Short abstract
Obesity is associated with chronic metabolic conditions that directly and indirectly cause kidney parenchymal damage. A review of the literature was conducted to explore existing evidence of the relationship between obesity and chronic kidney disease as well as the role of bariatric surgery in improving access to kidney transplantation for patients with a high body mass index. The review showed no definitive evidence to support the use of a transplant eligibility cut-off parameter based solely on the body mass index. Moreover, in the pre-transplant scenario, the obesity paradox is associated with better patient survival among obese than non-obese patients, although promising results of bariatric surgery are emerging. However, until more information regarding improvement in outcomes for obese kidney transplant candidates is available, clinicians should focus on screening of the overall frailty condition of transplant candidates to ensure their eligibility and addition to the wait list.
Keywords: Obesity, kidney transplant, bariatric surgery, chronic kidney disease, body mass index, obesity paradox
Introduction
Obesity is a chronic metabolic condition that does not intrinsically differ from other chronic diseases in the pre-transplant setting.1 Obesity can increase the risk of surgical complications after kidney transplantation and impact the community costs; however, the outcomes of being on dialysis are worse than those of undergoing transplantation.2 Therefore, a high body mass index (BMI) is no longer an absolute contraindication to transplantation.3
The increasing concern regarding the rising incidence and prevalence of chronic kidney disease (CKD) and obesity worldwide has recently increased the efforts to highlight a possible strategy with which to improve the outcomes of this patient population.4
The present review was performed to examine the current literature with a focus on the role of bariatric surgery (BS) in relation to kidney transplantation in patients with CKD and a high BMI.
CKD and obesity
CKD is characterised by alterations in the kidney parenchymal structure, function, or both that compromise patients’ health.5–7 The Kidney Disease: Improving Global Outcomes guideline classifies an individual as having CKD if abnormalities of kidney structure or function persist for more than 3 months, and the severity of CKD is based on the estimated glomerular filtration rate (eGFR) (a marker of renal excretory function) and the extent of albuminuria (an indicator of renal barrier dysfunction).8 CKD is now recognised as a health priority worldwide because of its negative impact on patients’ prognosis and quality of life and its cost to national health care systems.
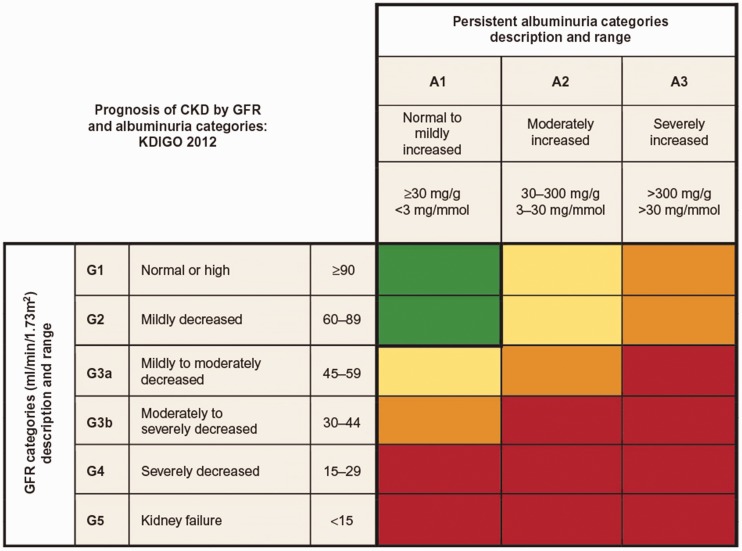
Patients with CKD can be classified by their level of kidney function (i.e., the eGFR) and the amount of protein present in the urine. A lower eGFR and higher amount of albumin present in the urine indicate a more advanced stage of CKD8 (Figure 1).
Figure 1.
Prognosis of CKD according to GFR and albuminuria categories: KDIGO 2012. Green indicates low risk (if no other markers of kidney disease are present), yellow indicates moderately increased risk, orange indicates high risk, and red indicates very high risk. CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes.
