Short abstract
Objective
This study aimed to evaluate the efficacy and safety profile of capecitabine and oxaliplatin (CAPOX) and 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) regimens as adjuvant treatment in patients with stage III colon cancer.
Methods
A total of 243 patients who received CAPOX and FOLFOX chemotherapy between 2014 and 2018 for stage III colon cancer in two centers were retrospectively studied. Among the patients, 106 (43.6%) and 137 (56.4%) were treated using CAPOX and FOLFOX regimens, respectively. Efficacy, treatment-related side effects, and overall survival rates with these two regimens were compared.
Results
The rate of disease progression was significantly higher in the presence of moderately/poorly differentiated histology, and KRAS and NRAS mutations. An increased number of metastatic lymph nodes and prolonged time from surgery to chemotherapy significantly increased disease progression. Patients who received CAPOX were significantly older than those who received FOLFOX. Disease progression, metastasis, and mortality rates were significantly higher in the FOLFOX arm than in the CAPOX arm. There was no significant difference in the overall survival rate between the two regimens.
Conclusion
The CAPOX regimen is preferred in older patients. Disease progression, metastasis, and mortality rates are higher with FOLFOX than with CAPOX.
Keywords: Colon carcinoma, CAPOX, FOLFOX, toxicity, overall survival, mortality
Introduction
Colorectal cancer is among the most common cancer worldwide and the third most frequent cause of cancer-related mortality. Currently, cancer statistics of Turkey are almost compatible with global data.1 A study from Turkey on colorectal cancer epidemiology with the largest group of patients (n = 968) was published by the Turkish Oncology Group in 2015.2 However, treatment and survival outcomes of these patients were not evaluated in this previous study.
Surgery is the mainstay of treatment of colorectal cancer. However, 5-year disease-free survival (DFS) is unfortunately approximately 49% in stage III cancer, despite recent advances in modern surgical techniques and treatment modalities. The 5-fluorouracil-based chemotherapy regimen has been used for treating high-risk stage II, stage III, and stage IV cases for almost 2 decades.3 Ten years previously, the addition of oxaliplatin to fluorouracil-folinic acid in patients with stage III colon cancer was named the FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) regimen. This regimen was shown to be advantageous for progression-free survival and overall survival (OS) compared with the regimen without oxaliplatin.4 Subsequently, a combination of capecitabine, which is a fluoropyrimidine analog, and oxaliplatin was used in stage III cancer and was found to be non-inferior to the FOLFOX combination.5,6 Currently, capecitabine and oxaliplatin (CAPOX) and FOLFOX protocols are considered to be equivalent in adjuvant treatment of stage III cases.5–7 There has been particular interest in the CAPOX protocol because of the absence of requirement of a vascular port or hospitalization and findings suggesting that CAPOX is more cost-effective compared with the FOLFOX regimen.7 However, no prospective, randomized studies have compared these two protocols in a head-to-head fashion in terms of efficacy, safety, and survival rates.
Therefore, in the present study, we aimed to compare the efficacy, treatment-related side effects, and survival rates of the FOLFOX and CAPOX regimens in patients with stage III colon cancer.
Material and methods
Data of patients who were followed at the Medical Oncology Units of the hospitals of Mugla Sitki Kocman University and Pamukkale University, and received adjuvant CAPOX and FOLFOX chemotherapy between January 2014 and January 2018 for stage III colon cancer were retrospectively analyzed. Written informed consent was obtained from each patient. The study protocol was approved by the Non-Interventional Research Ethics Board of Pamukkale University (approval date: 04/04/2018; no. 23479). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Inclusion criteria were the presence of stage III colon cancer and receiving the first-line treatment of CAPOX or FOLFOX. Exclusion criteria included metastatic disease at the time of diagnosis, coexisting rectal cancer, and administration of adjuvant chemotherapy in an external center.
Age at the time of diagnosis, Eastern Cooperative Oncology Group performance status, smoking status and alcohol use, comorbidities (including diabetes mellitus, hypertension, and coronary artery disease), prior elective or emergency surgery, localization of the tumor in the colon, T stage according to the American Joint Committee on Cancer classification,8 the number of excised lymph nodes, the number of involved lymph nodes (N stage), chemotherapy protocols selected, the number of previous chemotherapy cycles, time from surgery to chemotherapy, the number of adjuvant chemotherapies, disease progression, OS and progression-free survival rates, and chemotherapy-related side effects as assessed by the Common Terminology Criteria for Adverse Events version 49 were recorded.
