Short abstract
Objective
This study was designed to evaluate the neurotoxicity of dexmedetomidine combined with ropivacaine for continuous femoral nerve block in rabbits.
Methods
Thirty New Zealand rabbits were randomly divided into 5 groups of 6 rabbits each and received a continuous femoral nerve block with saline; 0.25% ropivacaine; or 1, 2, or 3 µg/mL of dexmedetomidine added to 0.25% ropivacaine (Groups A–E, respectively). Sensory and motor function was assessed after the nerve block. The rabbits were anesthetized and killed after 48 hours of a continuous femoral nerve block, and the femoral nerves were removed for light and electron microscopy analyses.
Results
The behavior scores were highest in Group A at 2 and 6 hours after injection. The scores were higher in Groups B and C than in Groups D and E at these same time points. All groups showed normal pathological tissues in the femoral nerves under optical microscopy. Under electron microscopy, histological abnormalities were observed only in Group E; none of the other groups exhibited pathological abnormalities. Quantitative analysis of the myelin sheath area revealed no significant difference in the axonal area, total area of the myelin sheath, or ratio of the total axonal area to the total area of the myelin sheath in all groups.
Conclusion
The lowest doses of dexmedetomidine (1 and 2 µg/mL) combined with 0.25% ropivacaine for continuous femoral nerve block resulted in no neurotoxic lesions, but the higher dose (3 µg/mL) resulted in neurotoxic lesions in this rabbit experimental model.
Keywords: Dexmedetomidine, femoral nerve block, demyelination, neurotoxicity, ropivacaine, rabbit model
Background
Alpha 2 agonists such as clonidine increase the duration of local anesthetic nerve blocks and enhance analgesic intensity.1 Studies have shown that clonidine prolongs the time of analgesia in peripheral nerve block anesthesia without significantly increasing the clinical risk.2,3 Dexmedetomidine is a selective α2 adrenoceptor agonist with a selectivity ratio of 1600:1 (α2:α1) and a receptor affinity eight times greater than that of clonidine. In recent years, greater numbers of studies have utilized dexmedetomidine as an adjunct to local anesthetics for peripheral nerve block and spinal anesthesia. Results have shown that the addition of local anesthetics to dexmedetomidine prolongs the time of the nerve block for postoperative analgesia without severe bradycardia, hypotension, respiratory depression, or other complications.4,5 The addition of dexmedetomidine to levobupivacaine may prolong the nerve block time and enhance the blocking effect or reduce the onset time in peripheral nerve blocks.4,6–11 Dexmedetomidine is now recognized as an auxiliary drug for use in local anesthetics for nerve blocks because of its advantages of enhancing the effects of these local anesthetics. However, the adverse effects of local application of dexmedetomidine to peripheral nerves have caused concern in recent years. Studies have shown that the addition of large doses of dexmedetomidine to bupivacaine enhances the analgesic effect and increases the duration of sciatic nerve block in rats, with no changes in axons or myelin sheaths, at 24 hours and 14 days.12 However, very few reports to date have focused on nerve injury and postoperative nerve injury in clinical trials.13,14 Additionally, although studies have been performed to evaluate the effects and safety of a single peripheral nerve block, few have been performed to assess continuous nerve block. Therefore, the present study was designed to evaluate the neurotoxicity caused by adding dexmedetomidine to ropivacaine for continuous femoral nerve block in an experimental rabbit model.
Material and methods
This study was approved by the Ethics Committee of Guangxi Medical University (2013-KY-GUIKE-056, Nanning, China). All animals received care in compliance with the Principles of Laboratory Animal Care formulated by the National Institutes of Health and the Dutch Law on Experimental Animal Care.
Animal model preparation
The study was performed on 30 New Zealand rabbits (4–6 months-old, body weight of 2.0–2.5 kg). All rabbits were randomly assigned to 5 groups of 6 rabbits each: the control group, in which rabbits received saline alone (Group A); a group in which rabbits received 0.25% ropivacaine alone (Group B); and groups in which rabbits received 1, 2, or 3 µg/mL of dexmedetomidine added to 0.25% ropivacaine (Groups C–E, respectively).
All rabbits were fasted for 8 hours prior to experimentation but were allowed free access to drinking water. The ear vein was catheterized, and general anesthesia was performed by intravenous injection of propofol (6–8 mg/kg) maintained with continuous infusion of 40 to 50 mg·kg−1·h−1 propofol with spontaneous breathing. After anesthesia, the rabbits were fixed in the supine position, and the skin of the left groin area was cleaned. The femoral nerve trunk, 0.5 cm below the midpoint of the inguinal ligament, was isolated and exposed after disinfection of the skin of the left groin area with iodophor. An epidural catheter was placed next to the femoral nerve and fixed at the muscle. After surgery, the incision was sprayed with penicillin powder to prevent infection. The incision was sutured and propofol administration was terminated. The catheter was connected to the analgesic pump for use, and 1 mL of 2% lidocaine was injected through the catheter after the rabbits awoke. The catheter position and the block effect were evaluated by observing lower limb reactions when the knee joint was pricked with an acupuncture needle.