The most common definition of obesity is based on the BMI of an individual: a BMI of 25 to 29 kg/m2 is defined as overweight, and a BMI of ≥30 kg/m2 is defined as obese.9 However, the BMI itself is neither the only nor the most reliable index with which to define obesity. Importantly, the BMI cannot discriminate between sarcopaenia and adiposity. This discrimination is of extreme importance in patients with CKD because the muscle mass and protein storage level are critical outcome determinants in patients undergoing dialysis; a high lean mass is associated with improved survival, and a high fat mass is associated with reduced survival.10,11 In addition, the BMI does not consider the fat distribution, which is directly related to the pathophysiology of CKD. In fact, a high amount of visceral rather than subcutaneous fat is the main contributor to the metabolic deregulation that eventually harms the kidneys as well as other organs. Thomas et al.12 conducted a meta-analysis in 2011 to investigate the relationship between metabolic syndrome and renal disease and found that visceral fat, as assessed by the waist circumference, predicted new-onset CKD. Several alternative parameters, such as the waist circumference and waist–hip ratio, have been shown to be superior to the BMI in terms of the correct classification of obesity; however, the waist–hip ratio is very easy to calculate and is used in most scientific and clinical settings.13
The prevalences of obesity and CKD as epidemic diseases are rising in parallel.14 Already in 2003, up to 60% of patients undergoing renal transplantation were obese in the United States.15 The latest estimates on the worldwide obesity epidemic reveal that the age-standardised prevalence of obesity is expected to increase from 11% to 18% in men and from 15% to 21% in women by 2025.16
Knowledge of the aetiological connections between obesity and CKD has been developing in recent years, although the mechanisms of these connections are still a matter of scientific debate.17 There is evidence of a direct causal connection between a high BMI and CKD, with more rapid progression of CKD to end-stage renal disease (ESRD) because of the underlying renal hyperfiltration driven by the excess weight;17 this condition is termed obesity-related glomerulopathy. This syndrome is characterised by glomerulomegaly in the presence or absence of focal and segmental glomerulosclerosis lesions. Obesity-related glomerulopathy–associated glomerulomegaly and focal and segmental glomerulosclerosis can superimpose on other renal pathologies or systemic inflammatory conditions that have been established as factors for exacerbation of CKD, such as hypertension and diabetes.18 Together, these mechanisms synergise with obesity to induce ESRD, specifically causing an increase in albuminuria.19 An analysis of about 600,000 adults with good renal condition revealed the role of obesity in the onset of CKD after adjustment for the main determinants of new-onset CKD.20 In obese adults (BMI of >30 kg/m2), the risk of a new-onset low eGFR and albuminuria increased by 28% and 51%, respectively; however, this risk was not present in the overweight population (BMI of 25–29 kg/m2), suggesting a positive, although non-linear, relationship between BMI and de novo CKD.20 According to the authors, the nephrotoxic effect of a high BMI is visible only above a certain BMI threshold; i.e., within the obesity range. This study also demonstrates that obesity contributes to CKD not only in terms of a declining eGFR but also in terms of new-onset albuminuria. Besides its role in de novo CKD development, obesity also seems to exacerbate existing renal disease, accelerating the decline to ESRD.21–24 This pathological process acts mainly through the renin-angiotensin-aldosterone system axis as demonstrated by the enhanced nephroprotective effect of renin-angiotensin-aldosterone system inhibitors in obese versus non-obese patients with ESRD.25
The burden of obesity is particularly worrisome in children. In the United States, the prevalence of obesity from 2011 to 2014 was 17.0% and that of extreme obesity was 5.8% among youth 2 to 19 years of age. This trend mirrors the situation in European countries.26,27
The emerging evidence of an aetiological connection between CKD and obesity as well as their epidemiological and economic impacts suggests the need for a thorough scientific and clinical approach to prevent and address renal disease. Such an approach should also involve weight-loss strategies, including both traditional conservative options and BS.
Impact of obesity on kidney transplant eligibility
The increasing prevalence and incidence of obesity, its independent role in the aetiology of CKD, and its contribution to many other risk factors for renal failure account for the increasing number of obese patients in need of renal replacement therapy. According to an Italian report, the proportion of obese patients undergoing haemodialysis ranges from 6% to 16%.28
Although not fully understood, a paradoxical role of the BMI is present in patients undergoing dialysis29: a higher BMI is associated with a lower mortality rate in patients undergoing dialysis, while a lower BMI increases mortality. More specifically, historical unintended weight loss is an independent predictor of death, showing a J-shaped association between the BMI and death with a normal BMI at the nadir of the curve.30 Although the pathophysiology is complex, obese persons might be more well-nourished and have better immune responses against devastating chronic infectious and other diseases that are often a cause of death in patients with a lower BMI undergoing dialysis.31 The frailty often underlying malnutrition is a state of low physiological reserve and multi-systemic dysregulation that leads to susceptibility to external stressors and adverse outcomes.32 The scarcity of lean body mass, also known as sarcopaenia, is part of the frailty syndrome and is modifiable through physical exercise, which is also useful in the context of a weight-loss strategy.33 The actual prevalence of frailty among patients undergoing dialysis ranges from 30% to 78%, and it is associated with significant adverse outcomes such as falls, hospitalisation, mortality, and loss of functional independence.34 In this context, obese and non-frail patients undergoing dialysis benefit from a higher BMI as a protector against external stressors in contrast to patients with sarcopaenia and frailty.
The reverse epidemiology of the BMI in patients undergoing dialysis has introduced controversy among transplant surgeons and clinicians regarding the admission of obese patients to renal transplantation, a scepticism that is also amplified by the evidence of poorer surgical outcomes in obese than non-obese patients. Furthermore, the obesity paradox does not extend to the post-transplant setting, in which obesity and weight gain lead to reduced survival and a higher incidence of cardiovascular disease (CVD), as in the general population.35
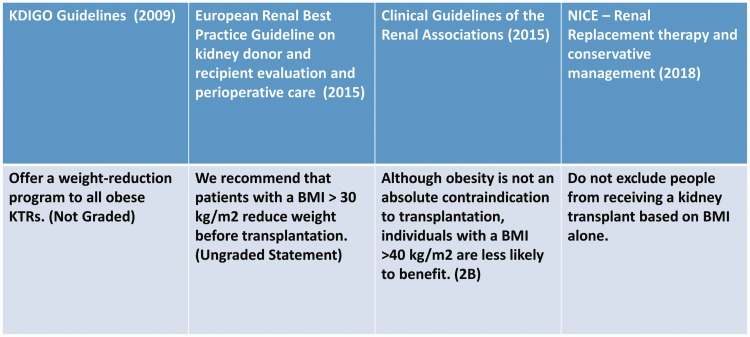
Current guidelines do not address this issue; in fact, although obesity is no longer recognised as an absolute contraindication for transplant surgery, cut-offs and risk stratification are still matters of debate (Figure 2). According to the European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care, patients with a BMI of >30 kg/m2 are recommended to reduce their body weight before transplantation.36 According to the Renal Association Clinical Practice Guideline, obese patients present technical difficulties and are at increased risk of perioperative complications; therefore, they should be screened rigorously for CVD and each patient should be considered individually.37 Additionally, individuals with a BMI of >40 kg/m2 are less likely to benefit from transplantation;37 thus, the allocation of the limited organ pool must be carefully considered. The recently published National Institute for Health and Care Excellence guidelines claim that transplantation provides better survival and higher quality of life in overweight patients undergoing dialysis and that there is not enough evidence to recommend exclusion based only on the BMI.3
Figure 2.
