Short abstract
Over-activation of autophagy due to increased levels of reactive oxygen species is a key mechanism of lipopolysaccharide-induced acute lung injury. Hydrogen-rich saline, an antioxidant, has been proved to be an effective agent for the prevention of acute lung injury, but the underlying mechanism remains unclear. Here, we investigated the mechanism through which hydrogen-rich saline prevents acute lung injury by focusing on autophagy regulation. The acute lung injury model was induced using lipopolysaccharide both in vivo and in vitro. The activation of autophagy was observed by detecting the expression of autophagy-related proteins using Western blotting. Lung histopathological changes, malondialdehyde content, wet/dry ratio, inflammatory cell count, protein content in bronchoalveolar lavage fluid, and cell viability were measured to evaluate the severity of acute lung injury. Intracellular reactive oxygen species levels were detected using dihydroethidium, which is a reactive oxygen species fluorescent probe. The results showed that the expression of Beclin-1 and microtubule-associated protein 1 light chain 3 II/I (LC3II/I ratio) was obviously increased after lipopolysaccharide administration. Pretreatment with hydrogen-rich saline markedly inhibited the expression of Beclin-1 and the LC3II/I ratio; ameliorated lung histopathological changes, malondialdehyde content and wet/dry ratio; and reduced protein content and infiltration of inflammatory cells in bronchoalveolar lavage fluid. These protective effects were also observed by pretreatment with autophagy inhibitor 3-methyladenine. Similar results were found in vitro. The production of reactive oxygen species and the activation of the AMPK/mTOR pathway were notably enhanced in MLE-12 cells after lipopolysaccharide treatment, and ameliorated by the hydrogen-rich medium. The activation of AMPK induced by lipopolysaccharide was also inhibited by pretreatment with N-acetyl-L-cysteine (a reactive oxygen species scavenger). In addition, the over-activation of autophagy was also suppressed by compound C (an AMPK inhibitor). In conclusion, hydrogen-rich saline alleviated lipopolysaccharide-induced acute lung injury by inhibiting excessive autophagy activation via the ROS/AMPK/mTOR pathway in mice. Hydrogen-rich saline may be a new therapeutic strategy for acute lung injury prevention and treatment in the future.
Impact statement
Acute lung injury (ALI), a common complication of many serious health issues, such as serious infection, burns, and shock, is one of the most common critical illnesses in clinical practice with a high mortality rate of 30–40%. There are still short of effective prevention and treatment measures. Evidence is growing that hydrogen-rich saline (HRS) may be an effective drug for the prevention and treatment of ALI. However, the mechanisms involved in have not been clearly understood. In this study, we investigated the underling mechanisms by focusing on autophagy regulation. The results showed that HRS ameliorated lipopolysaccharide-induced ALI in mice by inhibiting autophagy over-activation through ROS/AMPK/mTOR pathway. HRS may be a new therapeutic strategy for ALI prevention and treatment in the future.
Keywords: Acute lung injury, hydrogen-rich saline, autophagy, reactive oxygen species, lipopolysaccharide, mTOR
Introduction
Acute lung injury (ALI) and the more severe form acute respiratory distress syndrome (ARDS) are two common serious complications of many acute severe diseases, including serious infection, burns, trauma, and shock.1 Clinically, the characters of ALI/ARDS are progressive hypoxemia and respiratory failure, caused by injury of alveolar epithelial cells and pulmonary capillary endothelial cells.1 Although numerous therapeutic strategies have been applied, ALI/ARDS remain to be a major clinical challenge, and the mortality rate of ALI/ARDS is still as high as 30–40%.2 It is becoming increasingly important to develop new therapeutic strategies to improve the outcome of ALI/ARDS.
Autophagy is a fundamental process involved in cellular homeostasis, by which unsued proteins and damaged organelles are transported to lysosomes to be broken down and recycled.3,4 This process is a double-edged sword, however, as its over-activation can lead to autophagic cell death caused by excessive degradation of intracellular substances.5,6 Inhibiting autophagy has been shown to alleviate ALI in various types of ALI models.7,8 Autophagy regulation has become a potential target for the treatment of ALI.7,8 Various stimuli, such as nutrient and energy stress, hormone stimulation, hypoxia, and oxidative stress can trigger autophagy.3,4 In recent years, the effects of reactive oxygen species (ROS) in autophagy regulation have received more and more attention.9,10 Studies have shown that, by reducing ROS production, hydrogen (H2) can effectively inhibit autophagy activity and provide protective effects against numerous diseases.11,12
H2 is the lightest chemical element and can easily diffuse through membranes into cytosol and organelles, exerting anti-oxidation, anti-inflammation, and anti-apoptotic effects.13,14 Under physiological conditions, breathing low concentrations of H2 has been proven to be relatively safe, but as it has explosive property, the use of H2 is limited in clinical practice.15 In studies, H2 is often dissolved in a solvent like physiological saline (hydrogen-rich saline, HRS) under high pressure to observe its effects in the treatment and prevention of diseases.14,16 It has been demonstrated that HRS can provide protection against various diseases, including sepsis, stroke, multiple organ dysfunction syndrome, and ALI.15,17
It is still unknown, however, whether autophagy regulation has a role in the protective effects of HRS on ALI and how HRS regulates the activation of autophagy. Here, by focusing on autophagy regulation, we investigated the protective effects of HRS on LPS-induced ALI in mice, and explored the possible mechanisms.
