Short abstract
Background
To investigate the behaviour of the inflammatory marker neutrophil-to-lymphocyte ratio (NLR) in the presence of peripheral blood eosinophilia (PBE) in paediatric asthma patients with lower respiratory tract (LRT) infections.
Methods
This retrospective study enrolled consecutive patients aged ≥5 years who were diagnosed with asthma and whose haemogram values were available. The patients were further subdivided based on the presence or absence of LRT infections and allergies. NLR and C-reactive protein (CRP) were evaluated in relation to the presence or absence of PBE (≥4% eosinophils).
Results
A total of 991 patients were enrolled in the study. Patients with LRT infections had significantly higher leucocyte and neutrophil counts, a greater NLR and a higher level of CRP compared with patients without LRT infections. Overall, patients with PBE had significantly lower NLRs and CRP regardless of the presence or absence of an LRT infection. The PBE percentage showed moderate inverse correlations with NLR (r = −0.34) and CRP (r = −0.20).
Conclusion
The presence of PBE was significantly associated with lower NLR and CRP regardless of the presence or absence of an infectious condition.
Keywords: Lower respiratory tract infection, peripheral blood eosinophilia, allergic asthma, neutrophil-to-lymphocyte ratio
Introduction
Asthma is the most common chronic inflammatory airway disease affecting children and adults worldwide.1,2 Despite limited information about the incidence of pneumonia in asthmatic patients, studies have demonstrated that the risk of developing community-acquired pneumonia is high.3,4 Some investigations have found that the clinical course and prognosis of pneumonia do not vary in asthmatic patients on the basis of some known inflammatory markers, such as C-reactive protein (CRP), leukocytes and procalcitonin, which guide physicians in antibiotic initiation and disease progression.5–9
The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory indicator.10–12 In a study of children with allergic rhinitis, the NLR was found to be elevated with inflammation.13 In asthmatic children, an elevated NLR was also detected during acute exacerbations of asthma.14 However, data pertaining to the NLR during chronic inflammation in asthmatic children are limited.15 Some studies have shown that the NLR is correlated with the severity of infection in adults with chronic obstructive pulmonary disease;16–18 however, an increased NLR was uncommon in the presence of peripheral blood eosinophilia (PBE).17,18 The behaviour of the NLR in the presence of both PBE and infection is not well studied in children with asthma.
This study hypothesized that an increase in inflammatory markers may be mild in eosinophilic (peripheral blood eosinophil count ≥4%) asthmatic patients with lower respiratory tract (LRT) infections compared with non-eosinophilic patients. The study aimed to evaluate the effect of PBE (≥4%) on the NLR in asthmatic children with LRT infections.
Patients and methods
Patients
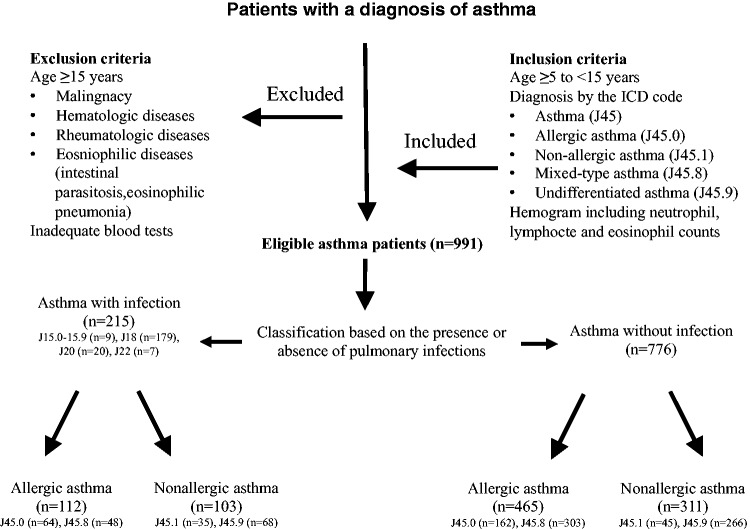
This retrospective, observational, cross-sectional study was undertaken in the paediatric outpatient clinic of Sureyyapasa Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey, which largely deals with pulmonary and allergic pulmonary diseases. The study included consecutive patients who presented with asthma over a 2-year period between September 2013 and September 2015 and who were ≥5 years of age. All of the patients had complete blood count data. The enrolment and exclusion criteria are summarized in a flow chart shown in Figure 1.