High BMI guidelines. No unanimous consensus has been reached regarding the management of obese candidates for kidney transplantation. BMI, body mass index; KDIGO, Kidney Disease: Improving Global Outcomes; KTRs, kidney transplant recipients; NICE, National Institute for Health and Care Excellence.
However, a high BMI is an important concern not only in terms of the preoperative and operative management of obese patients but also in terms of the post-transplant management. This is because an excess of visceral fat, a frequent finding in obese patients, is a driving force of CVD,38 which is recognised as the principal cause of morbidity and mortality after transplantation.39,40
Impact of obesity on waiting time for kidney transplantation
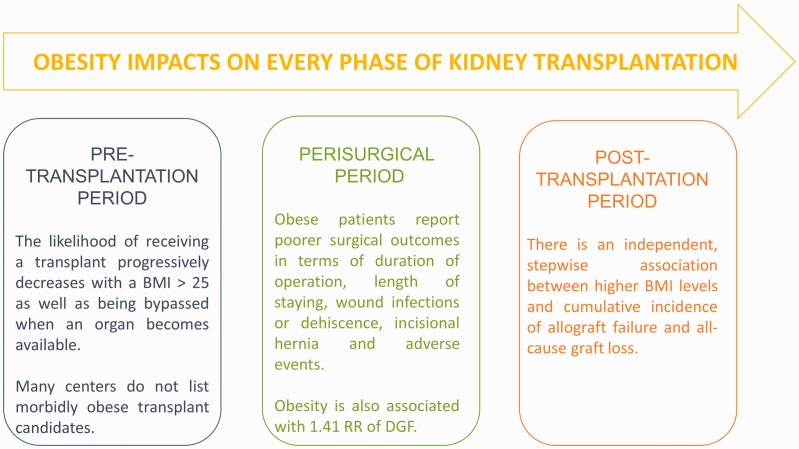
Less predictable and less documented is the role of obesity on the waiting time for kidney transplantation. In fact, besides any possible surgical and clinical complications caused by obesity and any transplant policy adopted by different centres in terms of transplant eligibility or presurgical weight-loss strategies, a BMI of >31 kg/m2 at the start of dialysis is associated with a lower likelihood of receiving a kidney transplant, with an L-shaped relationship between the BMI at the start of dialysis and overall mortality.41 More specifically, Segev et al.42 analysed a cohort of 132,353 patients on the United Network for Organ Sharing transplant waiting list and found that the likelihood of receiving a transplant progressively decreased with a BMI of >25 through >40 kg/m2 and that the likelihood of being bypassed when an organ became available progressively increased with a BMI of >25 kg/m2. The authors found that 21% of centres in the United States did not list a single morbidly obese candidate (BMI of >40 kg/m2) and that 15% of centres did not list a single severely obese candidate (BMI of >35 kg/m2).42
In addition, obese patients eligible for renal transplantation or already on a waiting list while attempting to lose weight should wait considerably longer.43 The consequence of this is either losing their pre-emptive status or prolonging the dialysis time, both of which are detrimental to allograft survival44 (Figure 3). These patients’ outcomes are even poorer if we consider that among those required to lose weight to be waitlisted, <5% are able to achieve the target weight and <50% of the obese patients initially waitlisted as inactive because of an inappropriate weight for renal transplantation (BMI of >35 kg/m2) achieve an active status within the following 6 years.45
Figure 3.
Obesity impacts every phase of kidney transplantation. BMI, body mass index; RR, relative risk; DGF, delayed graft function.
Interestingly, a Spanish study of 228 patients from non-hospital dialysis centres who were considered to have a non-active status on the waiting list because of incomplete immunological data or temporary contraindications demonstrated that obesity was the most frequent cause of non-inclusion; it was reported in 30% of the patients and was often associated with other comorbidities.46
Finally, a Canadian survey distributed to the Kidney Group of the Canadian Society of Transplantation showed that although many Canadian centres use independent BMI limits for transplant candidates [most commonly 40 kg/m2 (62%), followed by 35 kg/m2 (36%)], no or poor support is provided to patients committed to lose weight to be actively waitlisted; in fact, only 30% of the responders reported having a weight management programme in their centre.47
Impact of obesity on short- and long-term clinical and surgical outcomes
After having discussed eligibility and the waiting list, a complete evaluation of the extra burden faced by obese patients in need of a kidney transplant cannot avoid analysis of specific challenges that the anatomy and physiology associated with a high BMI present in terms of surgical and medical management during and after the transplant.
In 2014, Nicoletto et al.48 published a meta-analysis of observational studies including obese and non-obese kidney transplant recipients, focusing on follow-up and outcomes such as delayed graft function (DGF), acute rejection, graft or patient survival at 1 or 5 years after transplantation, and death by CVD. The analysis of 21 studies involving 9296 patients concluded that pre-transplantation obesity is associated with a 1.41 relative risk of DGF; however, no association was found between obesity and acute graft rejection.48 The association between obesity and the other outcomes included in the study (patient survival and death by CVD and all-cause mortality) depended on the time of surgery; no significant association was found for patients undergoing surgery after 2000, while obesity seemed to influence these outcomes before 2000.48 As reported by the authors, possible explanations for this distribution might be related to major changes and advancements in immunosuppressive therapy along with improved surgical and clinical management of obese patients and their complications (e.g., hypertension, CVD, diabetes, and a pro-inflammatory state).48 In this regard, alternative new drugs with better metabolic risk profiles (e.g., belatacept) look promising in terms of reducing drug-induced toxicities such as hypertension and diabetes49 with the potential of improving long-term renal function. Studies have shown higher mean non-high-density-lipoprotein cholesterol and lower mean triglyceride blood levels in de novo belatacept recipients than in patients treated with calcineurin inhibitors50 and have indicated that this might mitigate the metabolic risks associated with a high BMI.