Material and methods
Animals and treatments
Seven-week-old male C57BL/6 mice were provided by the Animal Research Center of Shanghai Jiaotong University, and housed with standard food and water ad libitum under a natural day/night cycle. The mice were acclimated for two weeks before the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the university.
Forty mice were randomly classified into five groups: Control group, saline group, LPS group, HRS+LPS group, and 3-Methyladenine (3-MA)+LPS group. The mice were intratracheally treated with LPS (10 mg/kg; 50 μL/per mouse) or an equal volume of saline to induce ALI. HRS was given intraperitoneally 1 h before LPS treatment. Twelve hours after LPS treatment, the mice were sacrificed and samples were collected.
HRS and hydrogen-rich medium preparation
H2 was dissolved in saline or DMEM/F-12 (Gibco, USA) under high pressure (0.4 MPa 6 h) to reach a supersaturated level and was stored at 4°C under atmospheric pressure.15,17 HRS and hydrogen-rich medium (HRM) were freshly prepared within 24 h prior to the experiments by the Department of Diving Medicine, Faculty of Navy Medicine, Second Military Medical University (Shanghai, China). H2 levels were measured according to the method described by Ohsawa et al.18 and showed an average level of 0.86 mmol/L.
Histopathological analysis
After the mice were sacrificed, the left lungs were excised and fixed in 4% paraformaldehyde. After dehydration, the lung tissues were embedded in paraffin wax. Then the tissues were sectioned followed by hematoxylin and eosin (H&E) staining. The histopathological features of ALI were evaluated under a microscope.
Bronchoalveolar lavage fluid acquisition and analysis
The lungs of the mice were lavaged with 0.3 mL precooled saline three times. The bronchoalveolar lavage fluid (BALF) was collected and centrifuged at 2000 r/min for 5 min. The cell pellets were then resuspended in PBS for a total cell count using a hemacytometer, and stained with H&E for cell classification. The protein concentration was quantified using a BCA protein assay kit (Beyotime, China).
Lung wet/dry ratio
The right lungs were excised and weighed to obtain the wet weight (W). Then, it was dried in an incubator at 60°C for 72 h and weighted to obtain the dry weight (D). The ratio of W/D was calculated to assess the lung tissue edema.
Cell culture
Mouse AECII cell line (MLE-12 cells) was purchased from the ATCC (Manassas, USA) and maintained in DMEM/F-12 (Hyclone, China) containing 2% fetal bovine serum (Gibco) and 100 U/mL penicillin and streptomycin (Gibco) in a humidified incubator containing 5% CO2 at 37°C.
Cell viability detection
Cell viability was measured using a CCK-8 assay according to the manufacturer’s instructions. Briefly, MLE-12 cells were incubated with 2 μg/mL LPS for 24 h, then the medium was replaced with CCK-8 solution, and the cells were incubated for an additional 1.5 h. The absorbance was measured with a microplate reader (BioTek, USA) at 450 nm.
ROS detection
Whole-cell ROS was detected using a widely used ROS fluorescent probe dihydroethidium (Thermo-fisher, USA). After LPS treatment, the cells were incubated with HBSS containing 5 μM dihydroethidium for 20 min. A microscope (Leica, Germany) was applied for acquiring fluorescence signal.
Western blotting analysis
The samples were lysed in a radio immunoprecipitation assay lysis buffer containing 1 mM phenylmethanesulfonyl fluoride, and separated by 10% or 12% SDS polyacrylamide gels before being electrotransferred onto a PVDF membrane. After being blocked with 5% (w/v) nonfat dry milk for 1 h at room temperature, the membranes were incubated with specific antibodies (Cell Signaling Technology, USA) overnight at 4°C, and then the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) for 2 h and detected with a chemiluminescence detection assay. The bands were analyzed with Image J software.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) and were analyzed using SPSS21.0. Differences between groups were compared with one-way ANOVA test. A P value less than 0.05 was considered to be statistically significant.