Figure 1.
The enrolment and exclusion criteria of paediatric patients (n = 991) with asthma who were included in this retrospective observational, cross-sectional study.
The study was approved by the Institutional Review Board of Sureyyapasa Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey (no. 2017/020) and was conducted in accordance with the ethical principles of the Declaration of Helsinki. Because of its retrospective design, written informed consent was not required from the parents. The study only retrieved the patients’ electronic data without ID information. For each retrospective study, written informed patient/parental consent was waived by the Institutional Review Board.
Diagnosis and ICD-10 coding for asthma
Asthma was diagnosed by paediatricians and paediatric pulmonary subspecialists (two pulmonary paediatricians, two allergy paediatricians and four paediatricians) according to international guidelines.19 Asthma was diagnosed if the airways were characterized by variable and recurring symptoms, reversible airflow obstruction and bronchospasm. The symptoms of wheezing, coughing, chest tightness and shortness of breath are accepted as the most common symptoms.
Lower respiratory tract infection was defined as an infectious process involving the lower respiratory tract (bronchitis/bronchiolitis) and pneumonia with the symptoms of coughing, fever, expectorations and the historical physician’s diagnosis based on a chest X-ray.
A specific ICD-10 code for asthma was assigned to each patient: asthma (J45), allergic asthma (J45.0), nonallergic asthma (J45.1), mixed-type asthma (which has either allergic or nonallergic clinical features, a combination of intrinsic and extrinsic asthma; J45.8) and undifferentiated asthma (exercise-induced asthma, acute exacerbation, cough variant asthma; J45.9). The patients were further classified into two groups based on the presence or absence of an LRT infection, with appropriate ICD coding. Infectious conditions included pneumonia (J15.0–J15.9, J18) and the other acute LRT infections (J20, J22). Patients with infectious or non-infectious conditions were also subdivided based on the presence or absence of allergy: allergic asthma (J45.0, J45.8) and nonallergic asthma (J45.1, J45.9) (Figure 1).
Data collection
The patients’ demographic (e.g. age, sex) and laboratory data were retrieved from the hospital electronic data system. The complete blood count included white blood cells, neutrophils, lymphocytes, eosinophils, monocytes, basophils, erythrocytes, haemoglobin, haematocrit, platelets and mean platelet volume (MPV). If present, biochemistry data and CRP were also recorded.
Calculations
The NLR, calculated by dividing the number of neutrophils by the number of lymphocytes, was used as a dichotomous variable (NLR: 1.5; 2.0; 3.0; 4.0; 5.0; 6.0; 7.0; 8.0; 9.0; 10.0). The ratio of platelet count (PC) to MPV was determined (PC/MPV). The PBE percentage was calculated as a dichotomous variable (eosinophil %: 2; 4; 5; 10). CRP was calculated as a dichotomous value (CRP, 5 mg/l; 10 mg/l). Comparisons were made based on the presence or absence of LRT infections, allergies and PBE.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). The demographic, clinical and laboratory data were analysed. For two-group comparisons, non-parametric continuous variables were expressed as median and interquartile ranges (IQR, 25% and 75%) and compared using the Mann–Whitney U–test. Parametric continuous variables were expressed as mean ± SD and compared using Student’s t-test. Dichotomous variables were expressed as counts and percentages and compared using χ2-test. For four-subgroup comparisons, non-parametric continuous variables were analysed using the Kruskal–Wallis test. The non-parametric Spearman rank correlation test was used for the NLR and PBE percentage. A P-value < 0.05 was considered statistically significant.
Results
A total of 991 asthmatic patients (566 boys, 57.1%; median age, 7 years; interquartile range, 6–10 years) were enrolled in the study. Of these, 215 (21.7%) had LRT infections and 776 (78.3%) did not have LRT infections. The distribution of the asthma patients based on the presence or absence of allergies and LRT infections according to allergic ICD codes is shown in Figure 1.
