Short abstract
Objective
To test the antifibrotic effect of dermatan sulphate in a bleomycin-induced mouse model of pulmonary fibrosis.
Methods
C57 mice were randomly divided into four experimental groups: saline-treated control group, bleomycin-induced fibrosis group, prednisolone acetate group and dermatan sulphate group. Lungs were assessed using the lung index, and the extent of interstitial fibrosis was graded using histopathological observation of haematoxylin & eosin-stained lung tissue. Lung tissue hydroxyproline levels and blood fibrinogen levels were measured using a hydroxyproline colorimetric kit and the Clauss fibrinogen assay, respectively. Tissue-type plasminogen activator (tPA) was measured using a chromogenic tPA assay kit.
Results
Lung index values were significantly lower in the dermatan sulphate group versus the fibrosis group. Histopathological analyses revealed that dermatan sulphate treatment ameliorated the increased inflammatory cell infiltration, and attenuated the reduction in interstitial thickening, associated with bleomycin-induced fibrosis. Hydroxyproline and fibrinogen levels were decreased in the dermatan sulphate group versus the fibrosis model group. Dermatan sulphate treatment was associated with increased tPA levels versus controls and the fibrosis group.
Conclusions
Damage associated with bleomycin-induced pulmonary fibrosis was alleviated by dermatan sulphate.
Keywords: Dermatan sulphate, pulmonary fibrosis, bleomycin
Introduction
Pulmonary fibrosis is a common outcome of various drug-induced or traumatic causes of chronic interstitial lung diseases, and is characterized by severe dyspnoea and loss of pulmonary function.1,2 Pulmonary fibrosis comprises several different types, namely, pneumoconiosis, allergic pneumonia, sarcoidosis and idiopathic pulmonary fibrosis, and disease pathogenesis involves epithelial cell injury, inflammatory cell infiltration, as well as fibroblast recruitment and activation. Median survival in patients with pulmonary fibrosis is only 3–5 years,3,4 and disease related morbidity and mortality has risen gradually over recent years. Corticosteroids and other immunosuppressive drugs are used in the clinical treatment of pulmonary fibrosis, however, most have poor efficacy for patients, and mortality rate remains high at up to 40% within 5 years of diagnosis.5 Lung transplantation is effective as a means of treating pulmonary fibrosis, and may improve symptoms, improve quality of life and prolong life, however, its application is limited due to complications, infection, high costs, and particularly a lack of donated organ resources.6 Therefore, there is an urgent need to explore new effective strategies for ameliorating pulmonary fibrosis. Although no single animal model of pulmonary fibrosis encompasses all aspects of the human disease, the use of animal models has led to the reproduction of some manifestations of pulmonary fibrosis.7,8
Bleomycin comprises a family of complex glycopeptides with antitumour antibiotic effects that are used to treat many cancers, however, pulmonary fibrosis is a well-known side-effect in bleomycin-treated patients.9–11 Due to its early historical introduction, and thus more extensive experience in its use, the bleomycin-induced animal model of fibrosis is the best characterized in many important aspects of pulmonary fibrosis, and is an excellent model to verify the function of novel pulmonary fibrosis pathophysiological characteristics.12–14
Dermatan sulphate, like heparan sulphate, is an anticoagulant and antithrombotic glycosaminoglycan consisting of disaccharide units of alternating L-iduronic acid and (1/3)-N-acetyl-β-D-galactosamine,15 and plays an important role in the treatment of cardiovascular diseases.16,17 Dermatan sulphate is involved in regulating the clotting process without antagonizing serine protease and also has anti-oedema activity and promotes skin regeneration, while also promoting hematoma reabsorption and preventing skin keratinization and spike disease.18 Research has also revealed that dermatan sulphate is involved in cancer, infection, wound repair, fibrosis, pulmonary fibrosis and other pathological or physiological processes, and is of great interest in the fields of pathology and pulmonary fibrosis.18
The aim of the current study was to test the effect of dermatan sulphate treatment in a mouse model of bleomycin-induced pulmonary fibrosis. The effects of dermatan sulphate on development of pulmonary fibrosis in response to bleomycin were evaluated compared with healthy control mice, a fibrosis model control, and a fibrosis model treated with active comparator (prednisolone acetate), with the aim of revealing potential new options for the treatment of pulmonary fibrosis in clinical drug therapy.
Materials and methods
Animals
Adult C57 mice (male and female, n = 120) weighing 18–22 g, were provided by Jinan Peng Yue Experimental Animal Breeding Co., Ltd (Jinan, China): animal production certificate number, SCXK (Lu) 2014 0007; and issuing unit, the Office of Science and Technology of Shandong Province, China. The animals were housed in cages with a 12-h:12-h light–dark cycle at an ambient temperature of approximately 20–26°C and relative humidity of approximately 40–70% with free access to food and water. The study was approved by Yantai Yuhuangding Hospital ethics committee, and all animal procedures performed in this study followed the regulation of ethical issues in animal experimentation.