The modified FOLFOX-6 regimen was used in this study. This protocol was composed of intravenous oxaliplatin at a dose of 85 mg/m2, intravenous leucovorin infusion at a dose of 400 mg/m2, and intravenous bolus 5-fluorouracil and continuous infusion of 5-fluorouracil for 46 hours at a dose of 2400 mg/m2 for 12 cycles once every 2 weeks. The CAPOX regimen was composed of intravenous oxaliplatin at a dose of 130 mg/m2 on the first day and oral capecitabine at a dose of 1000 mg/m2 every 12 hours on Days 1 and 14. This protocol was applied every 21 days for six to eight cycles.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 22.0 software (IBM Corp., Armonk, NY, USA). Descriptive data are expressed as mean, median, and percentages. The Student’s t-test for continuous variables and Fisher’s exact test for categorical variables were used. The Kaplan–Meier method was carried out to evaluate survival of the patients and the log-rank test was used for survival curves. OS and DFS were evaluated using the multivariate Cox proportional hazard model. A p value of ≤ 0.05 was considered statistically significant.
Results
Of the 243 patients included in the study, 146 (60.1%) were men and 97 (39.9%) were women. The mean age at the time of diagnosis was 61.7 years (range, 32–78 years). Demographic and clinical characteristics of the patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the patients.
| Number | % | |
|---|---|---|
| Age (years) | ||
| ≤64 | 146 | 60.1 |
| ≥65 | 97 | 39.9 |
| Sex | ||
| Male | 146 | 60.1 |
| Female | 97 | 39.9 |
| Smoking | ||
| No | 147 | 60.5 |
| Ex-smoker | 89 | 36.6 |
| Smoker | 7 | 2.9 |
| Alcohol | ||
| No | 231 | 95.1 |
| Yes | 12 | 4.9 |
| Diabetes mellitus | ||
| No | 206 | 84.8 |
| Yes | 37 | 15.2 |
| Hypertension | ||
| No | 129 | 53.1 |
| Yes | 114 | 46.9 |
| Coronary artery disease | ||
| No | 231 | 95.1 |
| yes | 12 | 4.9 |
Histopathological properties of the patients and mutations are presented in Table 2. Only a limited amount of mutation data from patients who had stage III disease at the time of the diagnosis were able to be obtained. This is because mutation analyses of KRAS, NRAS, and BRAF were often evaluated following the development of progression.
Table 2.
Histopathological properties and mutations of patients.
| Number | % | |
|---|---|---|
| Type of operation | ||
| Elective | 209 | 86.0 |
| Urgent | 34 | 14.0 |
| Tumor side | ||
| Right | 93 | 38.3 |
| Left | 150 | 61.7 |
| Differentiation | ||
| Good | 16 | 6.6 |
| Moderate | 85 | 35.0 |
| Poor | 142 | 58.4 |
| Lymphovascular invasion | ||
| No | 76 | 31.3 |
| Yes | 167 | 68.7 |
| Perineural invasion | ||
| No | 155 | 63.8 |
| Yes | 88 | 36.2 |
| KRAS mutation | ||
| No | 49 | 40.8 |
| Yes | 71 | 59.2 |
| NRAS mutation | ||
| No | 47 | 63.5 |
| Yes | 27 | 36.5 |
| BRAF mutation | ||
| No | 53 | 60.9 |
| Yes | 34 | 39.1 |
Among the patients, 106 (43.6%) and 137 (56.4%) were treated with the CAPOX and FOLFOX regimens, respectively. Anthropometric data before chemotherapy according to the regimens are shown in Table 3. Patients who received CAPOX were significantly older than those who received FOLFOX (p = 0.036).
Table 3.
