Short abstract
Objectives
Carotid endarterectomy (CEA) is efficient in preventing stroke for patients with significant carotid stenosis, but results in mild cognitive dysfunction. Dexmedetomidine is neuroprotective in stroke models. We hypothesized that dexmedetomidine may improve cognition after CEA.
Methods
Forty-nine patients scheduled for elective CEA were randomly assigned to intravenous dexmedetomidine treatment group (n = 25) and control group C (normal saline, n = 24). Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MOCA), as well as lactate, TNF-α, IL-6, and BDNF levels in blood, were assessed before, during, and after surgery.
Results
MMSE and MOCA scores showed subtle decline in both groups at 24 hours postoperatively; this decline remained at 48 hours postoperatively in group C. Both scores were higher in group D than in group C at 48 and 72 hours postoperatively. TNF-α and IL-6 were lower from 5 minutes post-clamping through 24 hours postoperatively in group D; lactate was lower at 5 minutes post-clamping in group D. BDNF was higher from 5 minutes post-clamping through 1 hour postoperatively in both groups, and remained high in group D at 24 hours postoperatively.
Conclusions
Dexmedetomidine improved recovery of cognition after CEA, potentially due to reduced inflammation and enhanced BDNF expression.
Keywords: Carotid endarterectomy, dexmedetomidine, cognitive dysfunction, brain-derived neurotrophic factor, Mini-Mental State Examination, Montreal Cognitive Assessment, stroke, tumor necrosis factor, lactate, interleukin 6
Introduction
Carotid endarterectomy (CEA) is a classical method for prevention of life-threatening strokes in patients with severe internal carotid artery stenosis (ICA), regardless of symptoms.1,2 However, there is increasing evidence that subtle cognitive dysfunction may occur in 27%–31% of patients after CEA; this may be caused by cerebral perfusion deficiency related to hemodynamic changes and cerebral emboli, as well as a variety of individual factors.3–5
Brain-derived neurotrophic factor (BDNF) is a well-known member of the neurotrophic family of growth-promoting proteins, which is essential to neuronal survival, axon growth, and synaptic plasticity.6 Elevation of BDNF in the central nervous system is known to prevent hypoxic neuronal death in animal models of stroke,7 and has been implicated in learning and memory processes,8 as well as other advanced neuronal functions. Moreover, BDNF can be detected in blood, and a direct correlation has been observed between cortical and serum levels of BDNF.9–11 Thus, the serum BDNF level may serve as an indicator of changes in BDNF levels in the central nervous system.
Dexmedetomidine (DEX) is a highly selective agonist of α2-adrenergic receptors with multiple effects on the human brain, including clinical sedation, anesthesia, and analgesia.12 Moreover, DEX has drawn widespread attention for its neuroprotective effects against stroke in animal models through its actions on α2-adrenergic receptors13–16; this neuroprotection is partly related to the DEX-induced elevation of BDNF levels in the cortex and hippocampus,17,18 as well as concurrent anti-inflammatory effects.19 However, for patients who have undergone CEA, the impacts of DEX on cognition and plasma BDNF level have not yet been determined.
Based on the above considerations, we hypothesized that DEX may improve cognition in aged patients after CEA. Here, we aimed to determine the effects of DEX administered intravenously during CEA on postoperative cognitive function, and to assess cerebral inflammation and plasma levels of BDNF, in order to elucidate whether DEX can prevent cognitive dysfunction in patients undergoing CEA.
Methods
The study protocol was approved by the institutional review board of Subei People’s Hospital (No. 2011032). Informed consent was obtained from patients undergoing elective CEA during the period from January 2013 to December 2014 in Subei People’s Hospital for severe carotid disease. In accordance with the recommendations of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the Asymptomatic Carotid Atherosclerosis Study, the inclusion criteria1 were as follows: male or female patients aged 65–80 years, with symptomatic ICA > 50% or asymptomatic ICA > 70%, as measured by ultrasound (non-invasive equivalent of the NASCET criteria). In addition, patients were required to understand and be willing to participate in the research.
Exclusion criteria were as follows: refusal of general anesthesia or enrollment in the study; history of mental disease and use of psychiatric drugs, or history of alcoholism and/or drug abuse; history of recent stroke (within 4 weeks) and modified Rankin Scale of > 1 before CEA. Reasons for abandonment of the protocol included the use of additional anesthetic drugs other than those indicated in the protocol; use of a shunt; severe hypotension and hemorrhage; administration of a second anesthetic within the first 24 h; and/or the inability to understand the contents of the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MOCA).