Table 1 presents the descriptive data for all of the groups and subgroups, along with the haemogram values and levels of inflammatory markers. Patients with and without an infectious condition were similar in age. There was a preponderance of males among the asthmatic patients without LRT infections. Asthma patients with LRT infections had significantly higher leucocyte and neutrophil counts, a greater NLR and a higher level of CRP compared with patients without LRT infection (P < 0.001 for all comparisons). On the other hand, asthmatic patients without LRT infections had a significantly higher rate of allergy history (e.g. allergic asthma, allergic rhinitis), a larger PBE percentage and a higher lymphocyte count (P < 0.05 for all comparisons). Analysis of the data across the four subgroups with or without allergies revealed similar age but different sex distributions. In asthmatic patients with LRT infections, the presence versus absence of allergies was associated with lower levels of inflammatory markers.
Table 1.
Descriptive characteristics of all groups and subgroups of paediatric patients (n = 991) with asthma including haemogram results and levels of inflammatory markers.
| Variables | Overall asthman = 991 | Infectiousn = 215(21.7%) | Noninfectious n = 776(78.3%) | Statistical significancea | Infectious | Noninfectious | Statistical significanceb | ||
|---|---|---|---|---|---|---|---|---|---|
| Allergicn = 112 | Nonallergicn = 103 | Allergicn = 465 | Nonallergicn = 311 | ||||||
| Boys | 566 (57.1) | 110 (51.2) | 456 (58.8) | P = 0.046 | 50 (44.6) | 60 (58.3) | 257 (55.3) | 199 (64.0) | P = 0.003 |
| Girls | 425 (42.9) | 105 (48.8) | 320 (41.2) | 62 (55.4) | 43 (41.7) | 208 (44.7) | 112 (36.0) | ||
| Age, years | 7 (6–10) | 7 (6–10) | 7 (6–10) | NS | 7 (6–9) | 7 (6–10) | 7 (6–10) | 7 (6–10) | NS |
| History of allergy | 577 (58.2) | 112 (52.1) | 465 (59.9) | P = 0.039 | – | – | – | – | |
| Leucocytes, ×109/l | 8.75 (7.09–10.79) | 9.50 (7.60–12.30) | 8.55 (6.96–10.43) | P < 0.001 | 9.53 (7.41–12.07) | 9.50 (7.84–12.82) | 8.52 (7.00–10.46) | 8.60 (6.90–10.40) | P = 0.005 |
| Neutrophils, ×109/l | 4.57 (3.36–6.67) | 5.37 (3.76–8.05) | 4.46 (3.23–6.22) | P < 0.001 | 5.35 (3.79–7.92) | 5.38 (3.71–8.08) | 4.43 (3.33–6.28) | 4.47 (3.17–5.96) | P = 0.033 |
| Lymphocytes, ×109/l | 2.73 (2.18–3.48) | 2.56 (2.05–3.34) | 2.77 (2.21–3.51) | P = 0.014 | 2.49 (2.03–3.33) | 2.60 (2.12–3.39) | 2.80 (2.16–3.46) | 2.74 (2.28–3.54) | P = 0.033 |
| Eosinophil, % | 2.50 (1.18–5.03) | 2.01 (0.77–4.60) | 2.60 (1.33–5.13) | P = 0.002 | 1.85 (0.78–4.76) | 2.10 (0.70–4.06) | 2.53 (1.20–5.70) | 2.66 (1.40–4.68) | NS |
| Haemoglobin, g/dl | 12.70 (12.12–13.27) | 12.54 (11.94–13.10) | 12.73 (12.16–13.30) | P = 0.006 | 12.54 (11.93–13.01) | 12.54 (12.03–13.21) | 12.64 (12.07–13.23) | 12.83 (12.27–13.38) | P = 0.001 |
| Haematocrit, % | 38.3 (36.6–40.1) | 37.9 (36.1–39.7) | 38.4 (36.8–40.2) | P = 0.014 | 37.8 (36.1–39.3) | 38.3 (36.2–39.8) | 38.3 (36.5–40.1) | 38.7 (37.1–40.4) | P = 0.011 |
| Platelets, ×103 | 323 (271–382) | 333.50 (278–397) | 321.00 (268–376) | NS | 312 (273–373) | 352 (278–412) | 319 (272–380) | 327 (263–369) | NS |
| MPV | 8.20 (7.65–8.81) | 8.18 (7.60–8.81) | 8.20 (7.67–8.81) | NS | 8.19 (7.56–8.80) | 8.18 (7.60–8.95) | 8.27 (7.75–8.87) | 8.11 (7.53–8.75) | NS |
| CRP, mg/l | 3.3 (3.3–13.3) | 7.5 (3.3–27.3)(n = 193) | 3.3 (3.3–8.8) (n = 461) | P < 0.001 | 6.6 (3.0–21.4) | 8.0 (3.3–33.2) | 3.0 (3.3–9.8) | 3.3 (3.3–6.1) | P < 0.001 |
| NLR | 1.62 (1.12–2.56) | 2.06 (1.32–3.26) | 1.55 (1.09–2.40) | P < 0.001 | 2.01 (1.26–3.42) | 2.14 (1.40–3.09) | 1.59 (1.10–2.50) | 1.47 (1.09–2.14) | P < 0.001 |
| Platelets/MPV | 38.47 (31.17–46.96) | 38.69 (32.93–47.72) | 38.44 (30.81–46.51) | NS | 38.39 (31.10–47.20) | 38.92 (34.05–55.13) | 37.88 (30.26–45.58) | 38.97 (32.35–46.61) | NS |
Data presented as n of patients (%) or median (interquartile range).