Anthropometric data before chemotherapy according to the regimens.
| Mean ± standard deviation | Median (minimum – maximum) | P | |
|---|---|---|---|
| Height (cm) | |||
| CAPOX (n = 106) | 163.73 ± 8.91 | 164 (136 − 178) | 0.142 |
| FOLFOX (n = 137) | 166.85 ± 9.34 | 168 (139 − 196) | |
| Weight (kg) | |||
| CAPOX (n = 106) | 72.5 ± 12.8 | 70.5 (45 − 99) | 0.645 |
| FOLFOX (n = 137) | 73.25 ± 12.36 | 71.5 (38 − 120) | |
| BMI (kg/m2) | |||
| CAPOX (n=106) | 27.06 ± 4.47 | 26.75 (17.78 − 43.16) | 0.196 |
| FOLFOX (n = 137) | 26.34 ± 4.14 | 26.1 (12.87 − 42.02) | |
| BSA (m2) | |||
| CAPOX (n = 106) | 1.77 ± 0.18 | 1.79 (1.32 − 2.1) | 0.335 |
| FOLFOX (n = 137) | 1.79 ± 0.16 | 1.8 (1.21 − 2.1) | |
| Age (years) | |||
| CAPOX (n = 106) | 63.37 ± 10.15 | 64 (39 − 83) | 0.036 |
| FOLFOX (n = 137) | 60.39 ± 9.72 | 62 (34 − 81) | |
| Operation to chemotherapy (days) | |||
| CAPOX (n = 106) | 45.6 ± 24.4 | 43 (13 − 229) | 0.602 |
| FOLFOX (n = 137) | 46.46 ± 21.69 | 44 (15 − 188) | |
CAPOX: capecitabine and oxaliplatin; FOLFOX: 5-fluorouracil, leucovorin, and oxaliplatin; BMI: body mass index; BSA: body surface area.
The effects of genetic mutations, type of surgery, location of involvement of the colon, and the degree of histological differentiation are shown in Table 4. Disease progression was significantly higher in patients with a KRAS mutation, an NRAS mutation, and moderately/poorly differentiated cases (p = 0.05, p = 0.023, and p = 0.002, respectively). Additionally, an increased number of metastatic lymph nodes and prolonged time from surgery to chemotherapy were significantly associated with disease progression (p = 0.0001 and p = 0.02, respectively).
Table 4.
Effects of genetic mutations, type of surgery, location of involvement, and the degree of histological differentiation.
| Progression |
|||
|---|---|---|---|
| No | Yes | P | |
| KRAS | |||
| Wild | 23 (52.27%) | 26 (34.21%) | 0.05 |
| Mutant | 21 (47.73%) | 50 (65.79%) | |
| NRAS | |||
| Wild | 21 (80.77%) | 26 (54.17%) | 0.023 |
| Mutant | 5 (19.23%) | 22 (45.83%) | |
| BRAF | |||
| Wild | 21 (70%) | 32 (56.1%) | 0.208 |
| Mutant | 9 (30%) | 25 (43.9%) | |
| Operation | |||
| Elective | 136 (87.18%) | 73 (83.91%) | 0.481 |
| Urgent | 20 (12.82%) | 14 (16.09%) | |
| Tumor side | |||
| Right | 59 (37.82%) | 34 (39.08%) | 0.846 |
| Left | 97 (62.18%) | 53 (60.92%) | |
| Differentiation | |||
| Good | 10 (6.41%) | 6 (6.9%) | 0.002 |
| Moderate | 67 (42.95%) | 18 (20.69%) | |
| Poor | 79 (50.64%) | 63 (72.41%) | |
| Operation to chemotherapy (days) | 44.57 ± 23.51 | 48.8 ± 21.53 | 0.022 |
| Total lymph nodes (n) | 14.8 ± 5.9 | 15.9 ± 5.7 | 0.143 |
| Metastatic lymph nodes (n) | 2.5 ± 3 | 3.3 ± 2.88 | 0.0001 |
Adverse events were evaluated using the Common Terminology Criteria for Adverse Events version 4 v4.03.9 No Grade 4 adverse events were observed in any of the patients. Patients who experienced no adverse events were grouped in one arm and those with Grades 1 to 3 adverse events were grouped in another arm. Adverse events secondary to CAPOX and FOLFOX regimens are shown in Table 5. The frequency of dose reduction of chemotherapy and the rate of treatment discontinuation were higher in the CAPOX arm than in the FOLFOX arm (p = 0.02 and p = 0.007, respectively). Hand-foot syndrome was significantly more common in the CAPOX arm than in the FOLFOX arm (p = 0.008).