All patients were randomly assigned into two groups: group C (control group) and group D (treated with DEX). In the evening before surgery, all patients took 25 mg of clorazepate orally; they did not take pre-medication orally on the day of surgery. Anesthesia was induced with intravenous doses of midazolam 0.05–0.1 mg/kg, fentanyl 2 µg/kg, etomidate 0.2–0.3 mg/kg, and rocuronium bromide 1.0 mg/kg; armored endotracheal intubation was then performed. Mechanical ventilation settings were tidal volume of 8 ml/kg, FiO2 of 0.50, and breathing frequency of 10–14 breaths/min, such that normal end-tidal CO2 was maintained. Anesthesia was maintained with intravenous infusion of remifentanil 0.1–0.3 ug·kg−1·min−1 and propofol 2–6 mg·kg−1·h−1; these were adjusted on the basis of bispectral index. Rocuronium bromide 0.1 mg/kg was intermittently administered to maintain muscle relaxation. In group D, additional infusions of DEX (0.3 µg/kg loading dose, 10 minutes before induction of anesthesia; 0.3 µg·kg−1·h−1 maintenance dose) were administered until 30 minutes before the end of surgery. Patients in the control group received equal volumes of normal saline. During surgery, mean arterial blood pressure (MAP) was maintained at 80%–120% of the baseline value; however, during clamping of the carotid artery, the MAP value was maintained at 125% of the baseline value. Maintenance of MAP was achieved by infusion of dopamine 2–8 µg·kg−1·min−1, and nitroglycerine 0.1–8.0 µg·kg−1·min−1. After unclamping of the carotid artery, the surgical field was infiltrated with ropivacaine 10 ml (5 mg/ml) for analgesia. All patients received tropisetron 5 mg intraoperatively. The anesthetists were not blinded to the study protocol; however, surgeons and post-anesthetic care unit staff were blinded.
Five-lead electrocardiography, pulse oximetry (Philips IntelliVue MP50, Philips Medicine Systems GmbH, Hamburg, Germany), end-tidal CO2 concentration, and bispectral index (A-2000 BIS monitor, version XP, Aspect Medical Systems, Newton, MA, USA) were used for intraoperative monitoring. The MAP and heart rate (HR) were recorded at 20 minutes before anesthesia; immediately after tracheal intubation; 5 and 15 minutes after clamping of the carotid artery; and 5 minutes after unclamping of the carotid artery. When the carotid artery stump pressure was ≤50 mmHg after clamping, a pipe was placed for shunting. A peripheral venous catheter and a radial artery catheter were inserted, followed by an arterial line for continuous monitoring of arterial blood pressure. An internal jugular vein catheter was then inserted in a retrograde manner into the jugular bulb to collect blood samples, at a location ipsilateral to the operated carotid. Blood samples were drawn from the jugular bulb at 20 minutes before anesthesia (T0), 10 minutes after tracheal intubation (T1), 5 minutes after clamping of the carotid artery (T2), 15 minutes after unclamping of the carotid artery (T3), 1 hour postoperatively (T4), and 24 hours (T5) postoperatively. Lactate blood gas analysis was performed at time points T0 through T3, using 1 mL of the blood samples. A portion of the samples for serum isolation were allowed to clot for 30 minutes at room temperature and then centrifuged at 10000 ×g for 15 minutes at 4°C; the resulting supernatant was stored at −80°C for further analysis. Serum BDNF, tumor necrosis factor alpha (TNF-α), and interleukin (IL)-6 at all time points were measured using enzyme-linked immunosorbent assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), in accordance with the manufacturer’s instructions.