Results
HRS inhibited the activation of autophagy in LPS-induced ALI
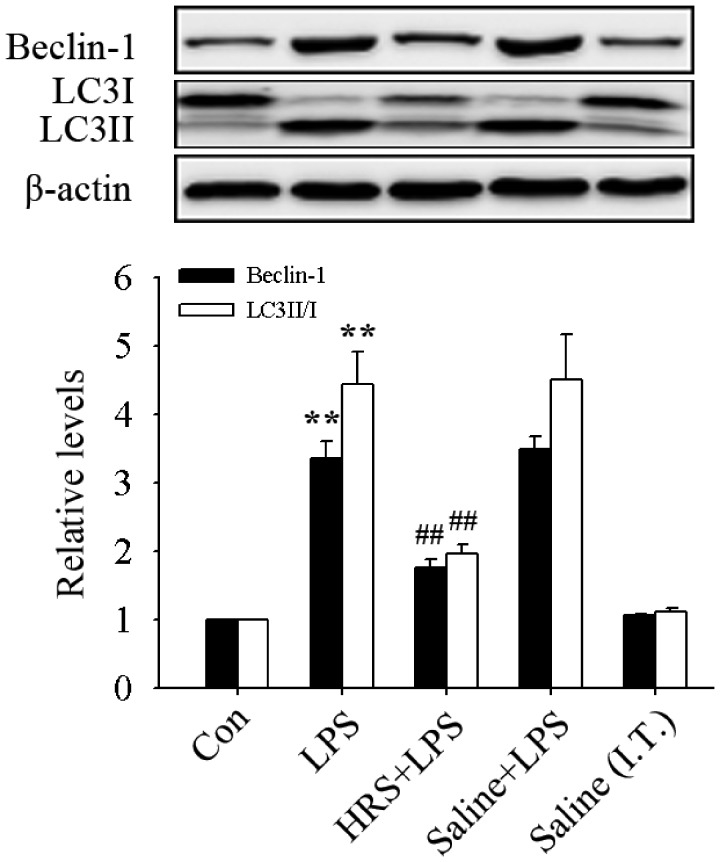
In this study, we first observed the effects of HRS on the LSP-induced autophagy activation in mice by detecting the expression of Beclin-1 and microtubule-associated protein 1 Light Chain 3 II/I (LC3II/I ratio). As shown in Figure 1, the expression of Beclin-1 and LC3II/I ratio significantly increased (all P < 0.01) in the LPS group. This further confirmed that autophagy is activated in LPS-induced ALI. HRS treatment significantly reversed these changes (all P < 0.01), suggesting that HRS can inhibit autophagy activation in LPS-induced ALI in mice.
Figure 1.
Effects of HRS on the expression of Beclin-1 and LC3II/I in LPS-induced ALI in mice. The mice were administrated intratracheal LPS (10 mg/kg, dissolved in saline; 50 μL/per mouse) or an equal volume of saline (Saline (I.T.)) to induce ALI. HRS (10 mL/kg) or an equal volume of saline was administrated intraperitoneally 1 h before LPS treatment. Twelve hours later, the mice were sacrificed and samples were collected. Data are represented as mean ± SD of three independent experiments. **P < 0.01 vs. Con group, ##P < 0.01 vs. LPS group. HRS: hydrogen- rich saline; Con: control; LPS: lipopolysaccharide.
HRS and autophagy inhibitor 3-MA alleviated LPS-induced ALI
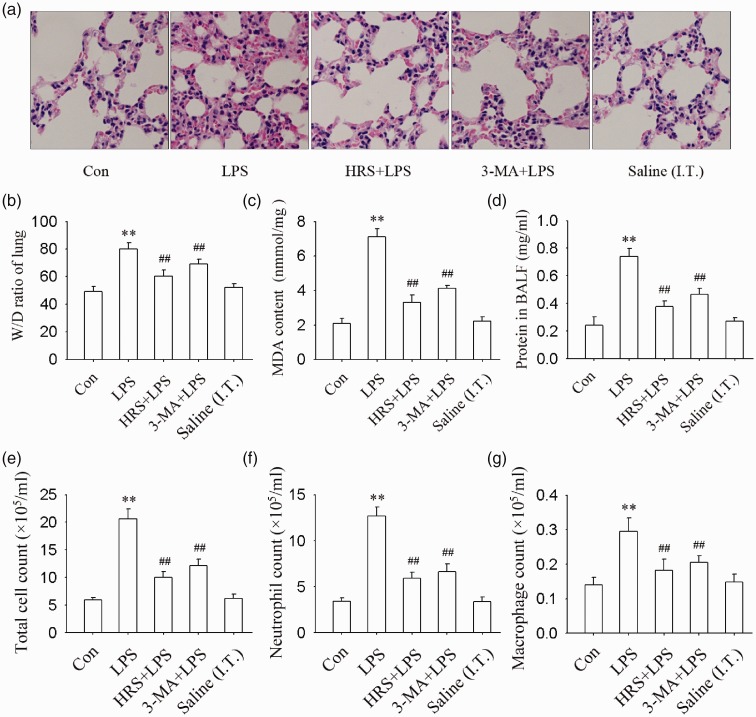
As shown in Figure 2, LPS treatment caused severe lung injury, which included the accumulation of neutrophils, the formation of hyaline membrane in lung tissue, and increased alveolar wall thickness. Total cells, neutrophils, macrophages, and levels of protein in BALF; W/D ratio; and the MDA content of lung tissue also significantly increased after LPS administration (all P < 0.01). Pretreatment with HRS or 3-MA (an autophagy inhibitor) significantly mitigated these changes (all P < 0.01). These results indicated that HRS alleviated LPS-induced ALI may through autophagy inhibition in mice.
