Short abstract
Interleukin (IL)-25 is shown to potentiate type-2 immunity and contribute to chronic airway inflammation and remodeling in allergic airway diseases. However, the role of IL-25 in idiopathic pulmonary fibrosis (IPF), dominated by nonatopic type-2 immunity, still remains largely unclear. Herein, we detected the expression levels of IL-25 and IL-17BR (IL-25’s receptor) by using lung tissue samples gained from IPF patients and normal subjects. Also, by directly intranasal (IN) instillation of IL-25 to mice, we examined the potential roles and mechanisms of IL-25 in the development of lung fibrosis. Furthermore, we tested whether IL-25 can directly activate human lung fibroblast by in vitro cell culture. Immunohistochemical, Western blot, and real-time reverse transcription-polymerase chain reaction (RT-PCR) showed that the mRNA and protein levels of IL-25 and IL-17BR are significantly higher in IPF patients when compared with normal controls. Intranasal instillation of IL-25 to mice markedly induces the expressions of alveolar IL-5 and IL-13. Furthermore, immunohistochemical analysis showed that the main components of the extracellular matrix including collagen I, collagen III and fibronectin are notably induced by IL-25 instillation in lung parenchyma (especially in alveolar epithelial cells [AECs]). Also, IL-25 potentiates the expression of connective tissue growth factor (CTGF) in AECs and the recruitment of lung fibroblast. By using Cell Counting Kit-8 and EDU incorporation assay, we found that IL-25 markedly enhances the proliferation of lung fibroblast. Finally, IL-25 potentiates fibroblast to produce several fibrogenic genes including collagen I/III, fibronectin, CTGF, α smooth muscle (α-SMA) and tissue inhibitor of metalloproteinase (TIMP)-1 as determined by RT-PCR assay. Collectively, we concluded that IL-25 is increased in IPF lungs and contributes to lung fibrosis by directly mediating AECs/fibroblast activation.
Impact statement
Our work focused on alveolar epithelial cells (AECs)-derived type-2 cytokine (interleukin [IL]-25) in the pathogenesis of idiopathic pulmonary fibrosis (IPF). We showed that IL-25 and IL-17BR (IL-25’s receptor) is upregulated in lung tissues (especially in AECs and lung fibroblasts) of IPF patients and contributes to lung fibrosis by directly activating lung fibroblasts and modulating epithelial–mesenchymal transition (EMT) of AECs. We suggest that IL-25 may be one of the master switches hidden in the milieu of abnormal epithelial–mesenchymal crosstalk. Treatment targeting IL-25 may be the potential and novel method for IPF patients.
Keywords: Alveolar epithelial cells, fibroblasts, interleukin-25, lung fibrosis, murine model
Introduction
Idiopathic pulmonary fibrosis (IPF) is a well-known yet untreatable fibrotic lung disease with increasing morbidity and hospital admissions rates.1–3 Usual interstitial pneumonia (UIP) is the histological features of IPF4; however, the precise molecular event that initiates and maintains UIP is currently unknown. It has been shown that dysfunctional epithelium-derived profibrotic milieu drives the progress of fibrotic lesions.5,6 Unfortunately, the master switch hidden in the profibrotic milieu is still not well elucidated.
Interleukin (IL)-25, a newly discovered member of IL-17 cytokine family, is produced by disordered epithelial cells and innate immune cells.7 It has been shown that IL-25 initiates a type-2 skewed immune response by excessive production of IL-4, IL-5, and IL-13,7 which contributes to the pathogenesis of allergic airway diseases (AADs).8–10 However, the potential role of IL-25 in allergen-independent lung inflammation and fibrosis driven by type-2 cytokine responses is not well interpreted. By using transgene mice, it was previously shown that IL-25 may mediate lung fibrosis by recruiting type-2 innate lymphoid cells (ILC2s).11 However, the potential profibrotic role of IL-25 as a cytokine itself still remains unknown. Also, little is known about the direct modulatory role of IL-25 on alveolar epithelial cells (AECs) and lung fibroblasts (two major cellular serial killers for IPF). Herein, we reported IL-25 is upregulated in IPF lungs and hypothesized that the fibrotic role of IL-25 may be mediated by the activation of (IL-17BR)+-AECs and (IL-17BR)+-fibroblasts.
Materials and methods
Study subjects and samples
All human study protocols were approved by the Ethics Committee of Capital Medical University, Beijing, China. All patients signed the informed written consent. Fibrotic lung tissues were obtained from nine patients with IPF who received pulmonary transplantation or diagnostic video-assisted thoracoscopic surgery. The diagnosis of IPF was followed with the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) statement published in 2011.2 Normal control lung tissues (n = 6) were obtained from healthy lung explants remaining after transplantation.
Murine study protocols
Mature female BALB/C mice were purchased from Vital River Laboratories (Beijing, China). They were fed in pathogen-free conditions at the Animal Center of Capital Medical University. The Animal Care and Use Committee of Capital Medical University approved the study procedures. To directly examine the potential profibrotic role of IL-25, mice were anaesthetized with isoflurane gas (4%) and administered by daily intranasal (IN) injection of 2 μg of recombinant mouse IL-25 (mIL-25, R&D System, Minneapolis, MN, USA) in 50 μL of sterile saline from day 0 to day 7. Thereafter, mIL-25 was intranasally administrated every 2 days for another 24 days. Control mice were intranasally injected with 50 μL of sterile saline instead in the same time points. On days 3, 7, 19, and 31 after the first challenge of mIL-25 or saline, mice were sacrificed for Bronchoalveolar Lavage Fluid (BALF) and lung tissue collection. The procedure of mIL-25 in nonatopic mice airway inflammation and remodeling was also depicted elsewhere.12
Fibroblast cultures
The human embryonic lung fibroblasts (MRC-5) were purchased from American Type Culture Collection (Rockville, MD, USA). The detailed information is shown in the supporting materials. Cells were challenged with the recombinant human IL-25 (hIL-25; 1, 10, 100 ng/mL), human transforming growth factor (TGF)-β1 (10 ng/mL; both from R&D Systems), or both. The corresponding vehicles were used for control study. Thereafter, cell proliferation and RNAs expression assays were performed.