The drugs used in the study were prepared by mixing 0.75% ropivacaine (AstraZeneca AB, Södertälje, Sweden) with normal saline or dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China). The control group received normal saline alone. Group B received 0.25% ropivacaine, and the other three groups received 1 µg/mL (Group C), 2 µg/mL (Group D), or 3 µg/mL (Group E) of dexmedetomidine together with 0.25% ropivacaine, respectively. The drugs were connected to an analgesia pump before the pump parameters (1-mL loading dose, 0.5-ml/h background infusion; additional 1 mL of drug was added every 6 h) were set for continuous femoral nerve block.
Neurobehavioral examination
After surgery, sensory and motor function was assessed with behavioral scores after the nerve block by subcutaneous injection of 0.5 mL of 10% formalin into the patella. The researcher who scored the behavioral assay was blinded to the experimental treatment. The animals’ behavioral scores were rated according to the following criteria: 0, the injected leg was able to bear a load, and there was no significant difference between the injected leg and normal leg; 1, the injected leg almost could not bear a load, and a clear limp was seen during movement; 2, the injected leg was raised and could not bear a load; and 3, scratching, licking, biting, or shaking of the leg was observed.
The analgesia onset time was defined as the time point at which the rabbit resumed walking after pain and injection of a background dose of 1 mL of drug through the epidural catheter.
Histopathological evaluation
After 48 hours of the continuous femoral nerve block, the rabbits were anesthetized and a 1-cm section of the femoral nerve around the tip of the epidural catheter was removed. The tissue was placed into a sample vial, placed in 4°C pre-cooled 2.5% glutaraldehyde for 2 hours, and rinsed three times (5 minutes each) with 4°C phosphate-buffered saline. The tissues were then fixed with 1% ricin at 4°C for 30 minutes, followed by three phosphate-buffered saline washes at 4°C for 5 minutes each. Dehydration was performed in an ethanol gradient at 4°C: 50% for 10 minutes, 70% for 10 minutes, 80% for 10 minutes, and 90% for 10 minutes, followed by a series of pyruvate concentrations: 50% for 10 minutes, 70% for 10 minutes, 90% for 20 minutes, and 100% for 10 minutes. EPON 618 epoxy resin (3 mL) and 100% acetone were added to the bottle and soaked at room temperature for 10 minutes to absorb the dehydrating agent. The embedding agent was removed, and 3 mL of pure EPON 618 epoxy resin embedding agent was added. The mixture was allowed to permeate overnight at room temperature. The embedding blocks were trimmed at 36°C to 60°C, and semi-thin slices were prepared for light microscopy analysis. After positioning, the ultrathin sections were prepared and stained with urinary uric acid and citric acid for electron microscopy analysis.
The prepared semi-thin slices were placed under a microscope to determine the required field at low magnification, and the eyepiece was then switched to a 100× oil objective to observe the cross section of the nerve fiber structure. Three fields of vision were randomly selected from each prepared slice. Myelin sheaths from five nerve fibers were randomly selected from each field of view to record the axonal area and total myelin area.
Statistical analysis
Data are expressed as mean ± standard deviation and were analyzed using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). One-factor analysis of variance was used to evaluate differences between the groups. A P value of <0.05 was considered statistically significant.
Results
At 2 and 6 hours after injection, the behavior scores in Group A were lower than those in the other groups. The scores in Groups B and C were higher than those in Groups D and E. There were no apparent differences between Groups B and C at the same time points, but the differences between Groups D and E were considered statistically significant (P < 0.05). Finally, there were also no apparent differences between Groups D and E at 2 and 6 hours after injection (Table 1).
Table 1.
Animal behavioral scores at different time points
| Group | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| A (n = 6) | 2.8 ± 0.4 | 2.5 ± 0.5*#Δ | 2.5 ± 0.5*#Δ | 2.3 ± 0.5*#Δ |
| B (n = 6) | 2.8 ± 0.4 | 1.8 ± 0.4* | 1.6 ± 0.5* | 1.3 ± 0.5* |
| C (n = 6) | 2.6 ± 0.5 | 1.3 ± 0.5* | 1.3 ± 0.4* | 1.0 ± 0.6* |
| D (n = 6) | 2.8 ± 0.4 | 0.6 ± 0.5*#Δ | 0.5 ± 0.5*#Δ | 0.3 ± 0.5*#Δ |
| E (n = 6) | 2.8 ± 0.4 | 0.5 ± 0.5*#Δ | 0.1 ± 0.4*#Δ | 0.1 ± 0.4*#Δ |
Data are presented as mean ± standard deviation.