MPV, mean platelet volume; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; NS, no significant between-group difference (P ≥0.05).
aMann–Whitney U-test; bKruskal–Wallis test.
Table 2 presents the leucocyte, NLR, CRP and PC/MPV values for non-eosinophilic and eosinophilic patients with PBE counts < 4% and ≥4%, respectively, together with four subgroups based on the presence or absence of an infectious condition. Overall, patients with PBE had significantly lower NLRs and CRP regardless of the presence or absence of an infectious condition (P < 0.05 for all comparisons). The PBE percentage showed moderate inverse correlations with NLR (r = –0.34, P < 0.001) and CRP (r = –0.20, P < 0.001).
Table 2.
The results of leucocyte, NLR, CRP and platelet/MPV based on the percentage of eosinophils (<4% and ≥4%) and in subgroups based on the presence and absence of lower respiratory tract infections in paediatric patients (n = 991) with asthma.
| Eosinophil<4% | Eosinophil≥4% | Statistical significancea | Eosinophil <4%Infection (–)n = 507 | Eosinophil ≥4%Infection (–)n = 269 | Eosinophil <4%Infection (+)n = 158 | Eosinophil ≥4%Infection (+)n = 57 | Statistical significanceb | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Leucocyte, ×109/l | 665 | 8.94 (7.18–11.20) | 326 | 8.58 (7.00–10.21) | P = 0.07 | 8.59 (6.90–10.65) | 8.48 (6.98–10.09) | 9.77 (7.84–12.52) | 8.96 (7.10–11.11) | P = 0.003 |
| NLR | 665 | 1.79 (1.21–2.93) | 326 | 1.36 (0.99–1.89) | P < 0.001 | 1.68 (1.16–2.72) | 1.32 (0.98–1.76) | 2.15 (1.15–3.44) | 1.69 (1.08–2.41) | P < 0.001 |
| CRP, mg/l | 447 | 3.9 (3.3–15.6) | 207 | 3.3 (3.3–9.1) | P = 0.001 | 3.3 (3.3–10.3) | 3.3 (3.3–7.1) | 9.5 (3.3–33.2) | 5.0 (3.3–15.4) | P = 0.001 |
| Platelet/MPV | 273 | 38.62 (30.37–47.20) | 107 | 38.46 (32.46–44.65) | NS | 37.42 (29.88–46.61) | 38.47 (32.09–46.33) | 39.03 (32.55–48.41) | 38.29 (33.98–40.54) | NS |
Data presented as n of patients and median (interquartile range).
NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; MPV, mean platelet volume; NS, no significant between-group difference (P ≥0.05).
aMann–Whitney U-test; bKruskal–Wallis test.
To estimate the risk of LRT infection in asthma patients, odds ratios for the PBE percentage, NLR and CRP are listed in Table 3. Higher NLR values (≥1.5) and higher levels of CRP (≥5 mg/l) were found to be significant markers of LRT infection risk; but the PBE percentage was not.
Table 3.