Cognitive assessment was performed using the MMSE and MOCA. The MMSE primarily measures cognitive function in the dominant cerebral hemisphere20,21; its scores range from 0 to 30, and a score of ≤23 is considered indicative of cognitive impairment. The MOCA was developed to rapidly screen for mild cognitive impairment, and is expected to overcome the limitations of the MMSE22; its scores range from 0 to 30 and are assigned for seven subsets: visuospatial/executive; naming; memory; attention; language, abstraction, and orientation. A MOCA score ≤25 is considered indicative of cognitive impairment. Neuropsychologic evaluations were performed by one trained clinical neuropsychologist who was blinded to the protocol; evaluations were performed at 1 day (t0) preoperatively, as well as at 24 hours (t1), 48 hours (t2), 3 days (t3), 7 days (t4), and 1 month (t5) postoperatively.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) 19.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The data are presented as mean and standard deviation for quantitative variables; categorical variables are shown as counts and percentages. To assess differences between groups, comparisons of normally distributed variables were performed by using one-way analysis of variance (ANOVA); comparisons of non-normally distributed variables were performed by using the Kruskal–Wallis test. Within each group, comparisons of normally distributed variables were performed by using repeated-measures ANOVA; comparisons of non-normally distributed variables were performed by using the Friedman test or the Wilcoxon signed-rank test. The chi-squared test was used to assess statistical differences in the ratio between the two groups. Differences with p < 0.05 were considered to be statistically significant.
Results
Fifty-six patients who underwent CEA were enrolled in the study (n = 28 per group). Technical success was observed in 49 patients (24 in group C and 25 in group D). In group C, two patients were excluded for excessive bleeding and two patients were excluded because of shunt use. In group D, two patients were excluded because of shunt use and one patient died of myocardial infarction. Table 1 shows the demographic and clinical data of the patients in this study. There were no significant differences between groups in terms of age, sex, American Society of Anesthesiologists’ grade, years of education, comorbid disease, medications, intraoperative adverse events (hypotension, hypoxia, or blood transfusion), the incidences of postoperative delirium and agitation, anesthesia duration, clamping duration, endarterectomy site, or the duration of postoperative hospitalization. However, during the surgery, patients who required vasoconstrictors (phenylephrine and ephedrine) and atropine were comparable in the two groups. The usage of nitroglycerine was slightly higher in group C than in group D, while the dosage of propofol was slightly lower in group D than in group C (p < 0.05).
Table 1.
Patient demographics and clinical data.
| Variable | Group C (n = 24) | Group D (n = 25) |
|---|---|---|
| Age (years) | 72 ± 5 | 70 ± 3 |
| Sex (M/F) | 15/9 | 17/8 |
| ASA grade (II/III) | 16/8 | 15/10 |
| Years of education | 9 ± 3 | 10 ± 3 |
| Preoperative MMSE score | 27.0 ± 0.8 | 27.5 ± 0.7 |
| Lateral carotid stenosis ≥70%, n (%) | 3 (12.5%) | 5 (20.0%) |
| Previous CVA or CEA, n (%) | 6 (25.0%) | 3 (12.0%) |
| Peripheral artery disease, n (%) | 3 (12.5%) | 3 (12.0%) |
| Coronary artery disease, n (%) | 8 (33.3%) | 10 (40.0%) |
| Hypertension, n (%) | 20 (83.3%) | 22 (88.0%) |
| Diabetes mellitus, n (%) | 6 (25.0%) | 9 (36.0%) |
| Hyperlipidemia, n (%) | 15 (62.5%) | 18 (72.0%) |
| Smoker, n (%) | 14 (58.3%) | 12 (48.0%) |
| Antiplatelet drugs, n (%) | 15 (62.5%) | 18 (72.0%) |
| Antihypertensive therapy, n (%) | 10 (41.7%) | 17 (68.0%) |
| Hypotension/hypoxia, n (%) | 0 (0%) | 0 (0%) |
| Blood transfusion, n (%) | 0 (0%) | 0 (0%) |
| Usage of vasoconstrictors, n (%) | 9 (37.5%) | 14 (56.0%) |
| Usage of nitroglycerine, n (%) | 13 (52.0%) | 5 (20.0%)* |
| Usage of atropine, n (%) | 3 (12.5%) | 8 (32.0%) |
| Anesthesia duration (minutes) | 123 ± 9 | 119 ± 4 |
| Clamp duration (minutes) | 31 ± 5 | 29 ± 4 |
| Endarterectomy site (left/right) | 14/10 | 12/13 |
| Dosage of propofol (mg) | 585 ± 23 | 542 ± 26* |
| Delirium, n (%) | 2 (8.3%) | 1 (4%) |
| Agitation, n (%) | 2 (8.3%) | 3 (12%) |
| Postoperative hospitalization (days) | 4.6 ± 1.1 | 4.8 ± 1.3 |
*p < 0.05, significant intergroup differences.
