Cedar H. Principles of DNA methylation and their implications for biology and medicine. Lancet (London, England) 2018; 392:777–86 [DOI] [PubMed] [Google Scholar]
- 35.Yin Y, Morgunova E, Jolma A, Kaasinen E, Sahu B, Khund-Sayeed S, Das PK, Kivioja T, Dave K, Zhong F, Nitta KR, Taipale M, Popov A, Ginno PA, Domcke S, Yan J, Schübeler D, Vinson C, Taipale J. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 2017; 356:eaaj2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Maurano MT, Qu H, Varley KE, Gertz J, Pauli F, Lee K, Canfield T, Weaver M, Sandstrom R, Thurman RE, Kaul R, Myers RM, Stamatoyannopoulos JA. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res 2012; 22:1680–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D, Kluz T, Fang L, Zhang X, Sun H, Jin C, Costa M. Hexavalent chromium (Cr(VI)) down-regulates acetylation of histone H4 at lysine 16 through induction of stressor protein nupr1. PLoS One 2016; 11:e0157317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng R-S, Hockman T, Crawford E, Anderson LM, Shiao Y-H. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol Carcinog 2004; 40:1–11 [DOI] [PubMed] [Google Scholar]
- 39.Lou J, Wang Y, Yao C, Jin L, Wang X, Xiao Y, Wu N, Song P, Song Y, Tan Y, Gao M, Liu K, Zhang X. Role of DNA methylation in cell cycle arrest induced by Cr(VI) in two cell lines. PLoS One 2013; 8:e71031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Wang T-C, Song Y-S, Wang H, Zhang J, Yu S-F, Gu Y-E, Chen T, Wang Y, Shen H-Q, Jia G. Oxidative DNA damage and global DNA hypomethylation are related to folate deficiency in chromate manufacturing workers. J Hazard Mater 2012; 213–214:440–6 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Wu W, Yao C, Lou J, Chen R, Jin L, Wu N, Gao M, Song P, Tan Y, Liu K. Elevated tissue Cr levels, increased plasma oxidative markers, and global hypomethylation of blood DNA in male Sprague–Dawley rats exposed to potassium dichromate in drinking water. Environ Toxicol 2016; 31:1080–90 [DOI] [PubMed] [Google Scholar]
- 42.Xiong J, Liu X, Cheng Q-Y, Xiao S, Xia L-X, Yuan B-F, Feng Y-Q. Heavy metals induce decline of derivatives of 5-methycytosine in both DNA and RNA of stem cells. ACS Chem Biol 2017; 12:1636–43 [DOI] [PubMed] [Google Scholar]
- 43.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res 2011; 21:381–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, Li Q, Arita A, Sun H, Costa M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol 2009; 236:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Wu J, Humphries B, Kondo K, Jiang Y, Shi X, Yang C. Upregulation of histone-lysine methyltransferases plays a causal role in hexavalent chromium-induced cancer stem cell-like property and cell transformation. Toxicol Appl Pharmacol 2018; 342:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Pérez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MÁ, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 2005; 37:391–400 [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama Y, Hieda M, Nishioka Y, Matsumoto A, Higashi S, Kimura H, Yamamoto H, Mori M, Matsuura S, Matsuura N. Cancer-associated upregulation of histone H3 lysine 9 trimethylation promotes cell motility in vitro and drives tumor formation in vivo. Cancer Sci 2013; 104:889–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell 2013; 152:1344–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidigal JA, Ventura A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol 2015; 25:137–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, Pegoraro V, Motta V, Tarantini L, Cantone L, Schwartz J, Bertazzi PA, Baccarelli A. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect 2010; 118:763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Li P, Yu S, Zhang J, Wang T, Jia G. miR-3940-5p associated with genetic damage in workers exposed to hexavalent chromium. Toxicol Lett 2014; 229:319–26 [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Hu G, Li P, Tang S, Zhang J, Jia G. miR-3940-5p enhances homologous recombination after DSB in Cr(VI) exposed 16HBE cell. Toxicology 2016; 344–346:1–6 [DOI] [PubMed] [Google Scholar]
- 53.He J, Qian X, Carpenter R, Xu Q, Wang L, Qi Y, Wang Z-X, Liu L-Z, Jiang B-H. Repression of miR-143 mediates Cr(VI)-induced tumor angiogenesis via IGF-IR/IRS1/ERK/IL-8 pathway. Toxicol Sci 2013; 134:26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VonHandorf A, Sánchez-Martín FJ, Biesiada J, Zhang H, Zhang X, Medvedovic M, Puga A. Chromium disrupts chromatin organization and CTCF access to its cognate sites in promoters of differentially expressed genes. Epigenetics 2018; 13:363–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ovesen JL, Fan Y, Zhang X, Chen J, Medvedovic M, Xia Y, Puga A. Formaldehyde-assisted isolation of regulatory elements (FAIRE) analysis uncovers broad changes in chromatin structure resulting from hexavalent chromium exposure. PLoS One 2014; 9:e97849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLoughery Z, Luczak MW, Ortega-Atienza S, Zhitkovich A. DNA double-strand breaks by Cr(VI) are targeted to euchromatin and cause ATR-dependent phosphorylation of histone H2AX and its ubiquitination. Toxicol Sci 2015; 143:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ovesen JL, Fan Y, Chen J, Medvedovic M, Xia Y, Puga A. Long-term exposure to low-concentrations of Cr(VI) induce DNA damage and disrupt the transcriptional response to benzo[a]pyrene. Toxicology 2014; 316:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]