Figure 2.
Effects of HRS and autophagy inhibitor 3-MA on LPS-induced ALI in mice. The mice were received an intraperitoneal injection of HRS (10 mL/kg) or an equal volume of saline 1 h before LPS treatment. The mice were administrated intratracheal LPS (10 mg/kg, dissolved in saline; 50 μL/per mouse) or an equal volume of saline (Saline (I.T.)) to induce ALI. (a) Lung histopathological changes measured by H&E staining. (b) W/D ratio. (c) MDA content in lung tissue. (d) Protein content in BALF. (e) Total cell count in BALF. (f) Neutrophil count in BALF. (g) Macrophagy count in BALF. Data are represented as mean ± SD of three independent experiments. **P < 0.01 vs. Con group, ##P < 0.01 vs. LPS group. MDA: malondialdehyde; W/D: wet/dry ratio, BALF: bronchoalveolar lavage fluid; 3-MA: 3-Methyladenine. (A color version of this figure is available in the online journal.)
HRM inhibited LPS-induced autophagy activation in MLE-12 cells
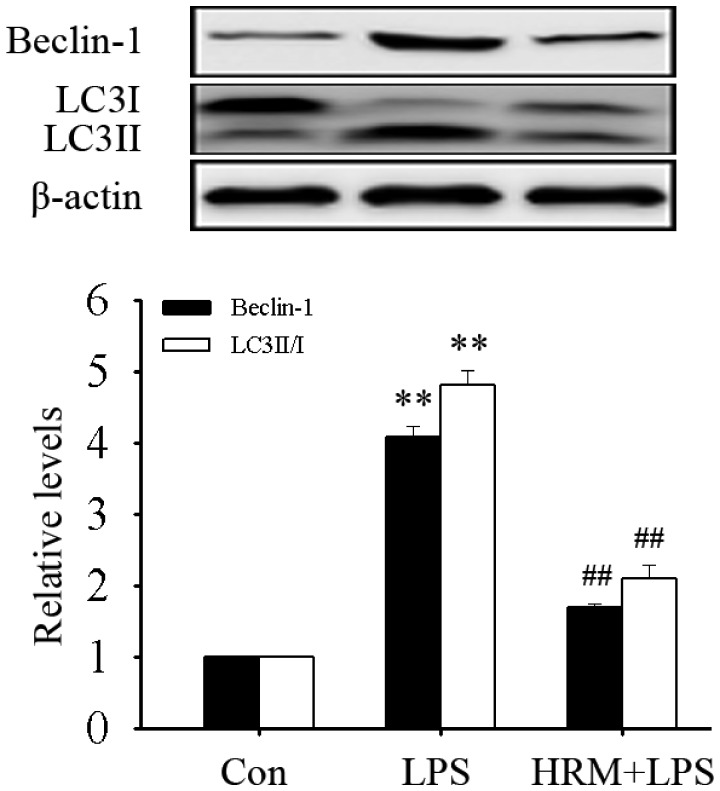
Alveolar type II epithelial cells (AECII) injury and dysfunction are a main cause of ALI. In this study, autophagy activation in mouse AECII cells (MLE-12 cells) after LPS treatment was further observed. As shown in Figure 3, LPS treatment significantly up-regulated the expression of Beclin-1 and the LC3II/I ratio (all P < 0.01). Pretreatment with HRM significantly attenuated these changes (all P < 0.01). These results indicated that HRM can inhibit the excessive activation of autophagy in AECII cells.
Figure 3.