Bleomycin fibrosis model and treatment protocol
The C57 mice were randomly divided into four groups (30 mice per group): healthy control group (control), bleomycin fibrosis model group (fibrosis model), prednisolone acetate-treated bleomycin fibrosis group (prednisolone acetate-treated group), and dermatan sulphate-treated bleomycin fibrosis group (dermatan sulphate-treated group). Bleomycin-induced pulmonary fibrosis was established following the procedures previously described, with minor modifications.1,19 Briefly, mice were anaesthetized using 0.1 ml/20 g of 1.5% pentobarbital sodium (Merck, Darmstadt, Germany) by intraperitoneal injection and then a tracheostomy was performed. Pulmonary fibrosis was induced with 5 mg/kg bleomycin (Selleck, Shanghai, China), injected intratracheally, in the fibrosis model group, the prednisolone acetate group, and the dermatan sulphate group. The healthy control group was injected with an equivalent volume of sterile physiological saline. Bleomycin was homogeneously distributed to the mouse lungs using an upright spin for 3–5 min. All mice received an intramuscular injection of 8000 U penicillin (Northeast Pharmaceutical group, Shenyang, China) at 1 day prior to surgery and at 3 days following surgery. From two days following surgery and bleomycin instillation (i.e., initiation of the bleomycin-induced pulmonary fibrosis model), 6.67 mg/kg prednisolone acetate (Selleck) was injected into the stomach of mice in the prednisolone acetate group, at the same time, 5.3 mg/kg dermatan sulphate (LaiYu Chemical Co., Laizhou, China) was injected in the stomach of the dermatan sulphate group, and an equivalent volume of physiological saline was injected into the stomachs of mice in the healthy control and fibrosis model groups. These treatments were continued once daily in all groups. Research has indicated that dermatan sulphate has significant antithrombotic activity, with a half maximum effective dose (ED50) of 2.0 mg/kg in healthy rabbits, with time- and dose-dependent activity.20 Therefore, the ED50 of dermatan sulphate in mice was calculated to be 5.3 mg/kg by conversion of body surface area between humans and animals in this study.
Lung specimen collection
A pulmonary function test system was used to analyse pulmonary fibrosis in the mice, including lung index, histopathology, and hydroxyproline, fibrinogen and tissue plasminogen activator assays. For pulmonary analyses, 10 mice in each group were randomly selected and killed by cervical dislocation, on days 7, 14 and 28 following the start of dermatan sulphate/prednisolone acetate treatment. A skin incision was then made in each mouse, along the centre line of the thorax and the sternum or ribs, to expose the lungs and allow observation of the pathological lung tissue. The left lung was fixed with 10% formaldehyde for 48 h, then dehydrated and paraffin-embedded using standard methods. Tissue sections of approximately 4 μm thickness were then cut, and stored at 4°C for subsequent histopathological analysis. The right lung was frozen in liquid nitrogen for hydroxyproline determination.
Lung index
Lung index was determined by finding the mass of the lung and the weight of the mouse to use in the following lung index formula:21 Lung index = lung mass (mg)/body weight (g).
Histopathology
The grade of lung fibrosis was determined by histopathology, and numerical fibrotic score was determined as described previously.22 Briefly, the left lung was fixed, embedded and sectioned as described above. Subsequent to dewaxing with xylene, hydration was performed using a series of graded concentrations of ethanol (100% ethanol for 5 min, 95% ethanol for 1 min, 80% ethanol for 5 min, 75% ethanol for 5 min and distilled water for 2 min). Standard haematoxylin and eosin (H&E) staining was performed at room temperature for 12 min, then the sections were dehydrated and cleared using two incubations with xylene at room temperature for 10 min each. Sections sealed with neutral resin were observed under a light microscope for histopathological changes. One slide was evaluated per lung and three fields of view per slide were observed. The degree of alveolitis and pulmonary fibrosis was classified as previously described.23 Briefly, tissues were divided into four grades, according to the extent of interstitial fibrosis using the following scale: 0, no significant fibrosis of the whole lung; 1, mild fibrosis (<20%); 2, moderate fibrosis (20–50%); and 3, severe fibrosis (>50%).
Hydroxyproline assay
The hydroxyproline content in lung tissue was determined by alkaline hydrolysis. The obtained lung tissues were homogenized on ice, centrifuged and the supernatant collected. The hydroxyproline content in the supernatant was analysed using a hydroxyproline colorimetric assay kit (Biovision, Milpitas, CA, USA) according to the manufacturer’s instructions.