Table 5.
Adverse events secondary to CAPOX and FOLFOX regimens.
| Chemotherapy |
|||
|---|---|---|---|
| CAPOX, n (%) | FOLFOX, n (%) | P | |
| Neutropenia | |||
| No | 54 (50.94) | 74 (54.01) | 0.634 |
| Yes | 52 (49.06) | 63 (45.99) | |
| Thrombocytopenia | |||
| No | 75 (70.75) | 104 (75.91) | 0.365 |
| Yes | 31 (29.25) | 33 (24.09) | |
| Anemia | |||
| No | 70 (66.04) | 100 (72.99) | 0.241 |
| Yes | 36 (33.96) | 37 (27.01) | |
| Neuropathy | |||
| No | 72 (67.92) | 103 (75.18) | 0.211 |
| Yes | 34 (32.08) | 34 (24.82) | |
| Hepatotoxicity | |||
| No | 103 (97.17) | 130 (94.89) | 0.52 |
| Yes | 3 (2.83) | 7 (5.11) | |
| Hand-foot syndrome | |||
| No | 90 (84.91) | 130 (94.89) | 0.008 |
| Yes | 16 (15.09) | 7 (5.11) | |
| Diarrhea | |||
| No | 58 (54.72) | 80 (58.39) | 0.566 |
| Yes | 48 (45.28) | 57 (41.61) | |
| Dose reduction of chemotherapy | |||
| No | 87 (82.08) | 126 (91.97) | 0.02 |
| Yes | 19 (17.92) | 11 (8.03) | |
| Discontinuation of chemotherapy | |||
| No | 95 (89.62) | 134 (97.81) | 0.007 |
| Yes | 11 (10.38) | 3 (2.19) | |
CAPOX: capecitabine and oxaliplatin; FOLFOX: 5-fluorouracil, leucovorin, and oxaliplatin.
The effects of the applied chemotherapy protocols on disease progression, development of metastasis, and the final health condition of patients with stage III colon cancer are shown in Table 6. Disease progression, development of metastasis, and the mortality rate of patients were significantly higher in the FOLFOX arm than in the CAPOX arm (p = 0.016, p = 0.001, p = 0.007, respectively).
Table 6.
Effects of chemotherapy protocols on disease progression, development of metastasis, and mortality rate.
| Chemotherapy |
P | ||
|---|---|---|---|
| CAPOX, n (%) | FOLFOX, n (%) | ||
| Progression | |||
| No | 77 (72.64) | 79 (57.66) | 0.016 |
| Yes | 29 (27.36) | 58 (42.34) | |
| Final status | |||
| Alive | 89 (83.96) | 88 (64.23) | 0.001 |
| Dead | 17 (16.04) | 49 (35.77) | |
| Metastasis | |||
| No | 78 (73.58) | 78 (56.93) | 0.007 |
| Yes | 28 (26.42) | 59 (43.07) | |
CAPOX: capecitabine and oxaliplatin; FOLFOX: 5-fluorouracil, leucovorin, and oxaliplatin.
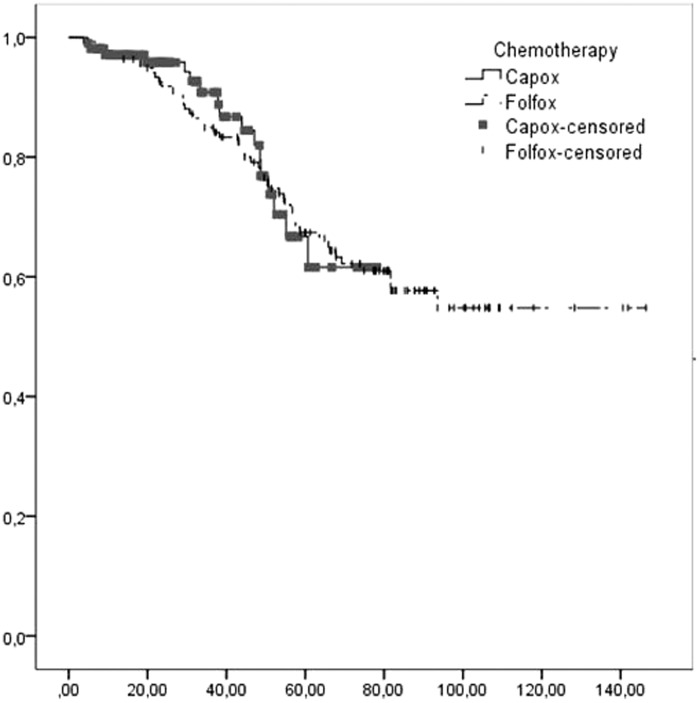
OS in high-risk patients for disease progression (TNM T4-N2-N3) was significantly shorter compared with that in low-risk patients (TNM T1-T2-T3-N1) (p = 0.006, Table 7). There was no significant difference in OS between the CAPOX and FOLFOX arms (Figure 1). Therefore, none of the chemotherapy regimens was superior to another in terms of OS in subgroup analyses.