HRM inhibited the activation of autophagy in MLE-12 cells. Culture medium was replaced with HRM 1 h before LPS (2 μg/mL) treatment. Data are represented as mean ± SD of three independent experiments. **P < 0.01 vs. Con group, ##P < 0.01 vs. LPS group. HRM: hydrogen-rich medium.
HRM and 3-MA alleviated LPS-induced MLE-12 cell injury
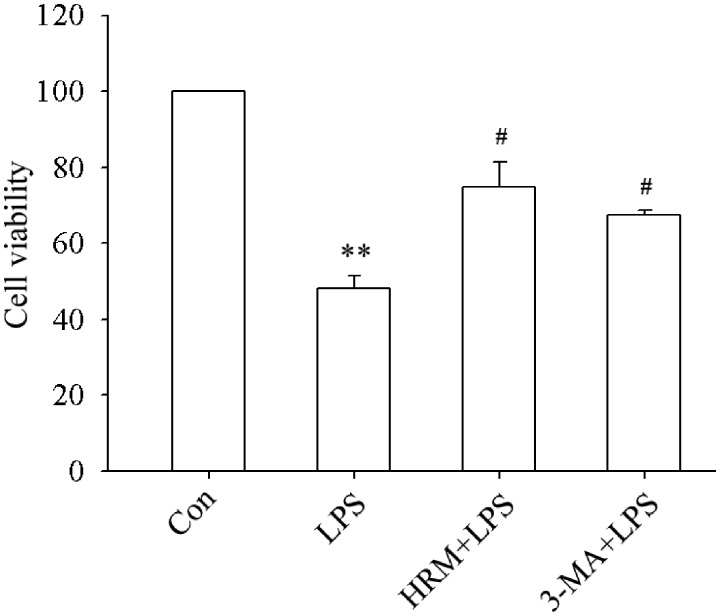
The effects of HRM on LPS-induced MLE-12 cells injury were evaluated by looking at the changes in cell viability. As shown in Figure 4, cell viability obviously decreased after LPS treatment (P < 0.01). Pretreatment with HRM or 3-MA significantly protected MLE-12 cells from LPS-induced injury (all P < 0.05). These findings suggested that HRM may produce significant protection in MLE-12 cells during ALI.
Figure 4.
Effects of HRM on LPS-induced MLE-12 cells injury. One hour before LPS administration, the culture medium was replaced with HRM in the HRM+LPS group and 3-MA (5 mM) were added into the culture medium of the 3-MA+LPS group. Data are represented as mean ± SD of three independent experiments. **P < 0.01 vs. Con group, #P < 0.05 vs. LPS group.
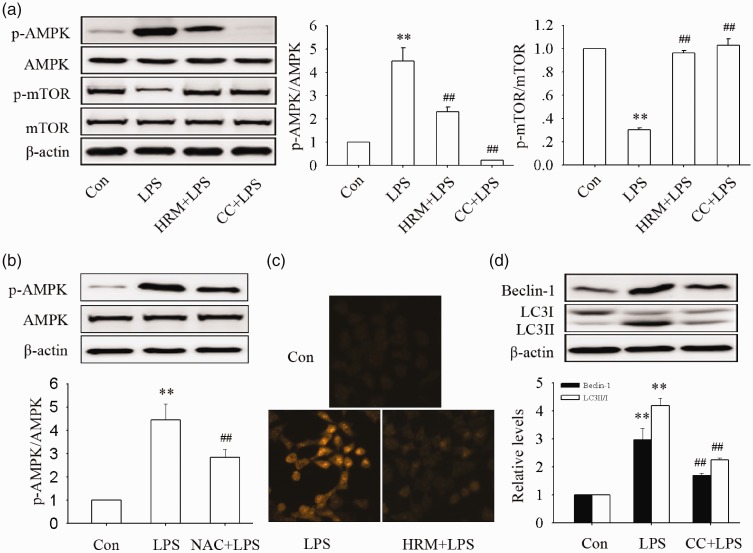
HRM may inhibit LPS-induced autophagy in MLE-12 cells via the ROS/AMPK/mTOR pathway
As shown in Figure 5, the intracellular ROS levels, the phosphorylation of AMPK, and the dephosphorylation of mTOR were significantly increased after LPS administration in MLE-12 cells (all P < 0.01). Pretreatment with HRM obviously reversed these changes (all P < 0.01). The phosphorylation of AMPK was also inhibited by pretreatment with NAC (a ROS scavenger, P < 0.01), and the dephosphorylation of mTOR was suppressed by pretreatment with AMPK inhibitor compound C (P < 0.01). Total AMPK and mTOR protein levels did not significantly changed in this study. These data suggested that HRM inhibited the AMPK/mTOR pathway activation may through ROS scavenging. The expression of Beclin-1 and the ratio of LC3II/I were also significantly inhibited by pretreatment compound C, indicating HRM inhibited LPS-induced autophagy in MLE-12 cells may via the ROS/AMPK/mTOR pathway.