Plasma fibrinogen and tissue-type plasminogen activator assays
On days 7, 14 and 28 following the start of dermatan sulphate/prednisolone acetate treatment, blood samples from the abdominal aorta of mice were collected into a centrifuge tube containing 0.109 mol/l sodium citrate anticoagulant at a volume ratio of 1/10. Samples were immediately centrifuged at 2 400 × g, and the plasma was collected and assayed. Plasma fibrinogen levels were measured using the Clauss fibrinogen assay.24 Plasma tissue-type plasminogen activator (tPA) levels were measured using a chromogenic substrate tPA assay kit (Selleck) according to the manufacturer’s instructions.
Statistical analyses
Results derived from the independent experiments are presented as mean ± SD, and were statistically analysed using SPSS software, version 17.0 (IBM, Armonk, NY, USA). Between-group differences in lung index, lung hydroxyproline levels, and plasma fibrinogen and tPA levels, were compared using one-way analysis of variance (ANOVA) and P values < 0.05 were considered to be statistically significant.
Results
Dermatan sulphate reduced the increase in lung index scores induced by bleomycin
Lung index values in the fibrosis model group were significantly higher than lung index values in the control group, at days 7, 14, and 28 (10.268 versus 7.321; 10.369 versus 6.259; and 10.632 versus 5.779; fibrosis model versus controls, respectively; all P < 0.01; Table 1). In the dermatan sulphate- and prednisolone acetate-treated fibrosis groups, lung index values were significantly lower than in the fibrosis model group at all time-points (Table 1). Lung index values at days 7, 14, and 28 following dermatan sulphate treatment, were significantly lower in the dermatan sulphate-treated group than in the fibrosis model group (7.586 versus 10.268 [P < 0.01]; 8.252 versus 10.369 [P < 0.01]; and 6.320 versus 10.632 [P < 0.05], dermatan sulphate-treated versus fibrosis model group, respectively; Table 1). There were no statistically significant differences between the control group and the prednisolone-treated or dermatan sulphate-treated groups at any time-points.
Table 1.
Lung index values in a bleomycin-induced C57 mouse model of pulmonary fibrosis, treated with dermatan sulphate or prednisolone acetate.
| Study group | Lung index, mg/g |
||
|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |
| Control | 7.321 ± 0.420 | 6.259 ± 0.634 | 5.779 ± 0.458 |
| Fibrosis model | 10.268 ± 1.556△ | 10.369 ± 1.782△ | 10.632 ± 4.369△ |
| Prednisolone acetate-treated | 7.703 ± 1.560** | 8.412 ± 1.325* | 6.365 ± 1.054* |
| Dermatan sulphate-treated | 7.586 ± 1.365** | 8.252 ± 1.205** | 6.320 ± 1.125* |
Data presented as mean ± SD.
Control, treated with saline only; Fibrosis model, bleomycin-induced pulmonary fibrosis; Prednisolone acetate-treated, bleomycin-induced pulmonary fibrosis treated with prednisolone acetate; Dermatan sulphate-treated, bleomycin-induced pulmonary fibrosis treated with dermatan sulphate.
△P < 0.01 versus control group; *P < 0.05, **P < 0.01 versus fibrosis model group.
Effects of dermatan sulphate on bleomycin-induced changes in lung histopathology
Alveolitis/pulmonary fibrosis scores were significantly increased in the bleomycin-induced fibrosis model group versus the control group at days 7, 14, and 28 (P < 0.01; Table 2). Increased alveolitis/pulmonary fibrosis scores in response to bleomycin-induced fibrosis were significantly attenuated in mice treated with prednisolone acetate and dermatan sulphate, shown by significantly lower scores for both treatments versus the fibrosis model group at days 7, 14 and 28 (P < 0.05; Table 2).
Table 2.
Alveolitis and pulmonary fibrosis scores in a bleomycin-induced C57 mouse model of pulmonary fibrosis, treated with dermatan sulphate or prednisolone acetate.
| Study group | Alveolitis and pulmonary fibrosis score |
||
|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |
| Control | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.20 ± 0.52 |
| Fibrosis model | 2.20 ± 0.54△ | 2.10 ± 0.59△ | 2.70 ± 0.45△ |
| Prednisolone acetate-treated | 1.10 ± 0.69** | 1.30 ± 0.56* | 1.20 ± 0.40** |
| Dermatan sulphate-treated | 1.50 ± 0.60** | 1.50 ± 0.41* | 2.00 ± 0.82* |
Data presented as mean ± SD.
Control, treated with saline only; Fibrosis model, bleomycin-induced pulmonary fibrosis; Prednisolone acetate-treated, bleomycin-induced pulmonary fibrosis treated with prednisolone acetate; Dermatan sulphate-treated, bleomycin-induced pulmonary fibrosis treated with dermatan sulphate.