T0: immediately after subcutaneous injection of 10% formalin (0.5 ml) under the tibia; T1: 2 hours after injection; T2: 6 hours after injection; T3: 24 hours after injection.
*P < 0.05 compared with T0, #P < 0.05 compared with Group B,ΔP < 0.05 compared with Group C.
The analgesia onset time in Groups A, B, C, D, and E was 21.3 ± 3.6, 16.1 ± 3.1, 13.1 ± 4.0, 9.1 ± 3.4, and 7.1 ± 1.2 minutes, respectively. The analgesia onset time was longer in Group A (control group) than in the experimental groups. Compared with Groups D and E, the time needed for restoration of walking was longer in Groups A, B, and C (P < 0.05). However, there were no apparent differences between Groups B and C.
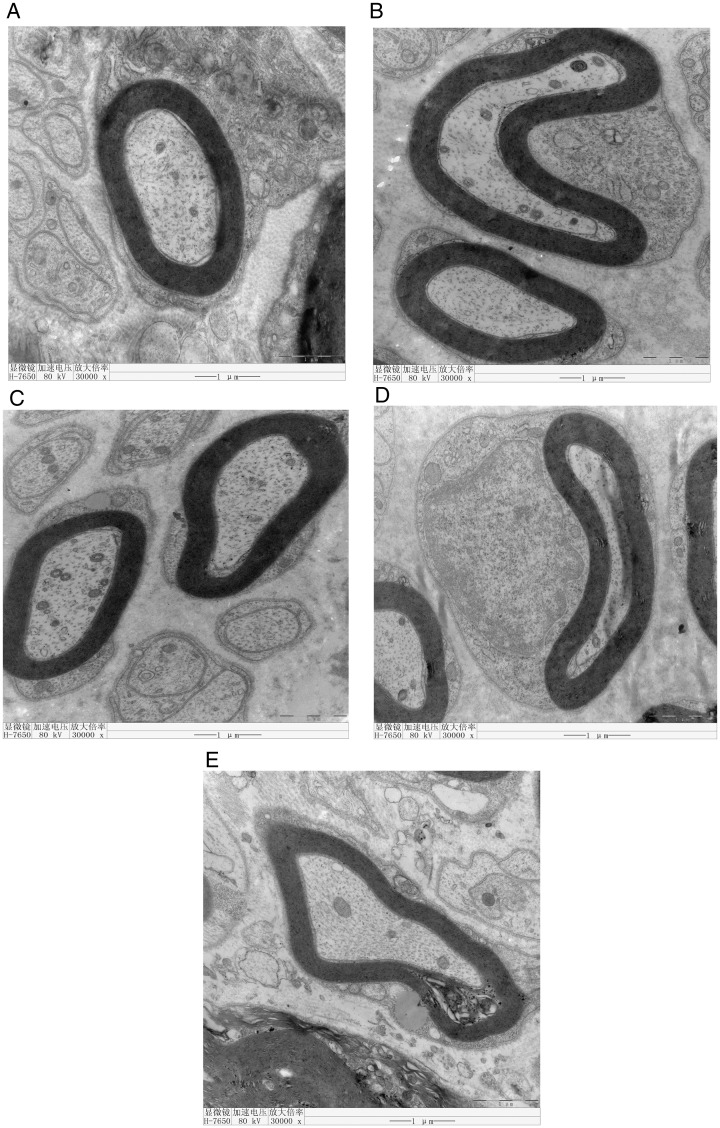
The structures of axons and myelin sheaths were clear under a light microscope. Electron microscopic examination of Groups A, B, C, and D showed that the myelin lamellar structure of the nerve fibers was rounded or oval with a compact structure. The structure of Schwann cells was clear, and the cytoplasm was uniform. The ultrastructure of the mitochondria and microfilaments was also clear. However, the myelin lamellar structure was loose and vacuolated in Group E (Figure 1).
Figure 1.
Electron microscopic examination of Groups A, B, C, and D shows that the myelin lamella of nerve fibers is rounded or oval with a compact structure. The structure of Schwann cells is clear and the cytoplasm is uniform. The ultrastructure of mitochondria and microfilaments is clear. However, the myelin lamella is loose and vacuolated in Group E.