CVA, cerebrovascular accident; CEA, carotid endarterectomy; MMSE, Mini-Mental State Examination.
MAP and HR in the two groups are shown in Table 2. Consistent with the requirements for blood pressure regulation in CEA, MAP was approximately 120%–125% of baseline in both groups during carotid clamping (p < 0.05 for both). Compared with group C, MAP and HR were both more stable after tracheal intubation in group D. HR decreased significantly in group D during the carotid clamping period (p < 0.05). However, HR remained in the normal range (>60 beats/min) during most of the surgery.
Table 2.
Hemodynamic data of patients in dexmedetomidine-treated and control groups.
| Variable | Groups |
Time points |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| MAP (mmHg) | Group D | 102 ± 10 | 104 ± 12 | 119 ± 12* | 121 ± 9* | 103 ± 8 |
| Group C | 104 ± 9 | 114 ± 13# | 120 ± 10# | 120 ± 12# | 102 ± 10 | |
| HR (beats/minute) | Group D | 78 ± 6 | 80 ± 9 | 70 ± 7* | 68 ± 8* | 73 ± 8 |
| Group C | 75 ± 8 | 89 ± 8# | 77 ± 9 | 78 ± 7 | 78 ± 8 | |
HR, heart rate; MAP, mean arterial blood pressure. The data were recorded at 20 minutes before anesthesia (0), immediately after tracheal intubation (1), 5 and 15 minutes after clamping of the carotid artery (2–3), and 5 minutes after unclamping of the carotid artery (4). Data are mean ± standard deviation. *p < 0.05, significant intragroup differences from baseline in group D; #p < 0.05, significant intragroup differences from baseline in group C.
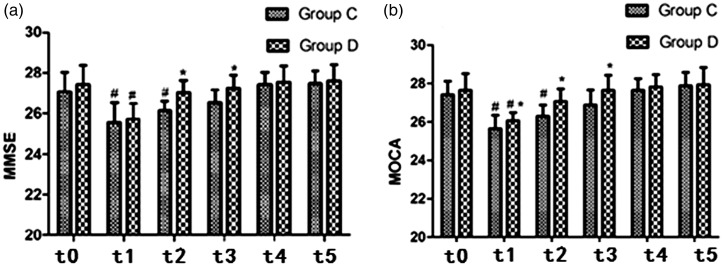
MMSE and MOCA scores in the two groups are shown in Figure 1a and 1b. Compared with baseline, a subtle decline in both scores was observed at t1 and t2 in group C (p < 0.05 for both); there was a mild decrease in group D at t1 (p < 0.05). Both scores were higher in group D than in group C at t2 and t3 (p < 0.05); MOCA scores were slightly higher in group D than in group C at t1 (p < 0.05). The proportions of patients showed reductions in their initial MMSE and MOCA scores were also significantly higher in group C within the first 72 hours postoperatively (t1–3).
Figure 1.
Mini-Mental State Examination (a) and Montreal Cognitive Assessment (b) scores in the two groups. One day before the surgical procedure (t0), 6 hours postoperatively (t1), 24 hours postoperatively (t2), 72 hours postoperatively (t3), 7 days postoperatively (t4), and 1 month postoperatively (t5). *p < 0.05, significant intergroup differences; #p < 0.05, significant intragroup differences from baseline in both groups. MMSE, Mini-Mental State Examination; MOCA, Montreal Cognitive Assessment.