Figure 5.
Effects of HRM on the activation of the ROS/AMPK/mTOR pathway. NAC (5 mM), compound C (CC, 10 μM), and HRM were administrated 1 h before LPS treatment. Protein samples were harvested 24 h after LPS administration. (a) The phosphorylation of AMPK and mTOR. (b) The effects of NAC on the phosphorylation of AMPK. (c) Levels of intracellular O2•− was detected by dihydroethidium. (d) The expression of Beclin-1 and LC3II/I. Data are represented as mean±SD of three independent experiments. **P < 0.01 vs. Con group, ##P < 0.01 vs. LPS group. NAC: N-acetyl-L-cysteine. (A color version of this figure is available in the online journal.)
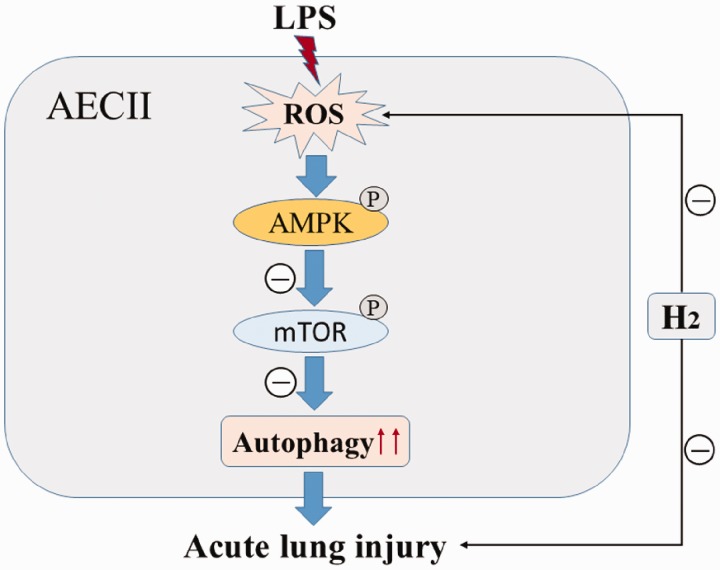
Figure 6.
H2 Mechanism for preventing ALI induced by LPS. Under basal conditions, autophagy is negatively regulated by mTOR. LPS treatment increased intracellular ROS formation, which inhibited the mTOR activity by activating AMPK. Inhibition of mTOR triggered the excessive activation of autophagy and led to ALI. Pretreatment with H2 can inhibit excessive activation of autophagy after LPS treatment by scavenging intracellular ROS and ameliorate ALI. AECII: alveolar type II epithelial cell. (A color version of this figure is available in the online journal.)
Discussion
Gram-negative bacterial infection is considered to be the main cause of ALI.15 It has been proven that LPS is the principal active component of bacterial endotoxin and is present in the outer membranes of Gram-negative bacteria. LPS can enhance the production of intracellular ROS and increase the release of inflammatory cytokines, which can recruit and activate inflammatory cells into the lung, initiating the inflammatory cascade and inducing alveolar capillary damage and alveolar epithelial cell damage.19,20
In recent years, the role of ROS in ALI/ARDS has received increased attention as it not only causes severe oxidative damage to cells but also initiates cell death pathways, such as apoptosis and autophagy over-activation.21,22 In this study, we observed the generation of ROS by detecting intracellular O2•−, which is the precursor form of ROS.23,24 The results showed that intracellular O2•− markedly increased after LPS treatment. At the same time, the in vivo results also showed that MDA levels, an indicator of oxidative damage, also significantly increased in lung tissue. These results further confirmed that oxidative damage may play a key role in LPS-induced ALI.
Autophagy is a very important mechanism involved in the maintenance of cellular homeostasis,5 through which damaged or unused proteins and organelles are degraded and recycled to promote cell survival.5 However, over-activation of autophagy may induce autophagic cell death via excessively destroying intracellular substances.5,6 Studies have shown that autophagy over-activation contributed to various ALI conditions.7,25 In this study, we found that the expression of Beclin-1 and LC3I/II ratio observably increased after LPS treatment, both in vivo and in vitro, which further confirmed that autophagy was activated in LPS-induced ALI.