△P < 0.01 versus control group; *P < 0.05, **P < 0.01 versus fibrosis model group.
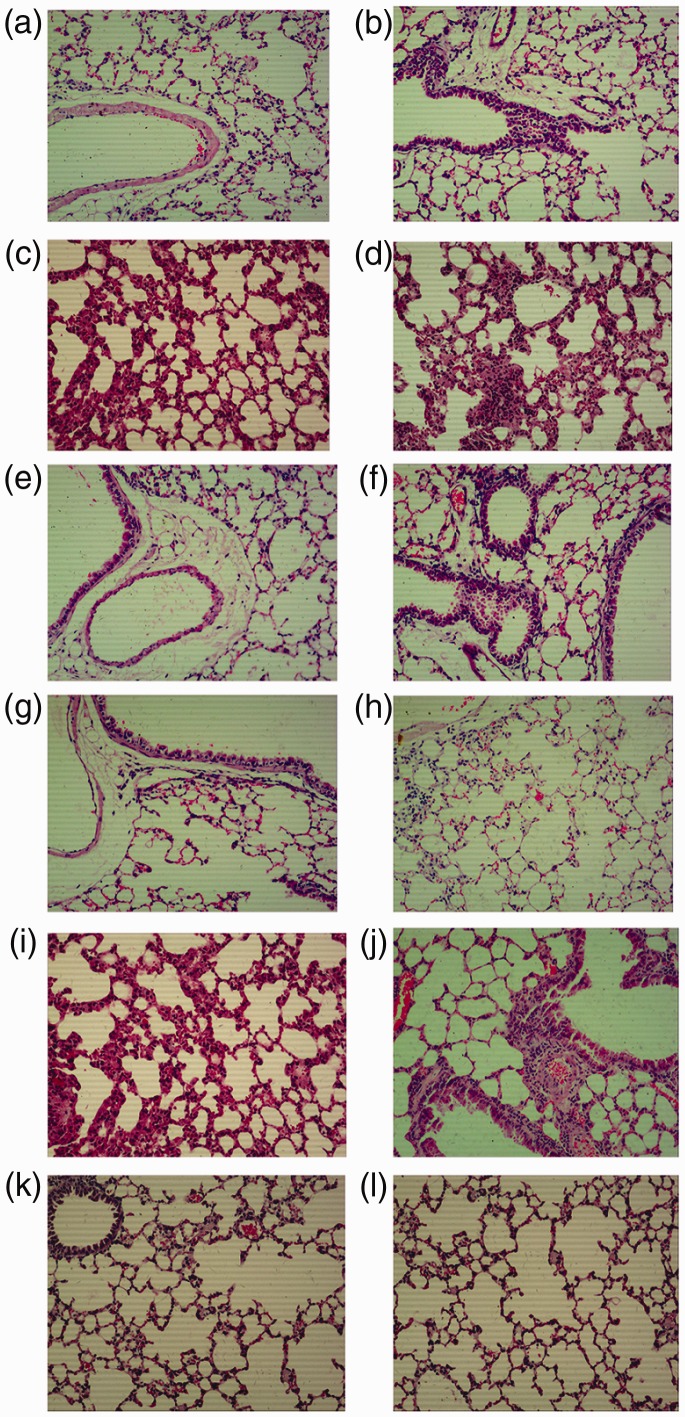
Haematoxylin and eosin stained lung tissue sections were evaluated for histopathological abnormalities on days 7, 14 and 28 following prednisolone acetate/dermatan sulphate treatment (Figure 1a–l). Under light microscopy, lung tissue sections from the control group showed normal alveolar spaces, normal thickening of the alveolar septa and high elasticity (Figure 1a, e and i). Lung sections from mice in the bleomycin-induced fibrosis group showed marked histopathological abnormalities, such as alveolar inflammation, abnormal alveolar structure, dense interstitial infiltration by lymphocytes, extensive thickening of the interalveolar septa, fibroblasts, plasma cells and neutrophils at day 7 (Figure 1b); alveolar inflammation and reduced alveolar space, with increased fibroblasts and matrix at day 14 (Figure 1f); and significant alveolar inflammation, spacious alveolar septum, many fibroblasts and a small number of macrophages, lymphocytes and plasma cells at day 28 (Figure 1j). Lung sections from mice treated with dermatan sulphate showed moderate amelioration of inflammatory cell infiltration, with normal alveolar structure and a few of macrophages, lymphocytes and plasma cells (Figure 1d, h and l). Lung sections from the prednisolone acetate group showed maximal suppression of inflammation, together with a reduction in interstitial thickening, also with normal alveolar structure and a few macrophages, lymphocytes and plasma cells (Figure 1c, g and k).
Figure 1.