Histologic analysis
Human and murine lung biopsies were processed for histological analysis. The detailed procedure is shown in the supporting information. The simplified pathology score for fibrotic lesions was as below: grade 1, no fibrotic lesion; grade 2, occasional small interstitial fibrotic lesion; grade 3, moderate fibrotic lesions; and grade 4, continuous interalveolar fibrosis. At least 10 high-power fields of each slide were analyzed in a blind fashion by two independent experienced pathologists.13
Immunohistochemistry
Immunohistochemistry was used for detecting the expressions of IL-25, IL-17BR, collagen I, collagen III, fibronectin, connective tissue growth factor (CTGF), and fibroblast growth factor receptor 4 (FGFR4) in fibrotic lung tissues from IPF patients or murine model. The detailed procedure is shown in supplemental materials.
BALF collection and harvest of lung homogenate
BALF and lung homogenate were collected for detecting the expression of IL-5 and IL-13. The detailed information is shown in the supporting materials.
Lung collagen assay
The soluble collagen in murine lung tissues was determined by using the Sircol Collagen Assay Kit (Biocolor, Carrickfergus, UK), according to the producer’s instructions.
Enzyme-linked immunosorbent assay
The BALF and lung homogenate concentrations of murine type-2 cytokines, IL-5 and IL-13, were measured by enzyme-linked immunosorbent assay (ELISA) as per the manufacturer’s instructions of the detecting kits (R&D Systems).
Western blot
Western blot (WB) was used for detecting the expression levels of lung IL-25, IL-17RA, and IL-17RB. The detailed protocol is shown in the supporting materials.
Real-time RT-PCR
Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the mRNA expression levels of target genes in lung tissues or fibroblast. The detailed information is shown in the supporting materials.
Cell proliferation assay
Cell proliferation was measured by Cell Counting Kit-8 (CCK-8) (Solarbio) and EDU Imaging Kit (ThermoFisher) as per manufacturer’s protocol.
Statistical analysis
All results were expressed as mean ± SEM. GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and figures drawing. Statistical significance of differences between two groups was determined by using independent Student’s t test. Comparisons between multiple treatment groups were performed by analysis of variance (ANOVA, one-way or two-way, as appropriate) with Bonferroni post hoc test. Differences were considered significant at P value <.05.
Results
IL-25 and IL-17BR expression is upregulated in lung tissues of IPF patients
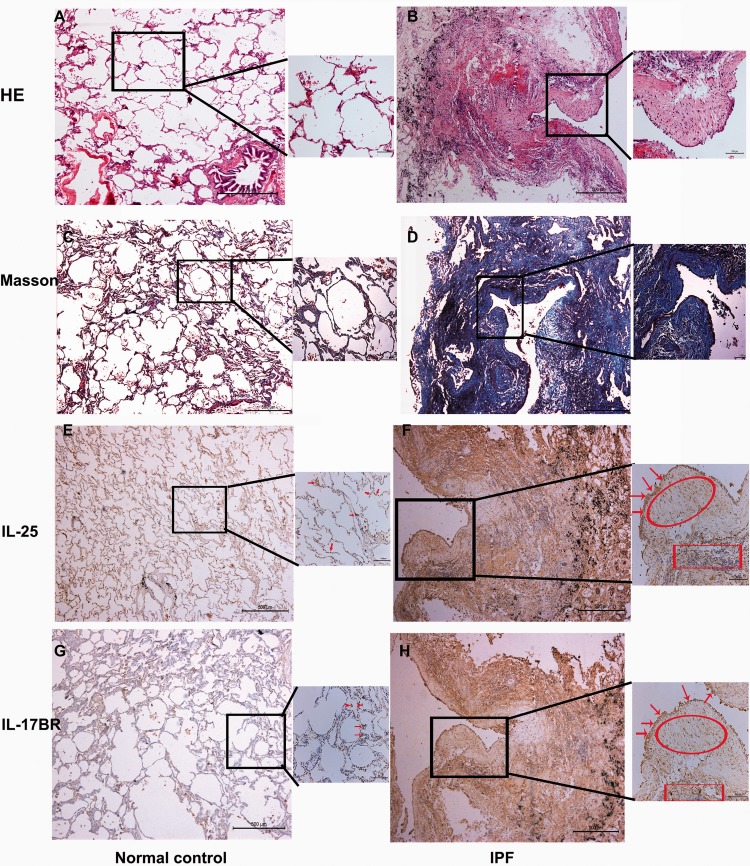
First, we showed a pattern of “UIP” in IPF lungs (Figure 1(b) and (d)). Specifically, the pathological features of fibrotic lung are characterized with areas of extensive fibrosis, architectural distortion, scattered honeycombing, and fibroblast foci (FF). The insets (in Figure 1(b) and (d)) show FF with high-power field. We next reported for the first time that IL-25/IL-17BR is occasionally expressed by AECs and alveolar macrophages in normal lung (Figure 1(e) and (g)). By contrast, strong IL-25 and IL-17BR immunostaining is shown in AECs/fibroblasts within FF and infiltrated inflammatory cells (lymphoid aggregates) in IPF lungs (Figure 1(f) and (h)). This is further confirmed by real-time RT-PCR and WB assay (Figure S1A–D). IgG-isotype control for immunohistochemical assay was shown in Figure S2.
Figure 1.