Odds ratios for markers of lower respiratory tract infection risk in paediatric patients (n = 991) with asthma.
| Odds ratio | 95% confidence interval | Statistical significance | |
|---|---|---|---|
| NLR | |||
| ≥1.5 | 2.18 | 1.58, 3.02 | P < 0.001 |
| ≥2 | 2.40 | 1.77, 3.27 | P < 0.001 |
| ≥4 | 2.57 | 1.65, 4.01 | P < 0.001 |
| ≥5 | 2.63 | 1.54, 4.47 | P < 0.001 |
| Eosinophil, % | |||
| ≥2 | 0.71 | 0.52, 0.96 | P = 0.025 |
| ≥4 | 0.68 | 0.49, 0.95 | P = 0.024 |
| ≥5 | 0.87 | 0.61, 1.24 | NS |
| CRP, mg/l | |||
| ≥5 | 2.27 | 0.61, 3.20 | P < 0.001 |
| ≥10 | 2.71 | 1.90, 3.86 | P < 0.001 |
| Leucocyte | |||
| ≥10.000/mm³ | 1.86 | 1.36, 2.53 | P < 0.001 |
| ≥12.000/mm³ | 2.13 | 1.49, 3.05 | P < 0.001 |
NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; NS, no significant association (P ≥ 0.05).
Discussion
The findings of this current study demonstrated that asthmatic children with LRT infections had a lower NLR and a lower level of CRP when PBE (PBE percentage ≥4%) accompanied the disease.
A recent study from Turkey evaluated the NLR in children with asthma (n = 469) and in controls (n = 170) without any allergic condition or infection.15 The NLR was also examined for asthmatic patients with allergic and infectious conditions.15 The study and control groups differed significantly with respect to the NLR: values were higher in the former (2.07 versus 1.77, P = 0.043).15 In addition, the NLR exhibited a weak inverse correlation with the percentage of eosinophils (r = –0.195, P = 0.001).15 In this current study, the NLR of asthmatic patients was very close to that reported previously for the control group and showed a moderate inverse correlation with the percentage of eosinophils.15 A previous study evaluated 164 patients with treated but uncontrolled asthma and found an increased NLR in cases of neutrophilic asthma.20 These authors also found significant correlations between the percentages of sputum eosinophils and neutrophils and the percentages of blood eosinophils and neutrophils, respectively.20 Based on their findings, the authors concluded that blood counts could be used for monitoring uncontrolled asthma.20 However, a study from the UK showed that the blood eosinophil count rarely reflects airway eosinophilia in children with severe asthma.21 They found that children with severe asthma were given higher doses of steroid medication, which caused unreliable blood cell differentiation.21 Two studies from Greece and Japan showed that the use of corticosteroids affects the amount of airway cells and in addition the peripheral blood cells.22,23 However, in the present study, the study populations were treated at outpatient clinics and the clinical status of the patients was not severe enough to use steroids. The medication data could not retrieved from the hospital database. These current results suggest a protective role of PBE against elevations in inflammatory markers such as CRP and NLR in paediatric asthma patients, regardless of the presence or absence of an LRT infection (Table 2).
A study from Australia investigated 50 asthmatic adults with or without systemic inflammation, which was defined as the presence of increased plasma levels of high sensitivity CRP and interleukin 6.24 Patients with systemic inflammation had an increased sputum neutrophil count compared with those without systemic inflammation.24 Airway neutrophilia was also significantly correlated with plasma CRP in patients with systemic inflammation.24
In a previous Turkish study, the NLR was also studied in 438 children with allergic rhinitis, 55% of whom had asthma, based on the severity of allergic rhinitis and compared with a control group.13 Patients with moderate-to severe allergic rhinitis had a significantly increased NLR compared with patients with mild allergic rhinitis and with healthy children.13
This current study had a number of limitations. First, it was conducted at a single centre. However, the current results are thought to be valid because they were obtained from a large sample size and from a disease-based hospital with specialist physicians. Secondly, the current results pertain only to asthmatic children within an age range of 5–15 years, so they cannot be generalized to children of all ages. Thirdly, this study had a retrospective design. Even though all of the data were retrieved from the electronic database of the single centre, some data might have been missing. Fourthly, information on allergy details (e.g. skin prick test, immunoglobulin E) was not retrieved. Nevertheless, the diagnosis of allergic airway diseases was made by specialists and allergic subspecialists. The data on allergies, NLR and PBE are thought to be reliable. Finally, one of the shortcomings of this study was the lack of data regarding the use of asthma medications.