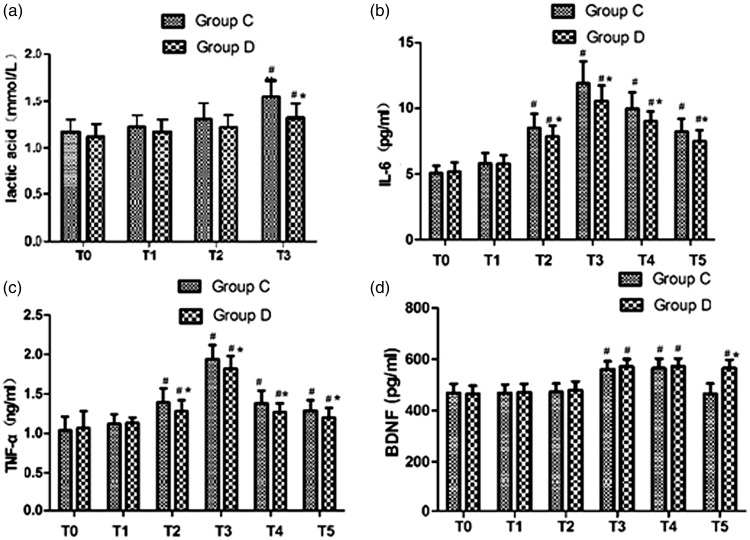
Compared with that in group C, the level of blood lactate was significantly lower in group D at T3 (p < 0.05), and was slightly higher at T3 than at baseline in both groups (Figure 2a). Serum TNF-α and IL-6 levels were lower in group D than in group C at T2 through T5 (p < 0.05 for all). (Figure 2b and 2c). Plasma levels of BDNF were comparable between groups: relative to baseline, they increased at T3 and T4 in both groups (p < 0.05 for both), and continued to remain high in group D at T5 (p < 0.05). However, the value of BDNF returned to baseline in group C at T5 (Figure 2d).
Figure 2.
Lactic acid (a), IL-6 (b), TNF-α (c), and BDNF (d) levels in blood from the jugular bulb in the two groups. Twenty minutes before anesthesia (T0), 10 minutes after tracheal intubation (T1), 15 minutes after clamping of the carotid artery (T2), 15 minutes after unclamping of the carotid artery (T3), 1 hour postoperatively (T4), and 24 hours postoperatively (T5). *p < 0.05, significant intergroup differences; #p < 0.05, significant intragroup differences from baseline in both groups.
Discussion
In the present study, the DEX-treated group (group D) exhibited a number of differences from the control group (group C), which suggested that DEX may be beneficial for the recovery of cognition in patients who have undergone CEA. In particular, group D demonstrated superior MMSE and MOCA scores, as well as measurements of cerebral inflammation (TNF-α, IL-6, and lactate content in jugular venous blood). Moreover, the infusion of DEX was associated with increased plasma BDNF levels.
For most aged patients undergoing CEA, hemodynamic fluctuation occurs frequently, and most such patients exhibit comorbid cerebrovascular disease.23 Thus, it is very important for these patients to maintain relatively stable circulation to avoid severe hypotension during carotid artery clamping. Hemodynamic assessment showed that MAP remained above baseline in both groups during carotid clamping; this may have been related to vasoconstrictor function, and could effectively increase cerebral blood flow.24 Moreover, MAP was more stable after tracheal intubation in group D than in group C; fewer patients were given nitroglycerine intraoperatively in group D, which further supported the ability of DEX to maintain stable circulation. Previous studies showed that improper application of DEX could result in severe hypertension or hypotension and bradycardia.25 However, in the present study, patients who required atropine for bradycardia, as well as the vasoconstrictor intervention, were comparable between the two groups. These findings support the safety of the intravenous administration of DEX in the dosage used in this study.
Many studies have focused on the neurocognitive course in patients undergoing CEA, but the results have been controversial, in that they showed either postoperative improvements or no functional changes; in some cases, patients have exhibited functional decline secondary to CEA.26–28 Notably, one study demonstrated that approximately 27%–31% of patients had subtle cognitive dysfunction after CEA.4 Similarly, in the present study, approximately 33% of patients showed reductions in MMSE and MOCA scores at 6 and 24 hours after surgery, compared with baseline. These functional deteriorations may be due to residual anesthetic effects, cerebral microemboli during and after surgery, oxidative stress, or prolonged global cerebral ischemia during carotid cross-clamping.29,30
The MMSE and MOCA are the most commonly used tests to assess mental abilities. Notably, the specificity of the MMSE is relatively high for detection of the effects of new ischemic events.31 The MOCA was developed as a tool to rapidly screen for mild cognitive impairment, in order to overcome limitations of the MMSE.32 Therefore, a combination of MMSE and MOCA was used in the present study to observe changes in the cognitive function of patients after CEA; we expected that this would increase the accuracy of the results. The trends of the two scores were similar, in that both decreased for a short period postoperatively (24 hours in group C, and 6 hours in group D). Moreover, the scores were significantly higher in group D at 24 and 72 hours postoperatively; MOCA scores were, significantly higher as early as 6 hours postoperatively, which suggested that MOCA was indeed more sensitive to changes in cognition. However, the two values did not differ between the two groups at 7 days or 1 month postoperatively. These findings indicated that infusion of DEX during CEA may be beneficial for improving the recovery of cognitive performance in the early period after CEA.