Representative photomicrographs showing haematoxylin and eosin (H&E) stained lung sections from a bleomycin-induced C57 mouse model of pulmonary fibrosis: (a–d) day 7; (e–h) day 14; and (i–l) day 28 following initiation of prednisolone acetate/dermatan sulphate treatment. Four study groups included controls treated with saline only (a, e, i), bleomycin-induced fibrosis model (b, f, j), bleomycin-induced fibrosis treated with 6.67 mg/kg prednisolone acetate (c, g, k) and bleomycin-induced fibrosis treated with 5.3 mg/kg dermatan sulphate (d, h, l). Original magnification, × 100.
Effect of dermatan sulphate on bleomycin-induced changes in lung hydroxyproline levels
In this study, hydroxyproline levels in lung tissues were measured as a marker of fibrosis, and were found to be significantly increased in lungs from the bleomycin-induced fibrosis group compared with the control group, at day 14 (0.62 versus 0.45; P < 0.05) and day 28 (0.64 versus 0.44; P < 0.05; Table 3). Treatment with dermatan sulphate was found to significantly attenuate the increased hydroxyproline levels associated with bleomycin at day 28 (0.54 versus 0.64; P < 0.01; Table 3). There were no statistically significant differences in lung hydroxyproline levels between any of the bleomycin-induced fibrosis groups (untreated, prednisolone-treated or dermatan sulphate-treated) at day 14.
Table 3.
Lung hydroxyproline levels in a bleomycin-induced C57 mouse model of pulmonary fibrosis, treated with dermatan sulphate or prednisolone acetate.
| Study group | Hydroxyproline levels, μg/g |
||
|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |
| Control | 0.40 ± 0.04 | 0.45 ± 0.16 | 0.44 ± 0.08 |
| Fibrosis model | 0.38 ± 0.05 | 0.62 ± 0.12△ | 0.64 ± 0.06△△ |
| Prednisolone acetate-treated | 0.33 ± 0.04 | 0.56 ± 0.10 | 0.63 ± 0.14 |
| Dermatan sulphate-treated | 0.28 ± 0.04** | 0.56 ± 0.12 | 0.54 ± 0.12** |
Data presented as mean ± SD.
Control, treated with saline only; Fibrosis model, bleomycin-induced pulmonary fibrosis; Prednisolone acetate-treated, bleomycin-induced pulmonary fibrosis treated with prednisolone acetate; Dermatan sulphate-treated, bleomycin-induced pulmonary fibrosis treated with dermatan sulphate.
△P < 0.05, △△P < 0.05 versus control group; **P < 0.01 versus fibrosis model group.
Effect of dermatan sulphate on the fibrinolytic system in mice with bleomycin-induced pulmonary fibrosis
Investigation of the effects of dermatan sulphate on the fibrinolytic system, through assay of plasma fibrinogen and tPA, revealed that treatment with dermatan sulphate or prednisolone acetate significantly reduced plasma fibrinogen levels and significantly increased plasma tPA levels compared with the fibrosis model group (P < 0.05; Tables 4 and 5). Although fibrinogen levels were not significantly lower than controls in the dermatan sulphate- or prednisolone acetate-treated groups (Table 4), plasma tPA levels were found to be significantly higher than controls in both treatment groups (P < 0.05; Table 5).
Table 4.
Plasma fibrinogen levels in a bleomycin-induced C57 mouse model of pulmonary fibrosis, treated with dermatan sulphate or prednisolone acetate.
| Study group | Fibrinogen, g/l |
||
|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |
| Control | 3.12 ± 0.32 | 3.14 ± 0.18 | 3.13 ± 0.25 |
| Fibrosis model | 6.23 ± 0.28△ | 6.58 ± 0.23△ | 7.30 ± 0.32△ |
| Prednisolone acetate-treated | 3.02 ± 0.24* | 2.98 ± 0.15* | 2.89 ± 0.23** |
| Dermatan sulphate-treated | 2.08 ± 0.54* | 1.96 ± 0.26** | 1.85 ± 0.14** |
Data presented as mean ± SD.
Control, treated with saline only; Fibrosis model, bleomycin-induced pulmonary fibrosis; Prednisolone acetate-treated, bleomycin-induced pulmonary fibrosis treated with prednisolone acetate; Dermatan sulphate-treated, bleomycin-induced pulmonary fibrosis treated with dermatan sulphate.
△P < 0.05 versus control group; *P < 0.05, **P < 0.01 versus fibrosis model group.
Table 5.