Another meta-analysis by Lafranca et al.51 included 56 studies and 5526 patients who were divided into those with a high BMI (>30 kg/m2) and low BMI (<30 kg/m2). The main outcomes analysed were survival (mortality, patient survival, and graft survival), renal function outcomes (DGF and acute rejection), and metabolic conditions [(new-onset diabetes after transplantation (NODAT) and hypertension)]; the other outcomes were mainly related to infections and surgery (duration of operation, length of stay, wound infection, incisional hernia, wound dehiscence, and adverse events). This latter group is of particular interest because a well-established study showed more surgical complications in obese than non-obese patients.52 Kidney transplant recipients with a BMI of >30 kg/m2 appeared to have worse graft and patient survival up to 3 years from transplantation; detrimental effects of a higher BMI on renal function were also evident in that the incidences of DGF and acute rejection were higher in patients with a high than low BMI.51 Additionally, metabolic outcomes in obese patients are different from those in non-obese patients because the incidence of NODAT and hypertension is higher in obese patients. Finally, concerning surgical outcomes, patients with a low BMI show significant better performance and fewer complications; the only exceptions are lymphocoeles and haematomas, possibly because these two conditions are not necessarily dependent on the BMI as observed by the authors themselves. Nevertheless, despite the poorer outcomes in patients with a high BMI, transplantation remains the most effective approach for patients with ESRD, although pre-transplant weight loss should be advised.51
Naik et al.53 performed a retrospective analysis in 2016 to clarify the adverse effects of obesity on long-term allograft survival in first-time kidney transplant recipients. The results showed an independent stepwise association between higher BMIs and the cumulative incidence of allograft failure and all-cause graft loss. For this reason, the authors suggested that despite the evidence of a beneficial effect of transplantation in patients with a high BMI, surgical and clinical decisions must be made with consideration of these outcomes and the centre’s expertise in handling this group of patients.53 Our centre’s experience during a 1-year follow-up did not show inferior outcomes in obese patients when compared with their overweight and non-obese counterparts. Another study also showed no difference in terms of NODAT or allograft loss, although overweight and obese patients had a lower eGFR at 3 and 6 months after transplantation.54 Because obesity is associated with an increased risk of steroid-induced diabetes and CVD risk factors,55 our centre’s policy is to withdraw steroids early (within the first week post-transplantation), which might contribute to amelioration of these poor outcomes in patients with a high BMI.54
In the last decade, research has shown that robotic kidney transplantation allows the performance of transplant surgery in patients with extremely high BMIs. Garcia-Roca et al.56 reported that 52.8% of procedures among their transplant candidates with a BMI of ≥45 kg/m2 was performed with the robotic technique. There are capital costs associated with this procedure, but the initial results show less severe postoperative pain and fewer wound complications, such as surgical site infections and hernia. These outcomes could be particularly advantageous in obese patients with respect to overall costs and rehospitalisation.
In summary, a higher BMI poses more challenges in terms of the perioperative and short- and long-term outcomes among patients in need of a kidney transplant, particularly regarding the enhanced risk of DGF and graft failure. There are probably three reasons for this increased risk: immunosuppression; a subclinical pro-inflammatory state, which is a well-known feature of patients with a high BMI; and a higher incidence of cardiovascular comorbidities. However, neither these challenges nor the obesity paradox is enough to deny transplantation to these patients. Indeed, the overall picture regarding the impact of the BMI on the chance and rate of successful transplantation remains unclear and greatly differs from centre to centre. For this reason, it is of utmost importance that each centre evaluates the possibility of transplantation in these patients based not only on the individual patient’s pathophysiological or anatomical characteristics but also on that particular centre’s experience in the management of possible complications. This should also be included in the patients’ consent form for the operation.
What remains to be answered is whether there are indications for BMI cut-offs or “optimal” weight loss before renal transplantation. In particular, we will attempt to collect evidence on the role and possible advantage of BS in patients with a high BMI to optimise the outcomes of these renal transplant candidates.
Pre-transplantation weight-loss strategy: the promising role of BS
As stated in the previous sections, the optimal management of obese and morbidly obese patients in need of a kidney transplant is largely controversial and unclear, and no guidelines have yet been developed to provide evidence-based indications.
However, two conflicting points are quite clear from our analysis. First, obesity is not a contraindication for transplantation, and there is no reason to exclude patients from transplant programmes based only on their BMI. Second, obesity is undoubtedly associated with additional issues in terms of waitlisting and the safety and success of the transplantation.
Ideally, weight loss before transplantation might provide beneficial effects, and some guidelines already suggest the activation of weight-loss strategies before the obese patient is actively enrolled in the transplant process.36 There is support for the efficacy of transplant facilitation through effective pre-transplant weight reduction in some bariatric programmes57 with an improvement in CKD risk categories, especially in patients with a moderate to high baseline risk.58
The need to reduce BMI: lifestyle change and diet versus BS
The increased rate of complications and suboptimal outcomes in obese and morbidly obese patients undergoing transplantation has led many centres to refuse patients with BMIs above a certain limit, with maximum BMI limits ranging from 30 to 40 kg/m2.59 Weight loss thus becomes unavoidable to be eligible for renal transplantation; however, regardless of the rules followed by each centre, pre-transplant weight loss should be strongly encouraged to accelerate candidacy and enhance surgical and renal outcomes in obese individuals with ESRD.60 Two main strategies are available to achieve this result: a conservative one involving mainly diet and exercise, and a more aggressive one involving BS (Table 1).