In conclusion, this current study showed that asthmatic children with LRT infections had a lower NLR and a lower level of CRP when PBE (PBE percentage ≥4%) accompanied the disease. In contrast, asthmatic children with PBE < 4% had higher NLR. Elevations in inflammatory markers due to LRT infections may be mild in paediatric asthma patients with PBE (≥4%). The present study suggests a protective role of PBE of ≥4% in mitigating the severity of inflammatory diseases through an inhibitory mechanism resulting in lower CRP and NLR. This protective role of PBE needs to be validated by further prospective studies.
Acknowledgements
All authors contributed to the study and helped to prepare the manuscript. We thank Associate Professor Dr Zuhal Karakurt for her contributions to the analysis and editing of the manuscript and Sinem Gungor and Sennur Keles for their support of the article (all based at Health Sciences Sureyyapasa Chest Disease and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey).
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol 2010; 126: 926–938. [DOI] [PubMed] [Google Scholar]
- 2.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143–178. [DOI] [PubMed] [Google Scholar]
- 3.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005; 352: 2082–2090. [DOI] [PubMed] [Google Scholar]
- 4.Wilson KM, Torok MR, Localio R, et al. Hospitalization for community-acquired pneumonia in children: effect of an asthma codiagnosis. Hosp Pediatr 2015; 5: 415–422. [DOI] [PubMed] [Google Scholar]
- 5.Terraneo S, Polverino E, Cilloniz C, et al. Severity and outcomes of community acquired pneumonia in asthmatic patients. Respir Med 2014; 108: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon RK, Yawn BP, Yoo KH, et al. Impact of asthma on the severity of serious pneumococcal disease. Epidemiology (Sunnyvale) 2011; S3: 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004; 39: 206–217. [DOI] [PubMed] [Google Scholar]
- 8.Van den Bruel A, Thompson MJ, Haj-Hassan T, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011; 342: d3082. [DOI] [PubMed] [Google Scholar]
- 9.Galetto-Lacour A, Zamora SA, Gervaix A. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referral center. Pediatrics 2003; 112: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 10.Günay E, Sarinç Ulaşli S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation 2014; 37: 374–380. [DOI] [PubMed] [Google Scholar]
- 11.Coşkun BN, Öksüz MF, Ermurat S, et al. Neutrophil lymphocyte ratio can be a valuable marker in defining disease activity in patients who have started anti-tumor necrosis factor (TNF) drugs for ankylosing spondylitis. Eur J Rheumatol 2014; 1: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen BB, Rifaioglu EN, Ekiz O, et al. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 2014; 33: 223–227. [DOI] [PubMed] [Google Scholar]
- 13.Dogru M, Evcimik MF, Cirik AA. Is neutrophil-lymphocyte ratio associated with the severity of allergic rhinitis in children? Eur Arch Otorhinolaryngol 2016; 273: 3175–3178. [DOI] [PubMed] [Google Scholar]
- 14.Nacaroglu HT, İsgüder R, Bent S, et al. Can neutrophil/lymphocyte ratio be a novel biomarker of inflammation in children with asthma? Eur J Inflamm 2016; 14: 109–112. [Google Scholar]
- 15.Dogru M, Yesiltepe Mutlu RG. The evaluation of neutrophil-lymphocyte ratio in children with asthma. Allergol Immunopathol (Madr) 2016; 44: 292–296. [DOI] [PubMed] [Google Scholar]
- 16.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184: 662–671. [DOI] [PubMed] [Google Scholar]
- 17.Saltürk C, Karakurt Z, Adiguzel N, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis 2015; 10: 1837–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis 2015; 10: 2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter PC. Current guidelines for the management of asthma in young children. Allergy Asthma Immunol Res 2010; 2: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XY, Simpson JL, Powell H, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy 2014; 44: 1137–1145. [DOI] [PubMed] [Google Scholar]
- 21.Ullmann N, Bossley CJ, Fleming L, et al. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy 2013; 68: 402–406. [DOI] [PubMed] [Google Scholar]
- 22.Karakonstantis S, Kalemaki D, Tzagkarakis E, et al. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect Dis (Lond) 2018; 50: 163–174. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa T, Sakagami T, Koya T, et al. Characteristics of eosinophilic and non-eosinophilic asthma during treatment with inhaled corticosteroids. J Asthma 2015; 52: 417–422. [DOI] [PubMed] [Google Scholar]
- 24.Fu JJ, Baines KJ, Wood LG, et al. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS 2013; 17: 187–199. [DOI] [PubMed] [Google Scholar]