Previous studies showed that carotid occlusion during CEA could result in lactate production due to cerebral ischemia33; this finding was inconsistent with our results. Higher levels of blood lactate were present in both groups after carotid unclamp. Additionally, significantly lower lactate levels were present in group D, compared with group C. This relative reduction of lactate production may be related to the amelioration of blocked cerebral blood flow or suppression of cerebral metabolism by DEX; such findings are consistent with the neuroprotective effects of DEX, which have been demonstrated in a series of animal experiments and clinical studies.13–16,34
The improvements in cognitive recovery and lactate accumulation by DEX were accompanied by significantly lower levels of TNF-α and IL-6. There is accumulating evidence that neuroinflammation plays a key role in cognitive dysfunction.35,36 A subset of inflammatory cytokines, such as TNF-α and IL-6, may have direct effects on neuronal functions, especially with respect to learning and memory.35,36 A recent study showed that increased levels of TNF-α were present in patients with mild cognitive impairment, compared with normal controls.37 In the present study, indicators of inflammation (TNF-α and IL-6) were increased after carotid unclamping and remained high through 24 hours postoperatively. This may be an important underlying cause of cognitive decline after CEA. There is evidence that DEX exerts anti-inflammatory effects, such as reduction of the production of inflammatory cytokines in critically ill patients with severe sepsis, as well as attenuation of increased plasma cytokine levels after endotoxin injection in animal models.38 Similarly, TNF-α and IL-6 levels in group D were significantly lower than those in group C through 24 ho postoperatively. This constitutes further evidence of the anti-inflammatory effects of DEX in relation to cerebral potential injury after CEA, and may be the basis for the improved cognitive performance observed with DEX treatment.
BDNF is mainly synthesized by neurons, and there is a large amount of BDNF present in the adult brain because it its crucial role in plasticity and cerebral functions. It has been established that BDNF can cross the blood brain barrier, and that the plasma level of BDNF correlates directly with the brain level of BDNF.39 Several studies have focused on the increased circulating BDNF levels in stroke patients,40–43 which are consistent with increased brain BDNF levels in animal models of stroke. The cerebral levels of BDNF could not be directly assessed in the present study; however, the blood samples for BDNF measurement were obtained from the jugular venous bulb, where blood collects from the cerebral hemispheres. Thus, the blood samples in this study may largely reflect cerebral levels of BDNF. Cerebral BDNF has been extensively studied in animal models of ischemic stroke, which consistently showed that brain BDNF levels increased rapidly in early periods of postischemic recirculation44–46; those findings suggested that BDNF participates in the early stress response after brain injury, and that it may have a relatively important neuroprotective role in either global or focal brain damage. Similarly, in the present study, the plasma BDNF level significantly increased after carotid unclamping in both groups; moreover, it remained higher in group D, and this enhancement lasted for an extended period, compared with that observed in group C. These findings constitute further evidence of the enhancement of BDNF levels following DEX treatment. A recent study proposed that DEX promoted astrocyte expression of BDNF in cultured astrocytes.18 An additional study suggested a central role for BDNF in neuroprotection against apathy and memory loss.47 BDNF is important for cognitive function, and lowered levels of circulating BDNF have been associated with lower cognitive test scores and mild cognitive impairment.48 Therefore, the present experimental results suggest that the neuroprotective effects of DEX with respect to cognitive performance may be partly related to the enhancement of BDNF levels.
There were several limitations in this study. First, only a small number of patients were included in this study; thus, the results should be validated in a larger cohort of patients. Second, the observation time was short in this study, and should be extended to determine the long-term effects of DEX administration in patients undergoing CEA. Third, the preoperative symptom severity of the patients was not fully analyzed in this study; this may impact cognition after CEA, and may have thus biased the results. In addition, postoperative administration of analgesic and antiemetic medications were not fully standardized, which may have affected the cognitive results. Larger and more rigorous studies should be performed in the future to overcome these limitations.