Table 7.
Overall survival according to risk groups.
| Alive, n (%) | Dead, n (%) | Mean | Standard deviation | 95% CI | P | |
|---|---|---|---|---|---|---|
| T4-N2-N3 | 31 (62) | 19 (38) | 68.930 | 6.900 | 55.410–82.460 | 0.006 |
| T1-T2-T3-N1 | 146 (76) | 47 (24) | 105.780 | 4.800 | 96.360–115.190 |
CI: confidence interval.
Figure 1.
Kaplan–Meier overall survival curve according to chemotherapy.
Discussion
Currently, the prognosis of colon cancer is best defined by the American Joint Committee on Cancer classification.8 Consistent with previous findings,8 disease progression was significantly higher in patients with a higher number of metastatic lymph nodes and high-risk patients (T4-N2-N3) in our study. Additionally, we found a negative effect of poor histological differentiation and the presence of KRAS/NRAS mutations on disease progression. In the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial, addition of oxaliplatin to fluorouracil and leucovorin increased 5-year progression-free survival from 67.4% to 73.3% and 6-year OS from 76% to 78.5%.4 Increased survival and a decreased mortality rate were achieved with adjuvant chemotherapy in patients with locally advanced (stage III) colon cancer.
An adjuvant treatment alternative in stage III patients is the CAPOX regimen. This regimen consists of intravenous oxaliplatin and capecitabine, which is an oral prodrug. Capecitabine is metabolized in the liver and reduced to 5-fluorouracil. Hospitalization of patients for 48 hours or placement of a subcutaneous vascular port is unnecessary because the CAPOX regimen contains no continuous infusion treatment. In a study by Aitini et al.7, the CAPOX regimen was more cost-effective compared with the FOLFOX regimen, as the adjuvant treatment of colon cancer. Therefore, the CAPOX regimen is primarily preferred in clinical practice. The use of capecitabine was also found to be non-inferior to 5-fluorouracil and folinic acid combination in the X-ACT study.5 Additionally, similar results were obtained with the CAPOX and FOLFOX treatment regimens in the NO169968 study.6 Although both combined chemotherapy regimens appear to have a similar efficacy profile, there has been no head-to-head prospective, randomized study that compared the two protocols in terms of efficacy, safety, and survival rates in the literature.
In a meta-analysis, Des Guetz et al.10 showed that a delayed time from surgery to the initiation of chemotherapy (longer than 8 weeks) decreased the OS rate in patients with stage III colorectal cancer. In the present study, we found no significant difference in the time from surgery to initiation of either chemotherapy regimen, which is inconsistent with previous findings.11 However, when the total patient group was considered, a delay in treatment caused a significant increase in the rate of disease progression.
The CAPOX regimen is preferred in patients with comorbid coronary artery disease. Additionally, the CAPOX regimen is mostly used in young patients,12 although this regimen was used more often in older patients in our study. In our study, the CAPOX regimen was predominantly used in older patients because of existing comorbidities, which may develop secondary to vascular port intervention in this patient population and there is a risk of development of nosocomial infections secondary to hospitalization for fluorouracil infusion treatment. Sara et al.13 also reported an increased incidence of coronary vasospasm, chest pain, angina, and myocardial infarction during fluorouracil bolus and continuous infusion compared with oral capecitabine use. We administered CAPOX in patients who were diagnosed with coronary artery disease to avoid the risk of cardiotoxicity.