Histological features and expression of IL-25/IL-17BR in IPF lung tissues. Lung tissues were harvested from nine IPF patients and six normal healthy controls. Histological micrograph of representative lung sections from normal control subjects (a) and IPF patients (b) were shown by HE staining. Fibroblastic foci were shown in the inset with higher resolution. Representative lung sections with Masson trichrome staining were shown in panels (c) and (d). Immunostaining for IL-25 and IL-17BR in normal controls (e and g) and IPF patients (f and h) were also shown here. Arrows indicate AECs and macrophages; scoop indicates fibrobastic foci; square indicates infiltrated aggregates of inflammatory cells. HE: hematoxylin and eosin; IL-25: interleukin 25; IL-17BR: interleukin-17 receptor B; IPF: idiopathic pulmonary fibrosis. (A color version of this figure is available in the online journal)
mIL-25 initiates the formation of alveolar type-2 cytokine milieu in vivo
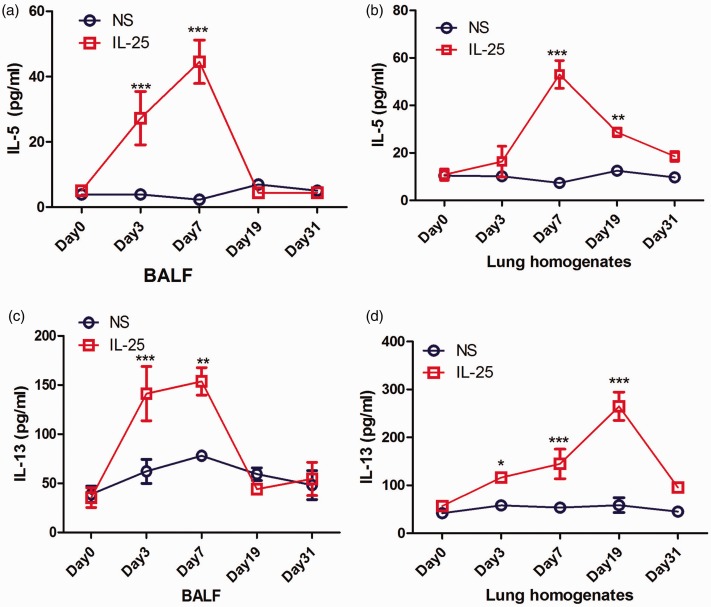
Next, we tested whether IL-25 can drive the type-2 immunity in lung parenchyma in vivo. We showed that the levels of BALF and lung IL-5/IL-13 are markedly increased and peaked at day 7 in mice treated with mIL-25 when compared with those treated with vehicle only (Figure 2(a) to (d)). However, the elevated levels of IL-5 and IL-13 decrease from day 19 to day 31, which ultimately show no difference between mIL-25- and saline-treated groups (Figure 2(a) to (d)).
Figure 2.
BALF and lung homogenate levels of IL-5 and IL-13 induced by mIL-25 challenge. Mice were intranasal injected with mIL-25 or saline and were sacrificed at days 3, 7, 19, and 31 for BALF and lung homogenate collection. ELISA was used to detect the BALF and lung homogenate levels of IL-5 (a and b) and IL-13 (c and d). Data are means ± SEMs (N = 5 per group) (mIL-25 vs. saline group at indicated time points, *P < .05, **P < .01, ***P < .001 calculated by two-way ANOVA with Bonferroni post hoc test). IL-25: interleukin 25; IL-5: interleukin 5; IL-13: interleukin 13; BALF: bronchoalveolar lavage fluid; NS: normal saline. (A color version of this figure is available in the online journal.)
mIL-25 induces significant lung inflammation and extensive lung fibrosis in mice
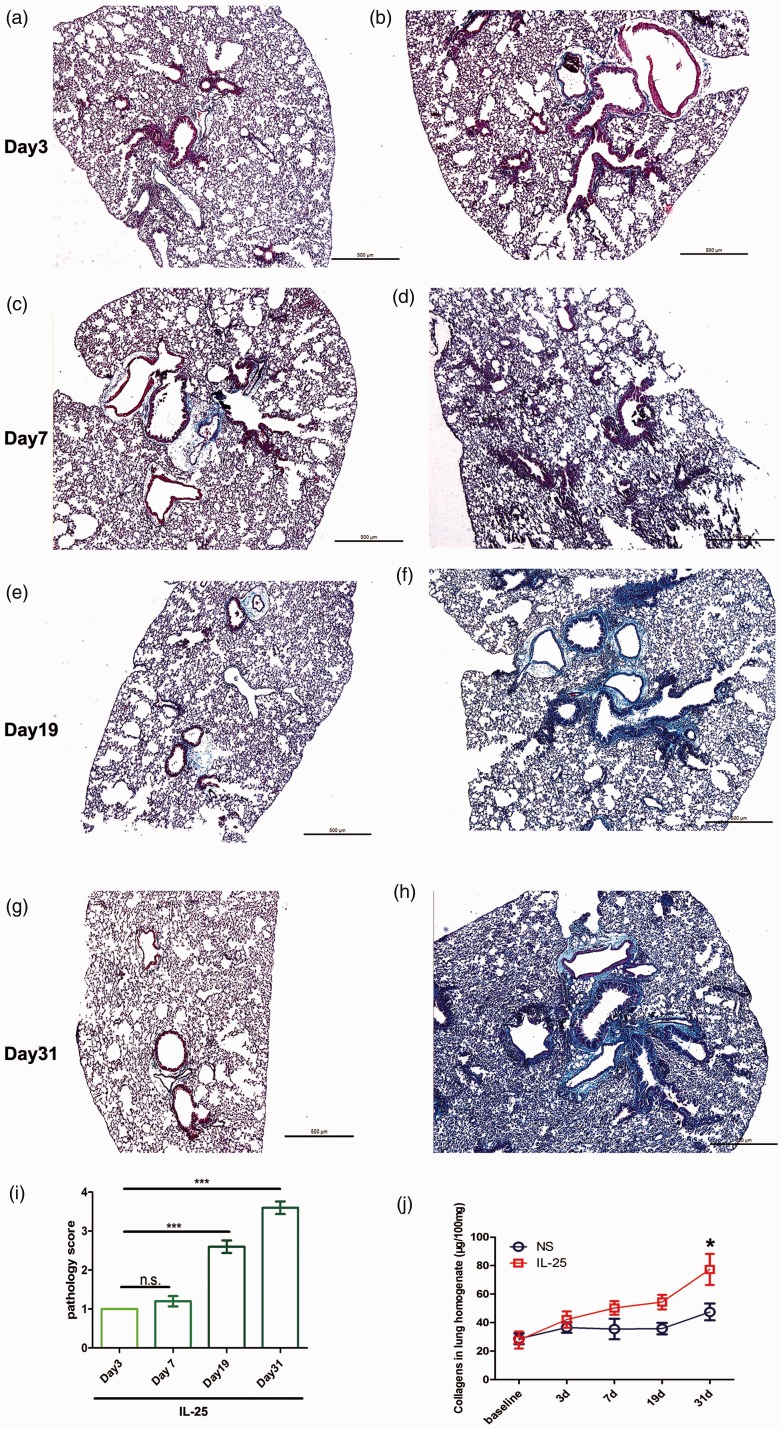