Table 1.
Pre-transplant weight-loss strategies.
| Lifestyle and nutritional interventions | Bariatric surgery |
|---|---|
| Lower cost and reduced aggressiveness (all patients eligible) | Best treatment option for severe obesity |
| Significant weight loss in the short term, but high drop-out rate and weight regain | Long-term results significantly increase impact of dietary interventions |
| No effect on drug absorption | Malabsorptive procedure could impact the transplant recipient’s immunosuppression dose |
| No complications described | Uncertainty about effect on kidney function |
The conservative approach has been preferred for many years because of its lower cost and reduced aggressiveness. Behavioural interventions that address both diet and physical activity show small but significant benefits on weight-loss maintenance;61 however, a considerable number of patients cannot reach the target weight either because of poor compliance62 or because of inadequate therapeutic plans.63 The first issue to be faced in such a conservative approach is the high drop-out rate registered during the follow-up of obese patients committed to diet and exercise. Another major concern is that despite an encouraging initial response in terms of weight loss, the long-term results are still a matter of debate because weight regain at different rates and in different patients has been quite frequently registered. Kidney transplant candidates should be referred to a dietician as soon as practicable, with regular follow-up to monitor the weight variance. Dietary advice should be individualised and include meal plans, exercise plans, and specific goals. A possible initial strategy of weight-loss therapy is to reduce body weight by approximately 10% from baseline, with weight loss of 1 to 2 kg per month. With success, further weight loss can be attempted if indicated through ongoing assessment.64
During the last two decades, the role of BS has become increasingly more important as demonstrated by the growing evidence in the literature.57,65–68 The promising results of BS are mainly due to the resultant weight loss, which allows patients to be waitlisted soon after the operation, as well as to the metabolic improvement in terms of diabetes and hypertension, which are deemed responsible for renal failure and suboptimal outcomes after transplantation. These improvements have been made possible by advancements in bariatric services that have reduced the complication rate and enhanced outcomes; these advantages have also been demonstrated in patients with CKD. Although promising,51 data regarding the renal impact and safety of BS in patients undergoing dialysis remain insufficient.69 BS seems to have a direct therapeutic effect on obesity-related glomerulopathy, reversing the glomerular hyperfiltration, albuminuria, and glomerulomegaly. The weight loss also acts at a systemic level, improving the inflammatory status of the obese condition and often resolving diabetes and hypertension, both of which are synergistic factors leading to ESRD.70
Although BS may improve long-term kidney outcomes, there is no universal agreement regarding the benefit and timing of BS in patients with ESRD. The potential adverse events described for this particular surgery must also be carefully evaluated in the short term and mid-term. Acute kidney injury, nephrolithiasis, and oxalate nephropathy71,72 in addition to the rapid weight loss after BS and overall frailty of patients undergoing dialysis could trigger adverse outcomes.
Although the performance of BS with the aim of increasing transplant eligibility by reducing the BMI in kidney transplant candidates is an interesting option, the effect of surgical weight loss on post-transplantation outcomes remains unknown.73 The literature also shows no evidence for a universally accepted BMI cut-off to suggest a bariatric procedure, although for kidney transplant candidates, a BMI ranging from 35 to 45 kg/m2 could be considered the threshold at which to implement weight-loss strategies such as BS.
Which bariatric procedure?
Having discussed the potential beneficial impact of BS on the management of candidates for renal transplantation, the next question to address deals with the choice of the most effective bariatric procedure. BS can be classified into three categories:
Malabsorptive procedures: These procedures create an artificial anatomical change that bypasses a portion of the small intestine with the effect of reducing the amount of nutrients and calories absorbed by the individual. Biliopancreatic diversion with or without a duodenal pouch is representative of malabsorptive procedures.
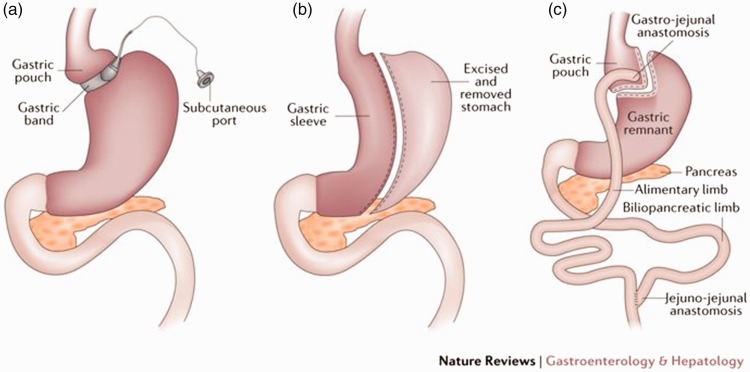
Restrictive procedures: The aim of these procedures is to reduce the amount of ingested food by reversible or irreversible, fixed or adjustable manipulation of the size of the stomach, providing less room for food and creating a rapid sense of fullness in the patients. The main restrictive procedures are placement of an adjustable laparoscopic gastric band (Figure 4(a)), performance of laparoscopic sleeve gastrectomy (LSG) (Figure 4(b)),74 and placement of an intragastric balloon.