The adverse event profile of the CAPOX and FOLFOX regimens is different among previous studies. Mamo et al.14 reported that nausea, diarrhea, neutropenia, and peripheral sensorial neuropathy were more frequent in the FOLFOX arm than in the CAPOX arm. However, Loree et al.15 found that mucositis and neutropenia in the FOLFOX arm and diarrhea and hand-foot syndrome in the CAPOX arm were more frequent. In our study, hand-foot syndrome was significantly more common in the CAPOX arm than in the FOLFOX arm. However, the rate of myelotoxic side effects, such as neutropenia, and other side effects (nausea, diarrhea, mucositis, neuropathy) were similar in both arms.
Loree et al.11 found that DFS was significantly higher in the CAPOX arm than in the FOLFOX arm in their first retrospective study that included 176 patients. These authors also reported a significantly higher DFS with CAPOX in their subsequent study that included 394 patients.15 However, there was no significant difference in the OS rate between the two studies. In our study, development of disease progression, development of metastasis, and the rate of mortality were higher in the FOLFOX arm than in the CAPOX arm. However, we found no significant difference in the OS rate between the CAPOX and FOLFOX arms.
Limitations of the present study include its retrospective nature, short duration of follow-up, and the low rate of expected events.
Conclusion
In this study, we used a real-life experience to describe baseline characteristics, the operation to chemotherapy interval, toxicity, and effect of FOLFOX and CAPOX on clinical outcomes in patients treated with either FOLFOX or CAPOX in the adjuvant setting in two different institutional practices in the western Anatolia region. The CAPOX regimen was preferred in older patients. Disease progression, metastasis, and the mortality rate were higher in the FOLFOX arm than in the CAPOX arm.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.http://kanser.gov.tr/daire-faaliyetleri/kanser-istatistikleri/2106-2014 y%C4%B1l%C4%B1-t%C3%BCrkiye-kanser-istatistikleri.html Ulaşılma tarihi 16/12/2017.
- 2.Aykan NF, Yalçin S, Turhal NS, et al. Epidemiology of colorectal cancer in Turkey: a cross-sectional disease registry study (A Turkish Oncology Group trial). Turk J Gastroenterol 2015; 26: 145–153. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990; 322: 352–358. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–3116. [DOI] [PubMed] [Google Scholar]
- 5.Twelves C, Scheithauer W, Mckendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012; 23: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 6.Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015; 33: 3733–3740. [DOI] [PubMed] [Google Scholar]
- 7.Aitini E, Rossi A, Morselli P, et al. Economic comparison of capecitabine-oxaliplatin and 5-fluorouracil-oxaliplatin in the adjuvant treatment of colon cancer. Cancer Manag Res 2012; 4: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed New York: Springer-Verlag, 2010. [Google Scholar]
- 9.National Institutes of Health. Common terminology criteria for adverse events.version 4. Bethesda: US Department of Health and Human Services, 2010. [Google Scholar]
- 10.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010; 46: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 11.Loree JM, Mulder KE, Ghosh SH, et al. CAPOX associated with toxicities of higher grade but improved disease free survival when compared with FOLFOX in the adjuvant treatment of stage III colon cancer. Clin Colorectal Cancer 2014; 13: 172–177. [DOI] [PubMed] [Google Scholar]
- 12.Sha A, Abadi S, Gill S. Utilization of capecitabine plus oxaliplatin and 5-fluorouracil/folinic acid plus oxaliplatin in the adjuvant treatment of stage IIB and stage III colon cancer: a multi-centre, retrospective, chart review study. J Oncol Pharm Pract 2017; 24: 501–506. [DOI] [PubMed] [Google Scholar]
- 13.Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol 2018; 10: 1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamo A, Easaw J, Ibnshamsah F, et al. Retrospective analysis of the effect of CAPOX and mFOLFOX6 dose intensity on survival in colorectal patients in the adjuvant setting. Curr Oncol 2016; 23: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loree JM, Sha A, Soleimani M, et al. Survival impact of CAPOX versus FOLFOX in the adjuvant treatment of stage III colon cancer. Clin Colorectal Cancer 2018; 17: 156–163. [DOI] [PubMed] [Google Scholar]