To determine the role of autophagy inhibition in the protection of LPS-induced ALI by HRS, we investigated the effects of HRS on the activation of autophagy and the protective effects of autophagy inhibitor 3-MA and HRS on LPS-induced ALI. The results showed that pretreatment with HRS significantly attenuated the activation of autophagy and that LPS-induced ALI was obviously alleviated by autophagy inhibitor 3-MA and HRS both in vivo and in vitro. This indicated that HRS protected the mice from LPS-induced ALI may through autophagy inhibition. In 2015, Zhang et al.26 found that autophagy was over-activated in LPS-induced ALI in rats and that pretreatment with HRS effectively inhibited the activation of autophagy and ameliorated LPS-induced ALI. In this study, however, the contribution of autophagy inhibition to the protective effects of HRS on ALI was not observed.26 In 2017, Zhang et al.26 according to the pathogenesis of ALI further speculated that HRS ameliorated LPS-induced ALI may through reducing excessive autophagy activation. They did not conduct an experiment to confirm their speculation, however.
H2 is an effective antioxidant for selectively reducing cytotoxic oxygen radicals and alleviating oxidative stress.27,28 Our results found that pretreatment with HRS significantly reduced MDA levels, an indicator of oxidative damage, in lung tissue, which verified the antioxidant effect of H2 in LPS-induced ALI. As AECII cells injury is an important cause of ALI and excessive autophagy activation of AECII cells is a key feature of aggravated ALI.29 We further observed the underlying mechanisms through which HRS regulates the activation of autophagy in MLE-12 cells, which is a cell line of mouse AEII cells.
Many signaling pathways participate in the regulation of autophagy.29 Among them, the AMPK/mTOR pathway has been shown to be particularly important.3,21,30 By negatively regulating mTOR activity, AMPK positively regulates the activation of autophagy.3,10 ROS is a very important activator of AMPK10,21 and to see whether ROS/AMPK/mTOR contribute to autophagy regulation by HRS, the levels of ROS and the phosphorylation of AMPK and mTOR were measured in this study. The results showed that LPS treatment significantly increased intracellular ROS levels, AMPK phosphorylation, and mTOR dephosphorylation. Pretreatment with HRS significantly reversed these changes. The phosphorylation of AMPK induced by LPS was also inhibited by pretreatment with a ROS scavenger NAC. In addition, we further observed the role of AMPK phosphorylation in autophagy activation by using AMPK specific inhibitor compound C. The results revealed that the expression of Beclin-1 and the LC3II/I ratio was obviously inhibited by pretreatment with compound C. These data led to the hypothesis that (as shown in Figure 6), during ALI, the activation of autophagy induced by LPS is associated with the accumulation of intracellular ROS, which causes the activation of the AMPK/mTOR pathway and the activation of autophagy. HRS effectively scavenged intracellular ROS, thus inhibiting autophagy over-activation and ameliorating LPS-induced ALI.
In conclusion, our results indicated that HRS ameliorated LPS-induced ALI by inhibiting autophagy over-activation via the ROS/AMPK/mTOR pathway.
Authors’ contributions
Jinghua Zhang and Jinsong Bo carried out the experiments and wrote the paper; Xuefen Wang, Jingnan Zhu and Yong Wang designed the study and revised the manuscript; Yong Wang supervised the whole study and revised the manuscript
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Herold S, Gabrielli NM, Vadasz I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2013; 305:665–81 [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Howard JP. Howard, Progress in modelling acute lung injury in a pre-clinical mouse model. Eur Respir J 2012; 39:1062–3 [DOI] [PubMed] [Google Scholar]
- 3.He L, Zhang J, Zhao J, Ma N, Kim SW, Qiao S, Ma X. Autophagy: the last defense against cellular nutritional stress. Adv Nutr 2018; 9:493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy J, Towers CG, Thorburn A. Thorburn. Targeting autophagy in cancer. Nat Rev Cancer 2017; 17:528–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y, Bowman JW, Jung JU. Autophagy during viral infection – a double-edged sword. Nat Rev Microbiol 2018; 16:341–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Zhang S, He L, Rong Y, Brier LW, Sun Q, Liu R, Fan W, Chen S, Yue Z, Kim J, Guan KL, Li D, Zhong Q. MTORC1-mediated NRBF2 phosphorylation functions as a switch for the class III PtdIns3K and autophagy. Autophagy 2017; 13:592–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slavin SA, Leonard A, Grose V, Fazal F, Rahman A. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2018; 314:388–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding D, Xu S, Zhang H, Zhao W, Zhang X, Jiang Y, Wang P, Dai Z, Zhang J. 3-Methyladenine and dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the inhibition of inflammation and autophagy. Exp Ther Med 2018; 15:3516–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Bravo-San PJ, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov 2017; 16:487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 2017; 44:532–53 [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Niu X, Guo Q, Dong Y, Xu J, Yin N, Qi Q, Jia Y, Gao L, He Q, Lv P. FoxO1-mediated autophagy plays an important role in the neuroprotective effects of hydrogen in a rat model of vascular dementia. Behav Brain Res 2019; 356:98–106 [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Yang H, Chi J, Xu Q, Zhao L, Yang W, Liu W, Yang W. hydrogen gas attenuates myocardial ischemia reperfusion injury independent of postconditioning in rats by attenuating endoplasmic reticulum stress-induced autophagy. Cell Physiol Biochem 2017; 43:1503–14 [DOI] [PubMed] [Google Scholar]
- 13.Dong A, Yu Y, Wang Y, Li C, Chen H, Bian Y, Zhang P, Zhao Y, Yu Y, Xie K. Protective effects of hydrogen gas against sepsis-induced acute lung injury via regulation of mitochondrial function and dynamics. Int Immunopharmacol 2018; 65:366–72 [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Cai J, Zhou J, Tao H, Zhang JH, Zhang W, Sun XJ. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. Crit Care Med 2011; 39:765–9 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang J. Zhang, Saturated hydrogen saline ameliorates lipopolysaccharide-induced acute lung injury by reducing excessive autophagy. Exp Ther Med 2017; 13:2609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo XY, Li XM, She CS, Lu XQ, Xiao CG, Wang SH, Huang GQ. Hydrogen-rich saline protects rat from oxygen glucose deprivation and reperusion-induced apoptosis through VDAC1 via Bcl-2. Brain Res 2019; 1706:110–5 [DOI] [PubMed] [Google Scholar]
- 17.Zou R, Wang MH, Chen Y, Fan X, Yang B, Du J, Wang XB, Liu KX, Zhou J. Hydrogen-rich saline attenuates acute lung injury induced by limb ischemia/reperfusion via down-regulating chemerin and NLRP3 in rats. Shock 2018. Epub ahead of print. DOI: 10.1097/SHK.0000000000001194 [DOI] [PubMed] [Google Scholar]
- 18.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13:688–94 [DOI] [PubMed] [Google Scholar]
- 19.Wang WC, Xia YM, Yang B, Su XN, Chen JK, Li W, Jiang T. Protective effects of tyrosol against LPS-induced acute lung injury via inhibiting NF-kappaB and AP-1 activation and activating the HO-1/Nrf2 pathways. Biol Pharm Bull 2017; 40:583–93 [DOI] [PubMed] [Google Scholar]
- 20.Lv H, Yu Z, Zheng Y, Wang L, Qin X, Cheng G, Ci X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-kappaB and activating HO-1/Nrf2 pathways. Int J Biol Sci 2016; 12:72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Yu S, Zhang K, Zhang Z, Li C, Gao B, Zhang W, Wang Y. Exogenous H2S inhibits autophagy in unilateral ureteral obstruction mouse renal tubule cells by regulating the ROS-AMPK signaling pathway. Cell Physiol Biochem 2018; 49:2200–13 [DOI] [PubMed] [Google Scholar]
- 22.Wang EX, Zou BY, Shi L, Du LY, Zhu YY, Jiang YM, Ma XD, Kang XH, Wang CY, Zhen YH, Sun LD. 7-O-geranylquercetin-induced autophagy contributes to apoptosis via ROS generation in human non-small cell lung cancer cells. Life Sci 2017; 180:102–13 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Huang G, Yu X, Xu W. A novel approach to estimate ROS origination by hyperbaric oxygen exposure, targeted probes and specific inhibitors. Cell Physiol Biochem 2018; 47:1800–8 [DOI] [PubMed] [Google Scholar]
- 24.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 2008; 4:278–86 [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Li H, Gong Y, Zheng H, Zhao D. Hydrogen sulfide ameliorated lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/Akt/mTOR pathway in mice. Biochem Biophys Res Commun 2018; 507:514–8 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Liu Y, Zhang J. Saturated hydrogen saline attenuates endotoxin-induced lung dysfunction. J Surg Res 2015; 198:41–9 [DOI] [PubMed] [Google Scholar]
- 27.Qian L, Shen J. Hydrogen therapy may be an effective and specific novel treatment for acute graft-versus-host disease (GVHD). J Cell Mol Med 2013; 17:1059–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res 2013; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Zhou F, Cheng L, Hu M, He Y, Zhang B, Liao D, Xu Z. MicroRNA-34a suppresses autophagy in alveolar type ii epithelial cells in acute lung injury by inhibiting FoxO3 expression. Inflammation 2017; 40:927–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43:67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]