Mixed procedures: These procedures include both restrictive and malabsorptive techniques (usually stomach size reduction and small intestinal bypass, respectively).75 A representative mixed procedure is Roux-en-Y gastric bypass (RYGB) (Figure 4(c)).
Figure 4.
Restrictive procedures. (a) Laparoscopic gastric banding. (b) Laparoscopic sleeve gastrectomy. (c) Laparoscopic Roux-en-y gastric bypass.
All three approaches have advantages and disadvantages, and it is beyond the objective of this review to develop a thorough analysis of all of them. From our viewpoint, it is enough to emphasise that a pure malabsorptive procedure is associated with important pharmacokinetic implications because the integrity of the enteric tract is important for both nutrient and drug absorption. This last point is of particular relevance because we are discussing pre-transplant BS and our hypothetical patient will require a life-long immunosuppression. For this reason, a mere malabsorptive surgery is hardly considered in the pre-transplant workup of obese patients.76 However, controversial results have also been reported in some restrictive procedures, such as laparoscopic gastric banding;77 this is probably due to the higher likelihood of gastric band erosion and dislodgment in immunosuppressed patients.78
Although diverse bariatric approaches have been reported in the management of transplant patients,79 the two most common are LSG and RYGB.
Thomas et al.80 recently published a single-centre retrospective analysis on the outcome of RYGB in 33 patients with ESRD before kidney transplantation with a mean pre-BS BMI of 43.5 ± 0.7 kg/m2. The authors found that 87% of the patients treated with RYGB were able to reach a BMI of <35 kg/m2 with a perioperative mortality rate of 0% and metabolic improvement in terms of diabetes and hypertension. These achievements allowed the patients to be eligible for renal transplantation. However, the post-transplant outcomes revealed that patients who had previously undergone RYGB had a higher incidence of biopsy-proven acute rejection than patients in the control group, and this is consistent with the fact that these patients had lower calcineurin inhibitor trough levels.80 The explanation, already mentioned in previous paragraphs, probably involves the mechanism of RYGB: in reducing the absorptive capacity of the intestinal tract, RYGB also negatively impacts the bioavailability of immunosuppressive drugs.81
The bottom line is that although RYGB has been proven safe and effective for reaching the BMI eligibility standard in patients with ESRD before transplantation, these patients require strict post-transplant monitoring and probably higher tolerable doses of immunosuppression to overcome reduced drug absorption and risks of biopsy-proven acute rejection.
The issue related to pharmacokinetics is not present in the other major type of BS for renal transplant candidates, namely LSG, because this is a restrictive procedure mainly affecting the size of the stomach. In 2018, Kim et al.82 published a single-centre retrospective analysis in which they compared the pre- and post-renal transplant outcomes of LSG in a cohort of 20 patients with ESRD versus a control group with a similar BMI who did not undergo LSG. The mean BMI of the treated patients before BS was 41.5 ± 4.4 kg/m2, which decreased to 32.3 ± 2.9 kg/m2 before kidney transplantation and was maintained thereafter; the incidence of 30-day readmission, complications, and mortality after LSG was 0%. Besides weight loss, some other beneficial effects of BS are also evident on comorbidities, particularly blood pressure. During the post-transplant follow-up, patients who underwent LSG had lower rates of NODAT, DGF, and other complications frequently encountered in obese patients who have undergone transplantation.82 Additionally, the overall postoperative course of these patients was not significantly different from that of patients in the control group.82 Therefore, LSG is recommended as a feasible and first-choice procedure in transplant candidates with a high BMI.
Conclusion
BS is a promising weight-loss strategy in obese patients with ESRD who are otherwise ineligible for kidney transplantation, and its beneficial effects also extend to the post-transplantation period.
LSG appears to provide some advantages over RYGB by avoiding the pharmacological issues related to malabsorptive procedures. There is no universal consensus about the appropriateness of timing or the indications for BS. The specific treatment of each obese patient with CKD is ultimately based on the centre’s policy and expertise.
Abbreviations
- BMI
body mass index
- BS
bariatric surgery
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DGF
delayed graft function
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- LSG
laparoscopic sleeve gastrectomy
- NODAT
new-onset diabetes after transplantation
- RYGB
Roux-en-Y gastric bypass
Author contributions
Maria Irene Bellini conceived the study, wrote the article, and searched the literature; Filippo Paoletti wrote the article and searched the literature; and Paul Herbert reviewed the article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics
This is a review of previously published articles. No human or animals were involved; therefore, no ethical approval was needed.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Khwaja A, El-Nahas M. Transplantation in the obese: separating myth from reality. Nephrol Dial Transplant 2012; 27: 3732–3735. [DOI] [PubMed] [Google Scholar]
- 2.Gill JS, Lan J, Dong J, et al. The survival benefit of kidney transplantation in obese patients. Am J Transplant 2013; 13: 2083–2090. [DOI] [PubMed] [Google Scholar]
- 3.https://www.nice.org.uk/ “Renal replacement therapy and conservative management” accessed 9 November 2018.