We showed that mice received mIL-25 develop significant and progressive lung inflammation (increased numbers of alveolar inflammatory cells and lymphoid follicles, Figure S3) and fibrosis (Masson staining, Figure 3) from day 7, peaks at day 31, compared with saline control mice. Specifically, IL-25 can induce significant peribronchial fibrotic reaction. Although less extensive, IL-25 can also induce remarkable fibrosis of lung parenchyma as indicated by the dark blue staining in the distal airway and alveolar spaces. By contrast, the lung slide is stained with brown red color in mice with saline instillation. Whole soluble lung collagen assay further elucidated that mIL-25 notably enhances lung collagen deposition (Figure 3(j)).
Figure 3.
Masson and soluble collagen assay show increased collagen deposition following mIL-25 intranasal instillation. Mice were intranasal injected with of mIL-25 or saline and were sacrificed at days 3, 7, 19, and 31 for lung tissue and homogenate collection. Representative low-power histology images show extensive collagen deposition by using Masson’s trichrome stain at indicated time points (a to h). Lung pathology score is shown in panel (i), data are means ± SEMs of five mice at indicated time points, NS: no significance, *P < .001 at days 7, 19, and 31 compared with day 3. Total lung soluble collagen in lung homogenate is determined by Sircol assay kit (j). Data are shown as means ± SEMs (N = 5 per group per experiment) *P < .05 compared between saline and IL-25 group at indicated time points, as calculated by ANOVA with Bonferroni post hoc test. IL-25: interleukin 25. (A color version of this figure is available in the online journal.)
mIL-25 enhances a profibrotic milieu by acting on AECs and recruiting fibroblast
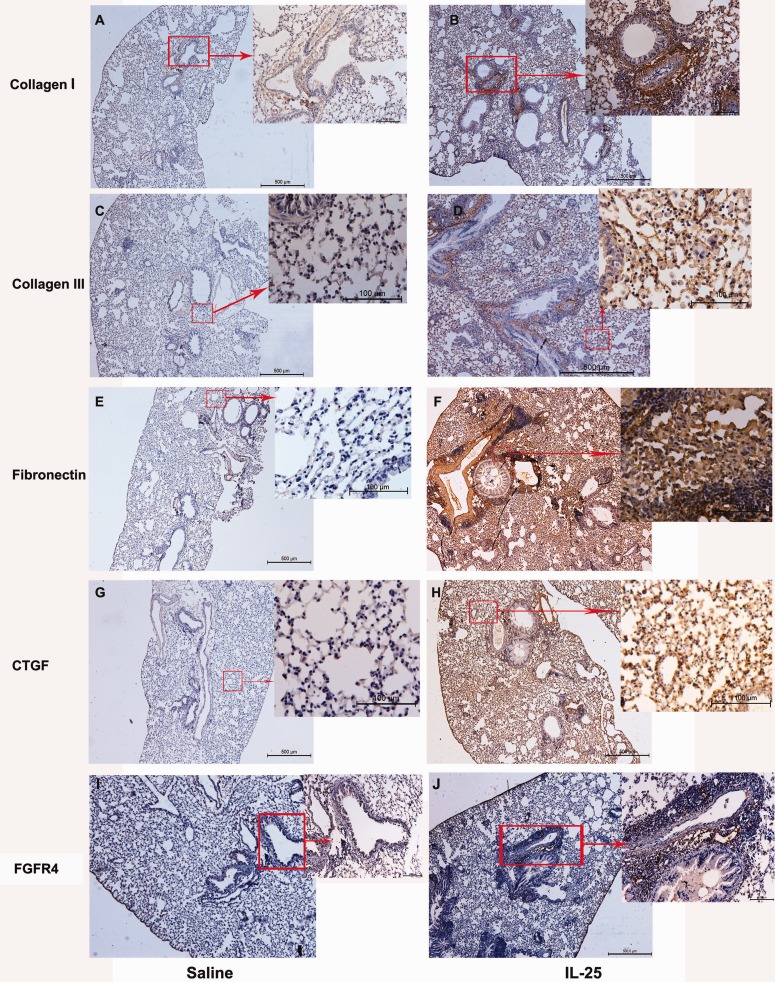
We next aimed to further explore the potential mechanisms that underlie IL-25’s profibrotic role. We firstly showed that mIL-25 notably induces the expression of extracellular matrix proteins including collagen I, collagen III, and fibronectin at day 31 compared with saline control mice (Figure 4(a) to (f)). The immunoreactivity for collagen I/III and fibronectin is not only limited to cells that resided in peribronchial/vascular areas but also significantly in injured AECs (Figure 4(a) to (f)). Furthermore, we also showed that the immunoreactivity for CTGF (major in AECs) is significantly higher at day 31 in mice treated with mIL-25 than those treated with saline only (Figure 4(g) and (h)). We also showed that fibroblast (FGFR4-positive cell) is recruited in the peribronchial areas following mIL-25 nasal inhalation compared with saline-treated mice (Figure 4(i) and (j)). IgG-isotype control for immunohistochemical assay was shown in Figure S4.