Plasma tissue plasminogen activator levels in a bleomycin-induced C57 mouse model of pulmonary fibrosis, treated with dermatan sulphate or prednisolone acetate.
| Study group | Tissue plasminogen activator, g/l |
||
|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |
| Control | 0.18 ± 0.01 | 0.17 ± 0.02 | 0.19 ± 0.02 |
| Fibrosis model | 0.27 ± 0.02△ | 0.30 ± 0.04△ | 0.31 ± 0.03△ |
| Prednisolone acetate-treated | 0.48 ± 0.03△,* | 0.50 ± 0.01△,* | 0.49 ± 0.03△,* |
| Dermatan sulphate-treated | 0.65 ± 0.06△△,* | 0.69 ± 0.03△△,* | 0.75 ± 0.02△△,* |
Data presented as mean ± SD.
Control, treated with saline only; Fibrosis model, bleomycin-induced pulmonary fibrosis; Prednisolone acetate-treated, bleomycin-induced pulmonary fibrosis treated with prednisolone acetate; Dermatan sulphate-treated, bleomycin-induced pulmonary fibrosis treated with dermatan sulphate.
△P < 0.05, △△P < 0.01 versus control group; *P < 0.05 versus fibrosis model group.
Discussion
Pulmonary fibrosis is characterized by inflammatory injury of alveolar epithelial cells, excessive proliferation of fibroblasts, extensive thickening of the interalveolar septa, and dense interstitial infiltration by lymphocytes,25,26 consistent with features of the bleomycin-induced pulmonary fibrosis model in the present study. Bleomycin was originally isolated from a strain of actinobacteria, Streptomyces verticillus, and has antineoplastic properties and induces pulmonary fibrosis by eliciting anti-inflammatory and a fibrotic phase in mice. Thus, a bleomycin-induced pulmonary fibrosis animal model was successfully developed to imitate the pathology of human pulmonary fibrosis, and to explore new pharmacological methods to inhibit pulmonary fibrosis.27,28
Research has shown that aucubin and oxymatrine alleviate bleomycin-induced pulmonary fibrosis in mice. For example, Zhou et al.1 found that aucubin exerted protective effects in their bleomycin-induced pulmonary fibrosis mouse model, and suggested that it may serve as a novel drug for pulmonary fibrosis by inhibiting inflammation, fibroblast proliferation, and differentiation. In another study,29 oxymatrine was reported to attenuate pulmonary fibrosis by inhibiting nitric oxide synthase expression and the transforming growth factor-β/Smad signalling pathway. A previous study found that dermatan sulphate is an important anticoagulant and antithrombotic glycosaminoglycan, which also has anti-inflammatory activity and has been used in traditional Chinese medicine.13 The antithrombotic activity of dermatan sulphate and anticoagulant activity through its activation of heparin cofactor II (HC II) have been investigated in previous studies. The effective concentration of dermatan sulphate was found to be 4.00 µg/mg, and in vivo antithrombotic activity was demonstrated through significantly longer clotting time of 58.12 min in the presence of dermatan sulphate compared with 19.79 min in a saline group.30,31 Dermatan sulphate inhibits thrombin activity and prevents thrombus formation by binding to HC II. Dermatan sulphate has also been shown to enhance fibrinolytic activity and reduce the formation of thrombus by regulating the release of tPA and plasminogen activator inhibitor.32,33 In a study investigating dermatan sulphate for treating the initial inflammatory response following arterial injury in mice, dermatan sulphate was found to inhibit thrombus formation and modulate inflammation.15 To the best of the present authors’ knowledge, there is currently no published research that investigates the validity of dermatan sulphate in treating bleomycin-induced pulmonary fibrosis in mice.
In the present novel investigation of a bleomycin-induced pulmonary fibrosis mouse model that compared dermatan sulphate-treated mice versus untreated mice with bleomycin-induced fibrosis, the authors demonstrated that dermatan sulphate exerts anti-inflammatory and antifibrotic actions in the lungs of mice with bleomycin-induced pulmonary fibrosis. These effects were evidenced by attenuation of the morphological fibrotic responses, and the reduced lung index values, neutrophil infiltration, and hydroxyproline and fibrinogen levels. In addition, the significantly increased tPA levels in the present study suggest that dermatan sulphate efficiently attenuated bleomycin-induced pulmonary fibrosis in this mouse model, as tPA is the major intravascular activator of fibrinolysis and a ligand for receptors involved in cell-signalling.34 The present findings suggest that dermatan sulphate treatment may represent a promising strategy for inhibiting the progression of pulmonary fibrosis in mice.
To the best of the authors’ knowledge, the present study is the first to show the effect of dermatan sulphate on lung index values in mice. Lung index values in the bleomycin-induced fibrosis group were increased, however, the magnitude was significantly reduced in the dermatan sulphate-treated group, suggesting that dermatan sulphate attenuates bleomycin-induced lung injury in mice.