The differences in the axonal area, myelin sheath area, and ratio of the axonal area to the total myelin sheath area in the femoral nerve of each group were not statistically significant (P < 0.05; Table 2).
Table 2.
Myelin area, axonal area, and their ratio in each group
| Group | Myelin area (µm2) | Axonal area (µm2) | Area ratio (%) |
|---|---|---|---|
| A | 143.71 | 43.471 | 29.721 |
| B | 158.21 | 47.571 | 29.221 |
| C | 132.08 | 38.428 | 27.448 |
| D | 137.00 | 34.230 | 25.050 |
| E | 145.26 | 41.746 | 27.706 |
Area ratio = axonal area/myelin area × 100%
Discussion
Our results show that the animal behavioral scores were significantly lower in Groups D and E than in Groups B and C, indicating that dexmedetomidine combined with ropivacaine in continuous femoral nerve blocks could increase the analgesic effects. Furthermore, the time needed for restoration of walking was shorter in Groups D and E than in the control group, demonstrating that the addition of dexmedetomidine to ropivacaine not only enhances the analgesic effect but also shortens the onset time of local anesthetics.
Overall, the present study showed that the addition of 1 and 2 µg/mL of dexmedetomidine to 0.25% ropivacaine to induce a femoral nerve block in rabbits for 48 hours did not cause histomorphological or cytopathological changes in the femoral nerve fibers, and the structural integrity of the nerve fiber myelin and axons remained intact. However, the addition of 3 µg/mL of dexmedetomidine to 0.25% ropivacaine resulted in loose and discrete local lamina in the myelin sheath of nerve fibers, suggesting nerve fiber demyelination.
In general, the normal nerve fiber myelin sheath structure is clearly observed under a 10 × 100 optical microscope. The structure is clear, the thickness is consistent, and the edge is regular. The axonal area in a cross-sectional area of nerve fibers (myelin area) accounts for a large proportion of the total area. However, the area of degeneration in the nerve fibers (myelin area) is significantly increased, the thickness of the lamellar layer is increased and loosened, the axons are decreased or absent, and the ratio of axons within the total axonal area is decreased. Therefore, we analyzed the myelin area using an Olympus image analyzer. The results showed no significant differences in the axonal area, myelin sheath area, or ratio of the axonal area to the myelin area in all groups. These results suggest that none of the doses of dexmedetomidine added to 0.25% ropivacaine to block the femoral nerve for 48 hours caused significant pathological damage to the femoral nerve fibers. However, the nerve fiber myelin sheath was loose and discrete under high-resolution transmission electron microscopy with the combination of 0.25% ropivacaine and 3 µg/mL of dexmedetomidine, suggesting that demyelination of nerve fibers occurred, although this pathological change was minor.
Dexmedetomidine is a highly selective α2 adrenergic agonist with sedative, hypnotic, anxiolytic, and analgesic effects as well as the ability to reduce the stress response.15 Numerous animal and clinical studies have shown that dexmedetomidine shortens the onset time of local anesthetics and prolongs the local anesthetic maintenance time as an adjunct to local anesthetics when applied singly in a peripheral nerve block.4,6,10,11 However, the related neurotoxicity due to local dexmedetomidine application remains controversial. Administration of dexmedetomidine via an epidural injection has been shown to lead to oligodendrocyte demyelination.12 In mice, however, a single sciatic nerve block with 0.5% bupivacaine (0.2 mL) and dexmedetomidine (5 µg/mL) revealed no pathological changes in the axonal or myelin sheath after 14 d.7 Dexmedetomidine alone did not exhibit neurotoxicity in a rat model of diabetes, but it significantly enhanced both the duration of nerve blockade and nerve toxicity produced by ropivacaine.9 Although results from experimental studies of peripheral neurotoxicity with dexmedetomidine are not consistent, clinical studies have shown that dexmedetomidine as an adjunct to local anesthetics singly used in peripheral nerve blocks does not result in clinically visible neurotoxicity-related adverse effects.16 Some studies have shown that dexmedetomidine has potential neuroprotective properties against local anesthetic-induced neurotoxicity.6,12,17,18 Brummett et al.12 found that dexmedetomidine can effectively reduce the neurological edema caused by bupivacaine 24 hours and 14 days after surgery. The neuroprotective mechanism of dexmedetomidine may be related to inhibition of macrophage oxidative stress and reduction of mRNA levels of interleukin 6 and tumor necrosis factor α.19 Furthermore, dexmedetomidine may suppress microglial activation, and it decreased proinflammatory cytokines in the spinal cord when administered systemically in rats with streptozotocin-induced diabetes.20 This finding of a protective rather detrimental effect with systemic dexmedetomidine is consistent with the protective effects afforded by intravenous infusion of dexmedetomidine.21
The mechanism of dexmedetomidine toxicity on peripheral nerves and the effect on local blood vessels remain poorly understood. The results of studies to date have shown that local dexmedetomidine injection leads to a dose-related decrease in local skin blood flow, and this change was not accompanied by systemic blood pressure or heart rate changes.22 This characteristic local blood flow change indicates that high doses of topical dexmedetomidine might result in decreased local blood supply to nerves, resulting in ischemic damage to nerve fibers. Our results showed that the nerve fiber demyelination caused by a larger dose (3 µg/mL) of continuous dexmedetomidine application for 48 hours could be related to local neurological ischemic damage. However, the exact mechanism remains unclear.