Figure 4.
Comparative sections of immunostaining for fibrotic genes in mice lung treated with mIL-25 and saline. Mice were intranasal injected with mIL-25 or saline and were sacrificed at days 3, 7, 19, and 31 for the preparations of immunohistochemical staining. Images are the representative immunostaining (N = 5 per group) for collagen I (a and b), collagen III (c and d), fibronectin (e and f), CTGF (g and h) and FGFR4 (i and j). Insets in panels (a) to (j) show positive immunostaining with higher magnification. CTGF: connective tissue growth factor; FGFR4: fibroblast growth factor receptor 4; IL-25: interleukin 25. (A color version of this figure is available in the online journal.)
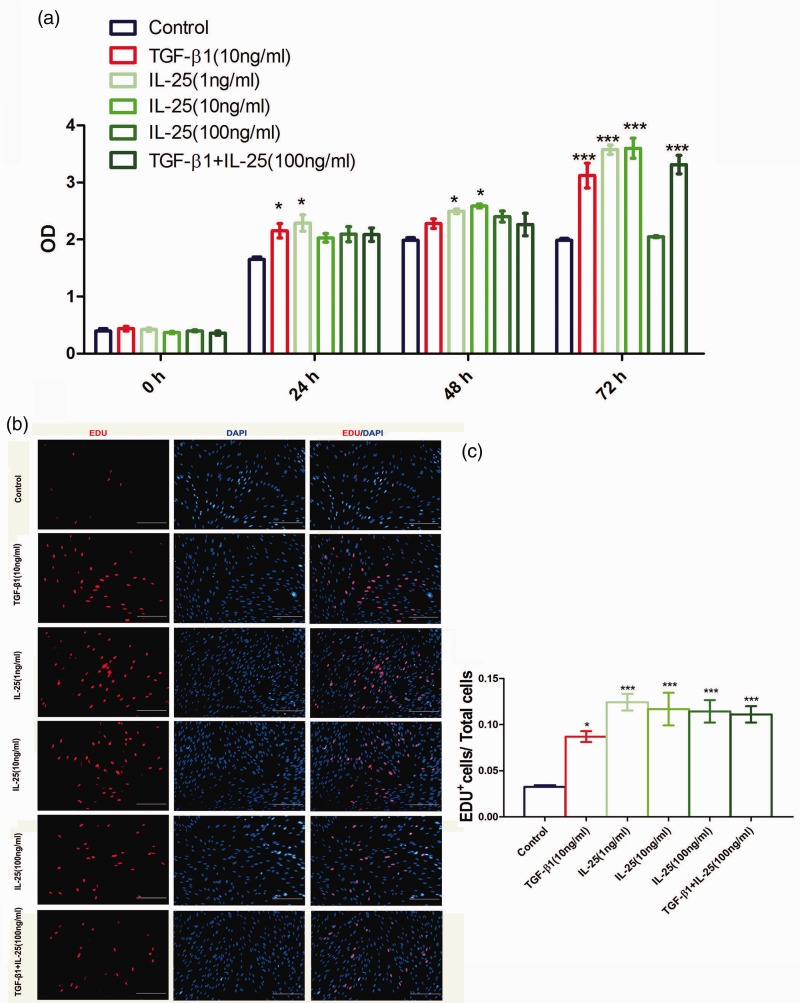
hIL-25 enhances proliferation of human lung fibroblast in vitro
We are interested in the pending topic that whether lung fibroblast is a direct cellular target for IL-25. As shown by CCK-8 assay in Figure 5(a), at as early as 24 h, both hIL-25 and TGF-β1 significantly increase the absorbance value, as compared with that in the vehicle control cells. However, hIL-25 plus TGF-β1 fails to induce further increase in the absorbance value (Figure 5(a) and (b)).
Figure 5.
IL-25 potentiates proliferation of human lung fibroblast in vitro. Cells were cultured in 96- or 24-well plates and treated with different dosages of hIL-25 and TGF-β1 or both for indicated time points. Then, cell proliferation of human lung fibroblast was detected by using CCK-8 (a) and EDU incorporation assay (b and c). The data are the average of three individual experiments and are shown as the means ± SEMs; *P < .05, ***P < .001 compared with vehicle-treated fibroblasts by using ANOVA with Bonferroni test. IL-25: interleukin 25; TGF-β1: human transforming growth factor-β1; DAPI: 4,6-diamino-2-phenyl indole OD: optical density. (A color version of this figure is available in the online journal.)