Previous studies have noted that a common criticism in the bleomycin-induced pulmonary fibrosis model is the role of inflammation, as it induces chronic inflammation and fibrosis, evidenced by the release of various inflammatory mediators.35–37 In the present study, bleomycin treatment was also found to cause evident chronic inflammation and fibrosis compared with the saline-treated control group, and this increased inflammation and fibrosis was significantly attenuated by dermatan sulphate treatment. H&E stained lung tissue sections from the control group showed that normal alveolar spaces, and normal thickening of the alveolar septa were present. However, sections from the lungs of mice in the bleomycin-induced fibrosis group showed marked histopathological abnormalities including alveolar inflammation and infiltration by neutrophils. Tissue sections from mice treated with dermatan sulphate showed moderate amelioration of inflammatory cell infiltration, together with a reduction in interstitial thickening. The present results concur with those previously reported.31,38
Hydroxyproline, known as the gold standard index of pulmonary fibrosis, is the product of collagen degradation and reflects the collagen content of lung tissue to assess the degree of pulmonary fibrosis.39 The present results verified previous data, and suggested that dermatan sulphate may represent a potential new medicine for decreasing hydroxyproline levels in the lung, and reducing lung injury. Fibrinogen levels were also attenuated with dermatan sulphate treatment, further supporting the potential for dermatan sulphate as a novel drug for treating bleomycin-induced pulmonary fibrosis in mice.
In conclusion, the present research revealed that dermatan sulphate could efficiently attenuate bleomycin-induced pulmonary fibrosis in a mouse model. The study provides evidence that dermatan sulphate may be a promising candidate for the prevention of bleomycin-induced lung damage. Further studies are needed to validate the results and clarify the mechanism with which dermatan sulphate attenuates bleomycin-induced pulmonary fibrosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Science and Technology Development Program for Chinese medicine of Shandong Province (2015-417).
References
- 1.Zhou Y, Li P, Duan JX, et al. Aucubin alleviates bleomycin-induced pulmonary fibrosis in a mouse model. Inflammation 2017: 40: 2062–2073. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Ren F, Cao J, et al. Intermittent hypoxia aggravated bleomycin-induced pulmonary fibrosis in a mouse model of sleep apnea. Chest 2016; 149: A584. [Google Scholar]
- 3.Ren L, Yang C, Dou Y, et al. MiR-541-5p regulates lung fibrosis by targeting cyclic nucleotide phosphodiesterase 1A. Exp Lung Res 2017: 43: 249–258. [DOI] [PubMed] [Google Scholar]
- 4.Chilakapati SR, Serasanambati M, Vissavajjhala P, et al. Amelioration of bleomycin-induced pulmonary fibrosis in a mouse model by a combination therapy of bosentan and imatinib. Exp Lung Res 2015; 41: 173–188. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou KM, Margaritopoulos GA, Siafakas NM. Pharmacological treatment of idiopathic pulmonary fibrosis: from the past to the future. Eur Respir Rev 2013; 22: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porteous MK, Lee JC. Primary graft dysfunction after lung transplantation. Clin Chest Med 2017; 38: 641–654. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol 2007; 170: 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo J, Lee C, Hwang HS, et al. Therapeutic advantage of inhaled tacrolimus-bound albumin nanoparticles in a bleomycin-induced pulmonary fibrosis mouse model. Pulm Pharmacol Ther 2016; 36: 53–61. [DOI] [PubMed] [Google Scholar]
- 9.Mikamo M, Kitagawa K, Sakai S, et al. Inhibiting Skp2 E3 ligase suppresses bleomycin-induced pulmonary fibrosis. Int J Mol Sci 2018; 19: E474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolzán AD, Bianchi MS. DNA and chromosome damage induced by bleomycin in mammalian cells: an update. Mutat Res 2018; 775: 51–62. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Dong H, Jiang L, et al. Bleomycin inhibits proliferation and induces apoptosis in TPC-1 cells through reversing M2-macrophages polarization. Oncol Lett 2018; 16: 3858–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabre A, Marchal-Sommé J, Marchand-Adam S, et al. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur Respir J 2008; 32: 426–436. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto N, Distler O. Molecular targets for therapy in systemic sclerosis. Fibrogenesis Tissue Repair 2012; 5: S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira PR, Oliveira-Junior MC, Mackenzie B, et al. Exercise reduces lung fibrosis involving serotonin/Akt signaling. Med Sci Sports Exerc 2016; 48: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 15.Godoy JA, Carneiro GD, Sielski MS, et al. Combined dermatan sulfate and endothelial progenitor cell treatment: action on the initial inflammatory response after arterial injury in C57BL/6 mice. Cytotherapy 2015; 17: 1447–1464. [DOI] [PubMed] [Google Scholar]