- 4.Bellini M, Ayathamattam J, Herbert P. The conundrum of high body mass index in kidney transplant patients. OBM Transplantation 2018; 2. DOI: 10.21926/obm.transplant.1804026. [Google Scholar]
- 5.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Vanholder R, Massy ZA, et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers 2017; 3: 17088. [DOI] [PubMed] [Google Scholar]
- 8.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization: The world health report 2002. - Reducing Risks, Promoting Healthy Life. [DOI] [PubMed]
- 10.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013; 84: 1096–1107. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G, Sehgal AR, Kashyap SR, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2011; 6: 2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen I, Heymsfield SB, Allison DB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr 2002; 75: 683–688. [DOI] [PubMed] [Google Scholar]
- 14.Kramer H, Luke A. Obesity and kidney disease: a big dilemma. Curr Opin Nephrol Hypertens 2007; 16: 237–241. [DOI] [PubMed] [Google Scholar]
- 15.Friedman AN, Miskulin DC, Rosenberg IH, et al. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis 2003; 41: 480–487. [DOI] [PubMed] [Google Scholar]
- 16.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camara NO, Iseki K, Kramer H, et al. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 2017; 13: 181–190. [DOI] [PubMed] [Google Scholar]
- 18.Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 19.Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med 2017; 11: 340–348. [DOI] [PubMed] [Google Scholar]
- 20.Garofalo C, Borrelli S, Minutolo R, et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int 2017; 91: 1224–1235. [DOI] [PubMed] [Google Scholar]
- 21.Lu JL, Molnar MZ, Naseer A, et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol 2015; 3: 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munkhaugen J, Lydersen S, Wideroe TE, et al. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis 2009; 54: 638–646. [DOI] [PubMed] [Google Scholar]
- 23.Iseki K. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 2004; 65: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28. [DOI] [PubMed] [Google Scholar]
- 25.Mallamaci F, Ruggenenti P, Perna A, et al. ACE inhibition is renoprotective among obese patients with proteinuria. Am Soc Nephrol 2011; 22: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaya B, Moneta MV, Pez O, et al. Country-level and individual correlates of overweight and obesity among primary school children: a cross-sectional study in seven European countries. BMC Public Health 2015; 15: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cattaneo A, Monasta L, Stamatakis E, et al. Overweight and obesity in infants and pre-school children in the European Union: a review of existing data. Obes Rev 2010; 11: 389–398. [DOI] [PubMed] [Google Scholar]
- 28.Postorino M, Mancini E, D'Arrigo G, et al. Body mass index trend in haemodialysis patients: the shift of nutritional disorders in two Italian regions. Nephrol Dial Transplant 2016; 31: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003; 63: 793–808. [DOI] [PubMed] [Google Scholar]
- 30.Yu E, Ley SH, Manson JE, et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med 2017; 166: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen KL, Young B, Kaysen GA, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 2004; 80: 324–332. [DOI] [PubMed] [Google Scholar]
- 32.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner JE. Is immunosenescence influenced by our lifetime “dose” of exercise? Biogerontology 2016; 17: 581–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson BM, Dutton M, Day E, et al. Frailty intervention trial iN end-stage patientS on haemodialysis (FITNESS): study protocol for a randomised controlled trial. Trials 2018; 19: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KY, Cho JH, Jung HY, et al. Effect of changes in body mass index on cardiovascular outcomes in kidney transplant recipients. Transplant Proc 2017; 49: 1038–1042. [DOI] [PubMed] [Google Scholar]
- 36.Abramowicz D, Cochat P, Claas FH, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015; 30: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 37.Dudley C, Harden P. Renal Association Clinical Practice Guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract 2011; 118: c209–c224. [DOI] [PubMed] [Google Scholar]
- 38.Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998; 97: 2099–2100. [DOI] [PubMed] [Google Scholar]
- 39.Young JB, Neumayer HH, Gordon RD. Pretransplant cardiovascular evaluation and posttransplant cardiovascular risk. Kidney Int Suppl 2010; 118: S1–S7. [DOI] [PubMed] [Google Scholar]
- 40.Piotti G, Gandolfini I, Palmisano A, et al. Metabolic risk profile in kidney transplant candidates and recipients. Nephrol Dial Transplant 2019; 34: 388–400. [DOI] [PubMed] [Google Scholar]
- 41.Lassalle M, Fezeu LK, Couchoud C, et al. Obesity and access to kidney transplantation in patients starting dialysis: a prospective cohort study. PloS One 2017; 12: e0176616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segev DL, Simpkins CE, Thompson RE, et al. Obesity impacts access to kidney transplantation. Am Soc Nephrol 2008; 19: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart A, Salkowski N, Snyder JJ, et al. Beyond “Median Waiting Time”: development and validation of a competing risk model to predict outcomes on the kidney transplant waiting list. Transplantation 2016; 100: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrol Dial Transplant 2005; 20: 167–175. [DOI] [PubMed] [Google Scholar]
- 45.Huang E, Shye M, Elashoff D, et al. Incidence of conversion to active waitlist status among temporarily inactive obese renal transplant candidates. Transplantation 2014; 98: 177–186. [DOI] [PubMed] [Google Scholar]
- 46.Toapanta-Gaibor NG, Suner-Poblet M, Cintra-Cabrera M, et al. Reasons for noninclusion on the kidney transplant waiting list: analysis in a set of hemodialysis centers. Transplant Proc 2018; 50: 553–554. [DOI] [PubMed] [Google Scholar]