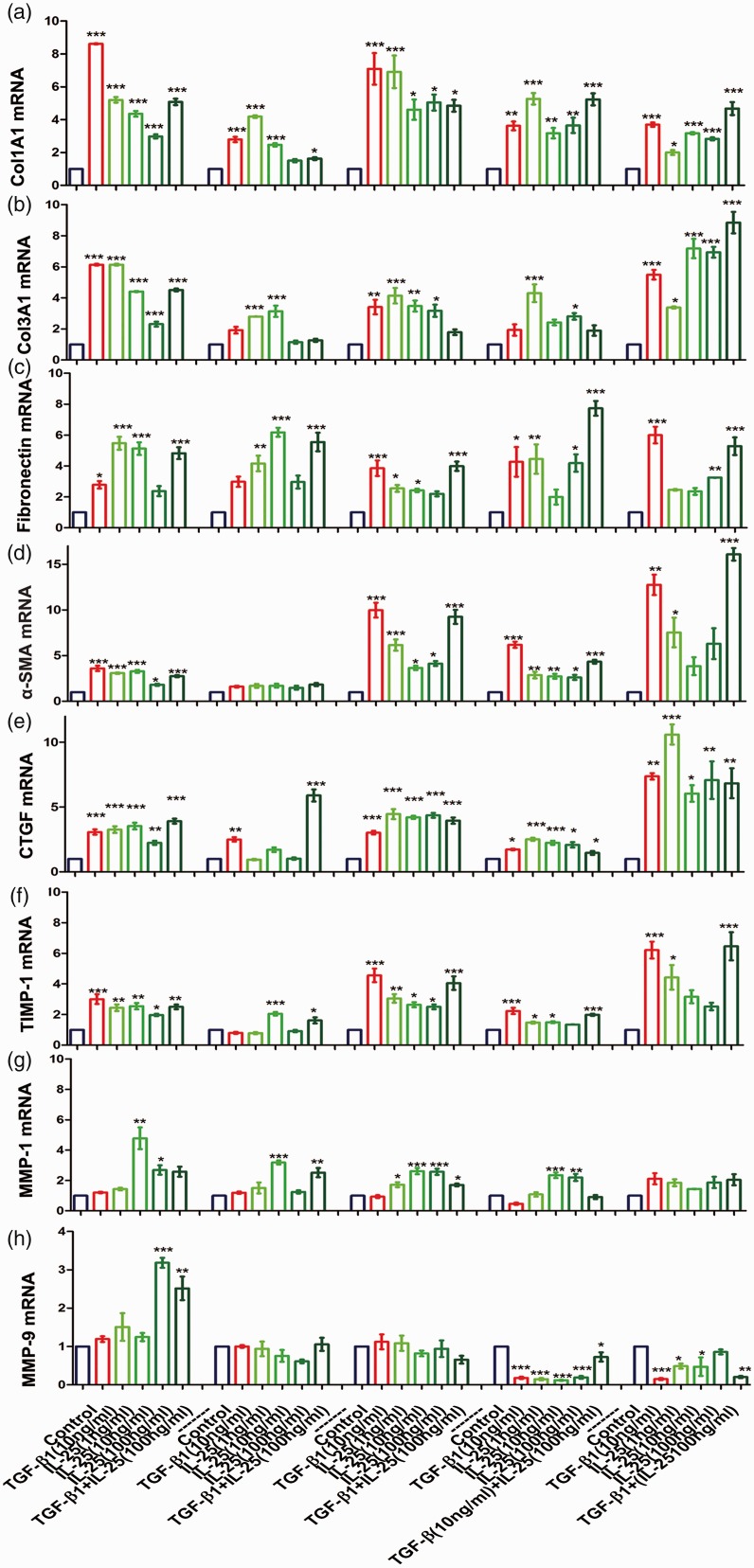
hIL-25 augments production of profibrotic genes in human lung fibroblast in vitro
Compared with vehicle-treated cells, both IL-25 and TGF-β1 induce a rapid and persistent increase in collagen I/III, fibronectin, α smooth muscle (α-SMA), CTGF, and tissue inhibitor of metalloproteinase (TIMP)-1 mRNA expression in fibroblasts (Figure 6). By contrast, hIL-25 or TGF-β1 treatment initially facilitates the mRNA expressions of matrix metalloproteinase (MMP)-1 and MMP-9 but eventually reduces their expressions when compared with vehicle controls (Figure 6(g) and (h)). However, no significant synergism is observed between hIL-25 and TGF-β1 in terms of mRNAs expression of these profibrotic genes.
Figure 6.
Effect of IL-25 on the expression of fibroblast-derived profibrotic mediators. Serum-starved human lung fibroblasts were stimulated with various dosages of hIL-25 for indicated time points. TGF-β1 was used for the positive control. COL1A1 (a), COL3A1 (b), fibronectin (c), α-SMA (d), CTGF (e), TIMP-1 (f), MMP-1 (g), and MMP-9 (h) mRNA levels were evaluated by real-time RT-PCR. Data are presented as a fold change compared with vehicle controls at the same time point. Values are means ± SEMs from three individual experimental replicates (*P < .05, **P < .01, ***P < .001 compared with vehicle-treated cells by using ANOVA). α-SMA: α smooth muscle; CTGF: connective tissue growth factor; IL-25: interleukin 25; TGF-β1: human transforming growth factor-β1; TIMP: tissue inhibitor of metalloproteinase; MMP: matrix metalloproteinase. (A color version of this figure is available in the online journal.)
Discussion
The role of IL-25 in developing type-2 immunity-mediated allergic airway inflammation and remodeling has been well demonstrated in human and murine models.7–10 The data presented here examined the potential role and mechanism of IL-25 in the context of nonallergen-driven fibrotic lung disease, i.e. IPF. We reported for the first time that IL-25/IL-25R axis is upregulated in injured AECs and lung fibroblasts that make up FFs. IL-25 exerts its profibrotic role by a hitherto unrecognized mechanism, i.e. by directly activating lung fibroblast and changing the phenotype of AECs.
By using immunohistochemical analysis, we found that both IL-25 and IL-17BR are markedly upregulated in injured AECs in FF of IPF lungs (Figure 1(f) and (h)), indicating that AECs represent as both cellular source and target of IL-25. This is in accordance with the concept that believes IL-25 is major derived from epithelial cells.14 Currently, numerous evidences support the hypothesis that repetitive cycles of alveolar epithelium injury and/or dysfunction drives the development of lung fibrosis by promoting the activation of lung fibroblast.6,15,16 Herein, we suggest that injured AECs mediate abnormal epithelial–mesenchymal cross talk by excessive secretion of IL-25 because we showed that IL-17BR (the receptor for IL-25) staining is also markedly localized in the fibroblasts with spindle appearance in the FF (Figure 1(f) and (h)). This is also in keeping with a previous in vitro finding which showed that human primary lung fibroblasts constitutively express IL-17BR.17 Collectively, our data support the notion that elevated IL-25/IL-17BR axis mediates epithelial–mesenchymal interaction (i.e. epithelium-derived IL-25 may activate fibroblast by binding binds IL-17BR which is expressed on fibroblast) in the context of lung fibrosis.