This study had some limitations. We were unable to observe more long-term pathological changes caused by the addition of large doses of dexmedetomidine to ropivacaine for continuous femoral nerve block in rabbits. Therefore, whether this pathological change is reversible remains unknown. Moreover, the observation point was a single time point, and we could not determine the specific timing of these pathological changes.
Conclusion
The lowest doses of dexmedetomidine (1 and 2 µg/mL) combined with 0.25% ropivacaine for continuous femoral nerve block resulted in no neurotoxic lesions, but the higher dose (3 µg/mL) resulted in neurotoxic lesions in this rabbit experimental model.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Guangxi Natural Science Foundation (2014GXNSFAA118242) and Sanmenxia Science and Technology Bureau Foundation (2016030312). These funding sources did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Congedo E, Sgreccia M, De Cosmo G. New drugs for epidural analgesia. Curr Drug Targets 2009; 10: 696–706. [DOI] [PubMed] [Google Scholar]
- 2.Singelyn FJ, Dangoisse M, Bartholomee S, et al. Adding clonidine to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Reg Anesth 1992; 17: 148–150. [PubMed] [Google Scholar]
- 3.Singelyn FJ, Gouverneur JM, Robert A. A minimum dose of clonidine added to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Anesth Analg 1996; 83: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 4.Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 2014; 39: 37–47. [DOI] [PubMed] [Google Scholar]
- 5.Hanoura SE, Hassanin R, Singh R. Intraoperative conditions and quality of postoperative analgesia after adding dexmedetomidine to epidural bupivacaine and fentanyl in elective cesarean section using combined spinal-epidural anesthesia. Anesth Essays Res 2013; 7: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Wang CS, Shi JH, et al. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med 2014; 7: 680–685. [PMC free article] [PubMed] [Google Scholar]
- 7.Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth 2013; 110: 438–442. [DOI] [PubMed] [Google Scholar]
- 8.Rancourt MP, Albert NT, Cote M, et al. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg 2012; 115: 958–962. [DOI] [PubMed] [Google Scholar]
- 9.Yu ZY, Geng J, Li ZQ, et al. Dexmedetomidine enhances ropivacaine-induced sciatic nerve injury in diabetic rats. Br J Anaesth 2019; 122: 141–149. [DOI] [PubMed] [Google Scholar]
- 10.Brummett CM, Padda AK, Amodeo FS, et al. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology 2009; 111: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg 2010; 111: 1548–1551. [DOI] [PubMed] [Google Scholar]
- 12.Brummett CM, Norat MA, Palmisano JM, et al. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology 2008; 109: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand 2006; 50: 222–227. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J 2009; 30: 365–370. [PubMed] [Google Scholar]
- 15.Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog 2015; 62: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirksey MA, Haskins SC, Cheng J, et al. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One 2015; 10: e0137312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tufek A, Kaya S, Tokgoz O, et al. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med 2013; 36: E95–E102. [DOI] [PubMed] [Google Scholar]
- 18.Bharti N, Sardana DK, Bala I. The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: a randomized controlled trial. Anesth Analg 2015; 121: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Lu Y, Zhang L, et al. Perineural dexmedetomidine attenuates inflammation in rat sciatic nerve via the NF-kappaB pathway. Int J Mol Sci 2014; 15: 4049–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Lin B, Zhong J. The therapeutic effect of dexmedetomidine on rat diabetic neuropathy pain and the mechanism. Biol Pharm Bull 2017; 40: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 21.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016; 388: 1893–1902. [DOI] [PubMed] [Google Scholar]
- 22.Yabuki A, Higuchi H, Yoshitomi T, et al. Locally injected dexmedetomidine induces vasoconstriction via peripheral alpha-2A adrenoceptor subtype in guinea pigs. Reg Anesth Pain Med 2014; 39: 133–136. [DOI] [PubMed] [Google Scholar]