In conclusion, this study preliminarily established that the neuroprotective effects of DEX with respect to cognitive performance in patients undergoing CEA may be partly related to the anti-inflammatory effects of DEX and its ability to enhance BDNF levels. However, the specific mechanisms for DEX-induced neuroprotection remain unclear; moreover, the present study did not perform a detailed analysis of the correlation among DEX, postoperative cognition, and BDNF levels, which should be implemented in a future study.
Acknowledgments
The authors thank Dr. Chunlin Wang for technical help with ELISA studies, and Professor Hualin Li of Yangzhou University for assistance with statistical analysis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the guiding programs of the Health Planning Commission of Jiangsu Province (Z 201621).
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 2.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 3.Berman L, Pietrzak RH, Mayes L. Neurocognitive changes after carotid revascularization: a review of the current literature. J Psychosom Res 2007; 63: 599–612. [DOI] [PubMed] [Google Scholar]
- 4.De Rango P, Caso V, Leys D, et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke 2008; 39: 3116–3127. [DOI] [PubMed] [Google Scholar]
- 5.Heyer EJ, Robert DLP, Quest DO, et al. Neuropsychological dysfunction in the absence of structural evidence for cerebral ischemia after uncomplicated carotid endarterectomy. Neurosurgery 2006; 58: 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sossin WS, Barker PA. Something old, something new: BDNF-induced neuron survival requires TRPC channel function. Nat Neurosci 2007; 10: 537–538. [DOI] [PubMed] [Google Scholar]
- 7.Grade S, Weng YC, Snapyan M, et al. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One 2013; 8: e55039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006; 110: 167–173. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Xu JM, Jiang X, et al. Effect of dexmedetomidine on plasma brain-derived neurotrophic factor: a double-blind, randomized and placebo-controlled study. Ups J Med Sci 2013; 118: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan W, Banks WA, Fasold MB. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998; 37: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 11.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 2002; 328: 261–264. [DOI] [PubMed] [Google Scholar]
- 12.Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine (Baltimore) 2015; 94: e1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyagi T, Nishikawa T, Tobe Y, et al. The combined neuroprotective effects of lidocaine and dexmedetomidine after transient forebrain ischemia in rats. Acta Anaesthesiol Scand 2009; 53: 1176–1183. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Kimura T, Nishikawa T, et al. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand 2010; 54: 377–382. [DOI] [PubMed] [Google Scholar]
- 15.Janke EL, Samra S. Dexmedetomidine and neuroprotection. Seminars in Anesthesia, Perioperative Medicine and Pain 2006; 25: 71–76. 10.1053/j.sane.2006.02.002 [DOI] [Google Scholar]
- 16.Eser O, Fidan H, Sahin O, et al. The influence of dexmedetomidine on ischemic rat hippocampus. Brain Res 2008; 1218: 250–256. [DOI] [PubMed] [Google Scholar]
- 17.Xu KL, Liu XQ, Yao YL, et al. Effect of dexmedetomidine on rats with convulsive status epilepticus and association with activation of cholinergic anti-inflammatory pathway. Biochem Biophys Res Commun 2018; 495: 421–426. [DOI] [PubMed] [Google Scholar]
- 18.Degos V, Charpentier TL, Chhor V, et al. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology 2013; 118: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 19.Tüfek A, Kaya S, Tokgöz O, et al. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med 2013; 36: E95–E102. [DOI] [PubMed] [Google Scholar]
- 20.Sabbagh M, Cummings J, Christensen D, et al. Evaluating the cognitive effects of donepezil 23 mg/d in moderate and severe Alzheimer's disease: Analysis of effects of baseline features on treatment response. BMC Geriatr 2013; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukonzo JK, Okwera A, Nakasujja N, et al. Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV‑positive patients with or without tuberculosis: A prospective cohort study. BMC Infect Dis 2013; 13: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisekovic S, Memic A, Pasalic A. Correction between MOCA and MMSE for the assessment of cognition in schizophrenia. Acta Inform Med 2012; 20: 186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TH, Marcantonio ER, Mageione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 24.Dudkiewicz M, Proctor KG. Tissue oxygenation during management of cerebral perfusion pressure with phenylephrine or vasopressin. Crit Care Med 2008; 36: 2641–2650. [DOI] [PubMed] [Google Scholar]