In line with our study, Hams et al. found that BALF level of IL-25 was significantly increased in IPF patients (n = 14).11 Unexpectedly, Lee et al. in a recent relatively large cohort of patients with IPF (n = 100) showed that BALF level of IL-25 is not different between normal controls and IPF group.18 The reason for the discrepancy between these studies is currently unknown. We suggested that the expression level of IL-25 may vary depending on disease severity and different disease phenotypes of IPF (e.g. patients with rapid progressive course or slow rapid progressive course or acute exacerbation). We found that notably upregulated levels of IL-25 correlates with extensive fibrotic lesion (severe IPF) in lung tissues of IPF patients (Figure 1). However, Lee et al.’s study recruited mild-to-moderate IPF patients with mean forced vital capacity % pred. at 75.0 (63.7–83.0).18 Therefore, we suggest that large cohort with different phenotypes of IPF patients is needed to further confirm the expression levels of IL-25 and its relationship to IPF.
We also assessed whether IN delivery with IL-25 contributes to lung fibrosis in mice. The delivery methods for developing murine lung fibrosis model include intratracheal route,19,20 IN route,21–23 oropharyngeal aspiration route,24 and subcutaneous route.25 We chose IN route because its simplicity, speed, and noninvasive nature. It was previously shown larger delivery volume and anesthesia can facilitate the relative distribution of the delivery agent to the lower respiratory tract (LRT).26 Thus, in our study, IL-25 was dissolved in 50 μL of sterile saline, and mice were anaesthetized with isoflurane gas in order to allow sufficient access of IL-25 to LRT and lung parenchyma.
We reported that IN instillation with IL-25 initiates a local type-2 immunity skew by upregulating the expression of IL-5 and IL-13. As shown in Figure 2, both BALF and lung IL-5 and IL-13 are rapidly induced by IL-25 IN instillation at day 3 and peak at day 7 (day 19 for lung IL-13) but all decrease to normal control level at day 31. By contrast, lung fibrosis induced by IL-25 develops at day 7 and gradually peaks at day 31 (Figure 3). This suggests that IL-25 drives lung fibrosis initially in a type-2 immunity-dependent fashion, which supports the notion that the Th1/Th2 paradigm in favor of Th2 correlates with fibrotic diseases.27 However, we hypothesize that with the progression of lung fibrosis, type-2 immunity may become dispensable and decreases to a relative low level because fibrotic lesions can self-propagate once (myo)fibroblast is fully activated and epithelial–mesenchymal interaction loop is well developed.
Most importantly, in our study, we hypothesize that IL-25 drives lung fibrosis by directly acting on injured AECs and fibroblast. First, both AECs and fibroblast express IL-17BR which binds with and respond to IL-25. Second, we found that following with IL-25 IN instillation, collagen I, collagen III, fibrobectin, and CTGF are not only induced in the areas of sub-bronchial epithelium but also induced in AECs in the distal areas (far away from large airways). This indicated that AECs starts to produce several mesenchymal markers in response to IL-25 (Figure 4(b), (d), (f), and (h)). Thus, we suggest that IL-25 may induce a process that mimics type-2 epithelial–mesenchymal transition (EMT) in vivo and promote AECs to change their phenotype in favor of profibrotic fashion.28 This was also confirmed by a previous study which showed the CTGF is confined predominantly to the proliferating type-II AECs in IPF lungs detected by immunohistochemical analysis.29 Third, we and previous study30 showed that IL-25 IN instillation significantly increased the numbers of lung fibroblast in the lung mesenchymal areas (Figure 4(j)). By in vitro cell culture, we reported for the first time that IL-25 notably potentiated the proliferations and several profibrotic gene expressions (including collagen I/III, fibronectin, CTGF, α-SMA, TIMP-1) of lung fibroblast (Figures 5 and 6). This indicates that IL-25 directly acts on lung fibroblast and may mediate lung fibrosis by augmenting activation/differentiation of fibroblast and inducing a skewing in favor of antiprotease system (i.e. TIMP-1 is upregulated whereas MMP-9 is downregulated). It is well known that the impaired proteases and antiproteases balance is implicated in the pathogenesis of IPF.31 Unexpectedly, we did not found a synergistic effect between IL-25 and TGF-β1, rather showed a somewhat antagonistic effect between them. This may be interpreted by the findings that TGF-β1 markedly inhibited IL-17BR mRNA expression in fibroblast.17 We also suggest that higher concentration of IL-25 may also counteract the proliferative effect of endogenous TGF-β1 because 10 ng/mL of IL-25 is more effective to promote the proliferation of lung fibroblast than 100 ng/mL at 72 h (Figure 5(a)). However, this needs further confirmative studies. Different from our studies, Hams et al.11 showed that ILC2 depletion obviously inhibited lung collagen deposition induced by IL-25. These data indicate various cellular targets of IL-25.
In conclusion, our study extends the current understanding of IL-25’ pathobiology beyond that of AADs. We reported that upregulated IL-25/IL-17BR axis contributes to lung fibrosis by promoting phenotype changes of AECs and activating fibroblast. However, we failed to assess the relationship between IL-25 and clinical features of IPF because of the small numbers of the cohort. Also, we did not examine whether IL-25 can directly induce EMT of AECs in vitro. The signaling pathways that underlie IL-25’s effects on fibroblast are remained elusive. Thus, how IL-25 contributes to the pathogenesis of IPF should be further determined.