- 16.He L, Giri TK, Vicente CP, et al. Vascular dermatan sulfate regulates the antithrombotic activity of heparin cofactor II. Blood 2008; 111: 4118–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicente CP, He L, Tollefsen DM. Accelerated atherogenesis and neointima formation in heparin cofactor II–deficient mice. Blood 2007; 110: 4261–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syx D, Van Damme T, Symoens S, et al. Genetic heterogeneity and clinical variability in musculocontractural Ehlers-Danlos syndrome caused by impaired dermatan sulfate biosynthesis. Hum Mutat 2015; 36: 535–547. [DOI] [PubMed] [Google Scholar]
- 19.Jules-Elysee K, White DA. Bleomycin-induced pulmonary toxicity. Clin Chest Med 1990; 11: 1–20. [PubMed] [Google Scholar]
- 20.Rasente RY, Imperiale JC, Lázaro-Martínez JM, et al. Dermatan sulfate/chitosan polyelectrolyte complex with potential application in the treatment and diagnosis of vascular disease. Carbohydr Polym 2016; 144: 362–370. [DOI] [PubMed] [Google Scholar]
- 21.Rodó C, Illescas T, Arévalo S, et al. Follow-up of fetuses with congenital diaphragmatic hernia: the quantitative lung index. Eur J Obstet Gynecol Reprod Biol 2018; 225: 22–25. [DOI] [PubMed] [Google Scholar]
- 22.Tawfik MK, Makary S. 5-HT7 receptor antagonism (SB-269970) attenuates bleomycin-induced pulmonary fibrosis in rats via downregulating oxidative burden and inflammatory cascades and ameliorating collagen deposition: comparison to terguride. Eur J Pharmacol 2017; 814: 114–123. [DOI] [PubMed] [Google Scholar]
- 23.Szapiel SV, Elson NA, Fulmer JD, et al. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979; 120: 893–899. [DOI] [PubMed] [Google Scholar]
- 24.de Maat MP, Arnold AE, van Buuren S, et al. Modulation of plasma fibrinogen levels by ticlopidine in healthy volunteers and patients with stable angina pectoris. Thromb Haemost 1996; 76: 166–170. [PubMed] [Google Scholar]
- 25.Kandhare AD, Bodhankar SL, Mohan V, et al. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Bio Interact 2015; 237: 151–165. [DOI] [PubMed] [Google Scholar]
- 26.Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. F1000Prime Rep 2014; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou XM, Feng YL, Wen FQ, et al. Simvastatin attenuates bleomycin-induced pulmonary fibrosis in mice. Chin Med J (Engl) 2008; 121: 1821–1829. [PubMed] [Google Scholar]
- 28.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 2011; 17: 355–361. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Lu W, Ma Z, et al. Oxymatrine attenuates bleomycin-induced pulmonary fibrosis in mice via the inhibition of inducible nitric oxide synthase expression and the TGF-β/Smad signaling pathway. Int J Mol Med 2012; 29: 815. [DOI] [PubMed] [Google Scholar]
- 30.Panagos CG, August DP, Jesson C, et al. Photochemical depolymerisation of dermatan sulfate and analysis of the generated oligosaccharides. Carbohyd Polym 2016; 140: 13–19. [DOI] [PubMed] [Google Scholar]
- 31.Westergren-Thorsson G, Hedström U, Nybom A, et al. Increased deposition of glycosaminoglycans and altered structure of heparan sulfate in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 2017; 83: 27–38. [DOI] [PubMed] [Google Scholar]
- 32.Moffatt P, Geng Y, Lamplugh L, et al. Absence of the dermatan sulfate chain of decorin does not affect mouse development. J Negat Results Biomed 2017; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honda T, Matsunaga E, Katagiri K, et al. Small dermatan sulphate proteoglycans in cultured fibroblasts from guinea-pig skin. Biomed Res 1987; 8: 175–183. [Google Scholar]
- 34.Wang H, Ji P, Zhao XS, et al. Recombinant human TNK tissue-type plasminogen activator (rhTNK-tPA) versus alteplase (rt-PA) as fibrinolytic therapy for acute ST-segment elevation myocardial infarction (China TNK STEMI): protocol for a randomised, controlled, non-inferiority trial. BMJ Open 2017; 7: e016838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Della Latta V, Cecchettini A, Del Ry S, et al. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol Res 2015; 97: 122–130. [DOI] [PubMed] [Google Scholar]
- 36.Lawson WE, Polosukhin VV, Stathopoulos GT, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 2005; 167: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limjunyawong N, Mitzner W, Horton MR. A mouse model of chronic idiopathic pulmonary fibrosis. Physiol Rep 2014; 2: e00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cores J, Hensley MT, Kinlaw K, et al. Safety and efficacy of allogeneic lung spheroid cells in a mismatched rat model of pulmonary fibrosis. Stem Cells Transl Med 2017; 6: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Gong L, Zhang L, et al. Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J Pharmacol 2006; 536: 287–295. [DOI] [PubMed] [Google Scholar]