- 25.Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs 2015; 75: 1119–1130. [DOI] [PubMed] [Google Scholar]
- 26.Akiko T, Naoya K, Naoki A, et al. Effect of carotid endarterectomy on cognitive function in patients with asymptomatic carotid artery stenosis. Acta Neurochir (Wien) 2013; 155: 627–633. [DOI] [PubMed] [Google Scholar]
- 27.Paraskevas KI, Lazaridis C, Andrews CM, et al. Comparison of cognitive function after carotid artery stenting versus carotid endarterectomy. Eur J Vasc Endovasc Surg 2014; 47: 221–231. [DOI] [PubMed] [Google Scholar]
- 28.Picchetto L, Spalletta G, Casolla B, et al. Cognitive performance following carotid endarterectomy or stenting in asymptomatic patients with severe ICA stenosis. Cardiovasc Psychiatry Neurol 2013; 2013: 342571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aleksic M, Huff W, Hoppmann B, et al. Cognitive function remains unchanged after endarterectomy of unilateral internal carotid artery stenosis under local anaesthesia. Eur J Vasc Endovasc Surg 2006; 31: 616–621. [DOI] [PubMed] [Google Scholar]
- 30.Weber CF, Friedl H, Hueppe M, et al. Impact of general versus local anesthesia on early postoperative cognitive dysfunction following carotid endarterectomy: GALA Study Subgroup Analysis. World J Surg 2009; 33: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 31.Arciniegas DB, Kellermeyer GF, Bonifer NM, et al. Screening for cognitive decline following single known stroke using the Mini‑Mental State Examination. Neuropsychiatr Dis Treat 2011; 7: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Zhou M, Zhou Y, et al. Effects of carotid endarterectomy on cerebral reperfusion and cognitive function in patients with high grade carotid stenosis: a perfusion weighted magnetic resonance imaging study. Eur J Vasc Endovasc Surg 2015; 50: 5–12. [DOI] [PubMed] [Google Scholar]
- 33.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012; 32: 1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sulemanji DS, Dönmez A, Aldemir D, et al. Dexmedetomidine during coronary artery bypass grafting surgery: is it neuroprotective? A pilot study: O‐28. Eur J Anaesthesiol. 2007; 24: 9. [DOI] [PubMed] [Google Scholar]
- 35.Qiao Y, Feng H, Zhao T, et al. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol 2015; 15: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Liu B, Zhang F, et al. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med 2014; 8: 4601–4605. [PMC free article] [PubMed] [Google Scholar]
- 37.Trollor JN, Smith E, Agars E, et al. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2012; 34: 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013; 25: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, Alam A, San CY, et al. Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: recent developments. Brain Res 2017; 1665: 1–21. [DOI] [PubMed] [Google Scholar]
- 40.Di LV, Profice P, Pilato F, et al. BDNF plasma levels in acute stroke. Neurosci Lett 2007; 422: 128–130. [DOI] [PubMed] [Google Scholar]
- 41.Jiménez I, Sobrino T, Rodríguezyáñez M, et al. High serum levels of leptin are associated with post-stroke depression. Psychol Med 2009; 39: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Zhang Z, Sun D, et al. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int J Geriatr Psychiatry 2011; 26: 495–502. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Lu T, Xu G, et al. Decreased serum brain-derived neurotrophic factor (BDNF) is associated with post-stroke depression but not with BDNF gene Val66Met polymorphism. Clin Chem Lab Med 2011; 49: 185–189. [DOI] [PubMed] [Google Scholar]
- 44.Sulejczak D, Ziemlińska E, Czarkowskabauch J, et al. Focal photothrombotic lesion of the rat motor cortex increases BDNF levels in motor-sensory cortical areas not accompanied by recovery of forelimb motor skills. J Neurotrauma 2007; 24: 1362–1377. [DOI] [PubMed] [Google Scholar]
- 45.Madinier A, Bertrand N, Mossiat C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One 2009; 4: e8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Béjot Y, Prigent-Tessier A, Cachia C, et al. Time-dependent contribution of non-neuronal cells to BDNF production after ischemic stroke in rats. Neurochem Int 2011; 58: 102–111. [DOI] [PubMed] [Google Scholar]
- 47.Failla MD, Juengst SB, Arenth PM, et al. Preliminary associations between brain-derived neurotrophic factor, memory impairment, functional cognition, and depressive symptoms following severe TBI. Neurorehabil Neural Repair 2016; 30: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimada H, Makizako H, Doi T, et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front Aging Neurosci 2014; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]