Supplemental Material
Supplemental material, Supplemental Material1 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material2 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material3 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material4 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material5 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material6 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
ACKNOWLEDGMENTS
The authors would like to thank Dr Xiujuan Yao, Department of Respiratory and Critical Care Medicine, Beijing Tong Ren Hospital for the excellent technical assistance in terms of murine model.
Authors’ contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; XX designed the protocol and drafted the manuscript. LS and LB made the experiments. DH and ZJ conceived the idea, analyzed and interpreted the data, revised the article, and final approved for the version to be published. All authors have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by Grants from National Natural Science Foundation of China (81470258) and by Science Foundation initiated by Capital Medical University, Beijing, China (PYZ2017024).
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018; 378:1811–23 [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, Fibrosis A. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schunemann HJ, American Thoracic Society, Japanese European Respiratory Society, Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192:e3–19 [DOI] [PubMed] [Google Scholar]
- 4.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017; 3:17074. [DOI] [PubMed] [Google Scholar]
- 5.Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 2018; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol 2018; 71–72:112–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, Dong C. IL-25 in allergic inflammation. Immunol Rev 2017; 278:185–91 [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 2007; 120:1324–31 [DOI] [PubMed] [Google Scholar]
- 9.Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, Kim DW. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2015; 135:1476–85.e7 [DOI] [PubMed] [Google Scholar]
- 10.Kim DW, Kim DK, Eun KM, Bae JS, Chung YJ, Xu J, Kim YM, Mo JH. IL-25 could be involved in the development of allergic rhinitis sensitized to house dust mite. Mediators Inflamm 2017; 2017:3908049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, Voehringer D, McKenzie AN, Donnelly SC, Fallon PG. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A 2014; 111:367–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao XJ, Huang KW, Li Y, Zhang Q, Wang JJ, Wang W, Liu J, Lv Z, An YQ, Ding YZ, Corrigan CJ, Wang W, Sun YC, Ying S. Direct comparison of the dynamics of IL-25- and ‘allergen’-induced airways inflammation, remodelling and hypersensitivity in a murine asthma model. Clin Exp Allergy 2014; 44:765–77 [DOI] [PubMed] [Google Scholar]
- 13.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004; 114:1308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PD, O’Byrne PM. Epithelial-derived cytokines in asthma. Chest 2017; 151:1338–44 [DOI] [PubMed] [Google Scholar]
- 15.Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta 2013; 1832:911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006; 3:364–72 [DOI] [PubMed] [Google Scholar]
- 17.Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J Allergy Clin Immunol 2006; 117:590–6 [DOI] [PubMed] [Google Scholar]
- 18.Lee JU, Chang HS, Lee HJ, Jung CA, Bae DJ, Song HJ, Park JS, Uh ST, Kim YH, Seo KH, Park CS. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm Med 2017; 17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010; 299:L442–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi K, Mizoguchi K, Kawarada S, Miyoshi A, Suzuki M, Chiba S, Deki T. Effects of erlotinib on lung injury induced by intratracheal administration of bleomycin (BLM) in rats. J Toxicol Sci 2010; 35:503–14 [DOI] [PubMed] [Google Scholar]
- 21.Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol 2001; 193:538–45 [DOI] [PubMed] [Google Scholar]
- 22.Elewa YHA, Ichii O, Takada K, Nakamura T, Masum MA, Kon Y. Histopathological correlations between mediastinal fat-associated lymphoid clusters and the development of lung inflammation and fibrosis following bleomycin administration in mice. Front Immunol 2018; 9:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Kashef DH. Nicorandil ameliorates pulmonary inflammation and fibrosis in a rat model of silicosis. Int Immunopharmacol 2018; 64:289–97 [DOI] [PubMed] [Google Scholar]
- 24.Egger C, Cannet C, Gerard C, Jarman E, Jarai G, Feige A, Suply T, Micard A, Dunbar A, Tigani B, Beckmann N. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS One 2013; 8:e63432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaru A, Kobayashi T, Hinneh JA, Baffour Tonto P, D’Alessandro-Gabazza CN, Fujimoto H, Fujiwara K, Takahashi Y, Ohnishi M, Yasuma T, Nishihama K, Yoshino M, Takao K, Toda M, Totoki T, Takei Y, Yoshikawa K, Taguchi O, Gabazza EC. Oligonucleotide-targeting periostin ameliorates pulmonary fibrosis. Gene Ther 2017; 24:706–16 [DOI] [PubMed] [Google Scholar]
- 26.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 2002; 282:L833–9 [DOI] [PubMed] [Google Scholar]
- 27.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004; 4:583–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jolly MK, Ward C, Eapen MS, Myers S, Hallgren O, Levine H, Sohal SS. Epithelial–mesenchymal transition, a spectrum of states: role in lung development, homeostasis, and disease. Dev Dyn 2018; 247:346–58 [DOI] [PubMed] [Google Scholar]
- 29.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J 2001; 17:1220–7 [DOI] [PubMed] [Google Scholar]
- 30.Yao X, Wang W, Li Y, Lv Z, Guo R, Corrigan CJ, Ding G, Huang K, Sun Y, Ying S. Characteristics of IL-25 and allergen-induced airway fibrosis in a murine model of asthma. Respirology 2015; 20:730–8 [DOI] [PubMed] [Google Scholar]
- 31.Menou A, Duitman J, Crestani B. The impaired proteases and anti-proteases balance in Idiopathic Pulmonary Fibrosis. Matrix Biol 2018; 68–69:382–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental material, Supplemental Material2 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental material, Supplemental Material3 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental material, Supplemental Material4 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental material, Supplemental Material5 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine
Supplemental material, Supplemental Material6 for IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts by Xuefeng Xu, Sa Luo, Biyun Li, Huaping Dai and Jinglan Zhang in Experimental Biology and Medicine