- 47.Chan G, Soucisse M. Survey of Canadian kidney transplant specialists on the management of morbid obesity and the transplant waiting list. Can J Kidney Health Dis 2016; 3: 2054358116675344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoletto BB, Fonseca NKO, Manfro RC, et al. Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation 2014; 98: 167–176. [DOI] [PubMed] [Google Scholar]
- 49.Durrbach A, Pestana JM, Florman S, et al. Long-term outcomes in belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant 2016; 16: 3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar D, LeCorchick S, Gupta G. Belatacept as an alternative to calcineurin inhibitors in patients with solid organ transplants. Front Med (Lausanne) 2017; 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafranca JA, IJermans JN, Betjes MG, et al. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Medicine 2015; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanthawar P, Mei X, Daily MF, et al. Kidney transplant outcomes in the super obese: a national study from the UNOS dataset. World J Surg 2016; 40: 2808–2815. [DOI] [PubMed] [Google Scholar]
- 53.Naik AS, Sakhuja A, Cibrik DM, et al. The impact of obesity on allograft failure after kidney transplantation: a competing risks analysis. Transplantation 2016; 100: 1963–1969. [DOI] [PubMed] [Google Scholar]
- 54.Bellini MI, Koutroutsos K, Galliford J, et al. One-year outcomes of a cohort of renal transplant patients related to BMI in a steroid-sparing regimen. Transplant Direct 2017; 3: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev 2016; 37: 37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Roca R, Garcia-Aroz S, Tzvetanov I, et al. Single center experience with robotic kidney transplantation for recipients with BMI of 40 kg/m2 or greater: a comparison with the UNOS registry. Transplantation 2017; 101: 191–196. [DOI] [PubMed] [Google Scholar]
- 57.Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis 2013; 9: 653–658. [DOI] [PubMed] [Google Scholar]
- 58.Friedman AN, Wahed AS, Wang J, et al. Effect of bariatric surgery on CKD risk. Am Soc Nephrol 2018; 29: 1289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potluri K, Hou S. Obesity in kidney transplant recipients and candidates. Am J Kidney Dis 2010; 56: 143–156. [DOI] [PubMed] [Google Scholar]
- 60.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation 2002; 73: 70–74. [DOI] [PubMed] [Google Scholar]
- 61.Dombrowski SU, Knittle K, Avenell A, et al. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014; 348: g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005; 29: 1168–1174. [DOI] [PubMed] [Google Scholar]
- 63.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–752. [DOI] [PubMed] [Google Scholar]
- 64.Chadban S, Chan M, Fry K, et al. The CARI guidelines. Nutritional management of overweight and obesity in adult kidney transplant recipients. Nephrology (Carlton) 2010; 15: S52–S55. [DOI] [PubMed] [Google Scholar]
- 65.Martin MJ, Bennett S. Pretransplant bariatric surgery: a new indication? Surg Obes Relat Dis 2007; 3: 648–651. [DOI] [PubMed] [Google Scholar]
- 66.Modanlou KA, Muthyala U, Xiao H, et al. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation. 2009; 87: 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Bahri S, Fakhry TK, Gonzalvo JP, et al. Bariatric surgery as a bridge to renal transplantation in patients with end-stage renal disease. Obes Surg 2017; 27: 2951–2955. [DOI] [PubMed] [Google Scholar]
- 68.Fried M, Hainer V, Basdevant A, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts 2008; 1: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Detwiler RK. Con: weight loss prior to transplant: no. Nephrol Dial Transplant 2015; 30: 1805–1809. [DOI] [PubMed] [Google Scholar]
- 70.Favre G, Schiavo L, Lemoine S, et al. Longitudinal assessment of renal function in native kidney after bariatric surgery. Surg Obes Relat Dis 2018; 4: 1411–1418. [DOI] [PubMed] [Google Scholar]
- 71.Troxell ML, Houghton DC, Hawkey M, et al. Enteric oxalate nephropathy in the renal allograft: an underrecognized complication of bariatric surgery. Am J Transplant 2013; 13: 501–509. [DOI] [PubMed] [Google Scholar]
- 72.Chang AR, Grams ME, Navaneethan SD. Bariatric surgery and kidney-related outcomes. Kidney Int Rep 2017; 2: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman CM, Woodle ES, Shi J, et al. Addressing morbid obesity as a barrier to renal transplantation with laparoscopic sleeve gastrectomy. Am J Transplant 2015; 15: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 74.Naik RD, Choksi YA, Vaezi MF. Consequences of bariatric surgery on oesophageal function in health and disease. Nat Rev Gastroenterol Hepatol 2015; 13: 111. [DOI] [PubMed] [Google Scholar]
- 75.Cerci M, Bellini MI, Russo F, et al. Bariatric surgery in moderately obese patients: a prospective study. Gastroenterol Res Pract 2013; 2013: 276183. DOI: 10.1155/2013/276183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers CC, Alloway RR, Alexander JW, et al. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant 2008; 22: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koshy AN, Coombes JS, Wilkinson S, et al. Laparoscopic gastric banding surgery performed in obese dialysis patients prior to kidney transplantation. Am J Kidney Dis 2008; 52: e15–e17. [DOI] [PubMed] [Google Scholar]
- 78.Buch KE, El-Sabrout R, Butt KM. Complications of laparoscopic gastric banding in renal transplant recipients: a case study. Transplant Proc 2006; 38: 3109–3111. [DOI] [PubMed] [Google Scholar]
- 79.Newcombe V, Blanch A, Slater GH, et al. Laparoscopic adjustable gastric banding prior to renal transplantation. Obes Surg 2005; 15: 567–570. [DOI] [PubMed] [Google Scholar]
- 80.Thomas IA, Gaynor JJ, Joseph T, et al. Roux-en-Y gastric bypass is an effective bridge to kidney transplantation: results from a single center. Clin Transplant 2018; 32: e13232. [DOI] [PubMed] [Google Scholar]
- 81.Tsunashima D, Kawamura A, Murakami M, et al. Assessment of tacrolimus absorption from the human intestinal tract: open-label, randomized, 4-way crossover study. Clin Ther 2014; 36: 748–759. [DOI] [PubMed] [Google Scholar]
- 82.Kim Y, Jung AD, Dhar VK, et al. Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients. Am J Transplant 2018; 18: 410–416. [DOI] [PubMed] [Google Scholar]