Short abstract
Morphological magnetic resonance imaging is currently the best imaging technique for local staging in patients with rectal cancer. However, morphological sequences have some limitations, especially after preoperative chemoradiotherapy (pCRT). Diffusion-weighted imaging has been applied to rectal cancer for detection of lesions, characterization of tissue, and evaluation of the response to therapy. In 2005, a non-Gaussian diffusion model called diffusion kurtosis imaging (DKI) was suggested.
Several electronic databases were evaluated in the present review. The search included articles published from January 2000 to May 2018. The references of all articles were also evaluated. All titles and abstracts were assessed, and only the studies of DKI in patients with rectal cancer were retained.
We identified 35 potentially relevant references through the electronic search. According to the inclusion and exclusion criteria, we retained five clinical studies that met the inclusion criteria.
DKI is a useful tool for assessment of tumor aggressiveness, the nodal status, and the risk of early metastases as well as prediction of the response to pCRT. The results of DKI should be considered in treatment decision-making during the work-up of patients with rectal cancer.
Keywords: Rectal cancer, magnetic resonance imaging, diffusion kurtosis imaging, diffusion-weighted imaging, preoperative chemoradiotherapy, retrospective review
Background
Rectal cancer is one of the most common oncological diseases worldwide. According to Siegel et al.,1 the incidence of colorectal cancer is usually comparable between the two sexes; from 2005 through 2014, the incidence rates of colorectal cancer decreased by about 2% to 3% annually. Declines in the incidence of cancer are due to modifications in risk factors and the increased use of screening tests. However, despite widespread use of screening, cancer in some patients is identified in an advanced stage.1 The standard of care for patients with locally advanced rectal cancer (LARC) is preoperative chemoradiotherapy (pCRT) followed by total mesorectal excision.2–4 In addition, for patients with a complete response to pCRT, there has been a surge of conservative management and the “wait-and-see” strategy.5 The benefit of this policy is a decreased morbidity rate and the opportunity to administer “true” organ-sparing therapy.5 In this setting, identification of the features that can accurately stratify patients according to their characteristics is required so that the most effective therapeutic strategy can be chosen.5 Morphological magnetic resonance imaging (mMRI), performed with T2-weighted sequences, is currently considered the best imaging technique for local rectal staging.3,4 However, morphological sequences have some limitations, especially after pCRT, because the tumor necrosis may not match the volumetric decrease. To overcome this limitation, functional parameters to evaluate tissue viability obtained by position emission tomography, dynamic contrast-enhanced MRI, and diffusion-weighted imaging (DWI) are being assessed. The practice of merging DWI into a standard MRI protocol is increasing because of the ability of DWI to detect and characterize lesions, which enhances its capability in the assessment of the treatment response,6–10 thus increasing clinical confidence and decreasing false positives.11,12 DWI assessment may be performed qualitatively or quantitatively with a mono-exponential analysis [apparent diffusion coefficient (ADC) map] or bi-exponential analysis [intravoxel incoherent motion (IVIM) model] or with diffusion kurtosis imaging (DKI) based on a kurtosis model. The DWI signal is due to the water mobility that indirectly reproduces tissue structures.10–12 The ADC map is a graphical illustration of the ratio of DW signal intensities. However, the ADC values may be limited by a lack of reproducibility.10–12 The ADC values can be more precisely assessed by obtaining a large number of b values.10–12 Traditionally, the DWI approach to data analysis was founded on the hypothesis that water molecules diffuse within a voxel following a single direction with Gaussian behavior without any restriction.13,14 However, according to the presence of microstructures, water molecules within biologic tissues exhibit a non-Gaussian phenomenon called DKI as proposed by Jensen and Helpern13 in 2010. This approach evaluates the kurtosis coefficient (K), which shows the deviance of diffusion from a Gaussian approach, and the diffusion coefficient (D) with the correction of non-Gaussian bias. Several studies have shown that DKI is more accurate than traditional ADC mapping in tumor detection and grading assessment.14–20 DWI using quantitative parameters (ADC and DKI) could serve as an imaging biomarker to more effectively choose patients who will benefit from more aggressive neoadjuvant treatment.3,8
The aim of this study was to provide an overview and update of the current status of DKI in patients with rectal cancer and evaluate the future perspectives.
Methods
Search criteria
Several electronic databases were used for the literature search: Scopus (Elsevier, http://www.scopus.com/), PubMed (US National Library of Medicine, http://www.ncbi.nlm.nih.gov/pubmed), Web of Science (Thomson Reuters, http://apps.webofknowledge.com/), and Google Scholar (https://scholar.google.it/). We used the following search terms: “rectal cancer” AND “diffusion kurtosis imaging” AND “prognostic factors,” “rectal cancer” AND “diffusion kurtosis imaging” AND “assessment post neoadjuvant therapy,” and “rectal cancer” AND “diffusion kurtosis imaging” AND “distant metastases.” The search included articles published from January 2000 to May 2018. Moreover, the reference lists of the identified articles were evaluated. All titles and abstracts were examined, and only the studies on DKI in patients with rectal cancer were retained. The inclusion criteria were clinical studies that assessed DKI in patients with rectal cancer for clinical pathologic evaluation, clinical studies that estimated DKI in patients with rectal cancer for prognostic assessment, and clinical studies that estimated DKI in patients with rectal cancer for post-neoadjuvant therapy assessment. Papers published in the English language from January 2000 to May 2018 were retained. The exclusion criteria were inaccessibility of the full text, overview articles, and articles with topics other than DKI in patients with rectal cancer.
Ethics
Study approval by an ethics committee or institutional review board was not required because of the retrospective nature of this review.
Results
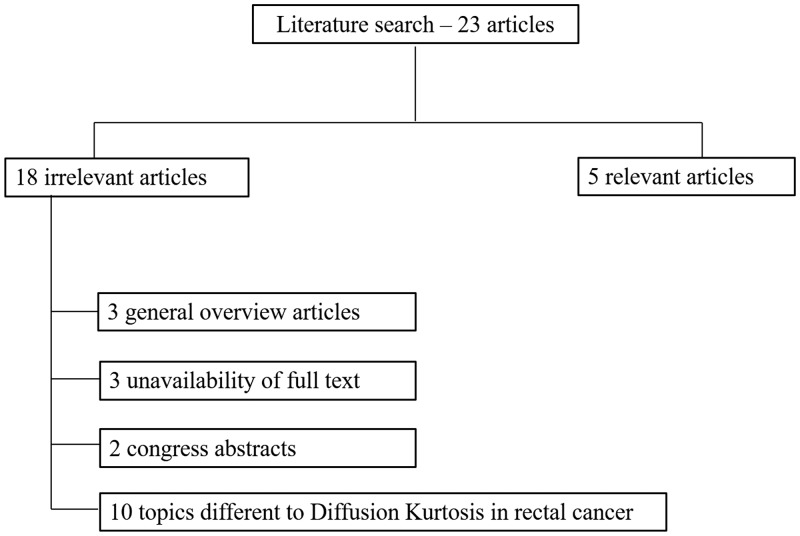
We evaluated 35 potentially pertinent references. After removing 12 duplicates, we obtained 23 papers. We omitted 18 papers that met the exclusion criteria. Finally, five articles met the inclusion criteria. A study flow diagram is shown in Figure 1.
Figure 1.
Studies included and excluded from the systematic review.
Discussion
The multidisciplinary management of patients with LARC is mainly based on risk stratification for local or distant recurrence. Prognostic features include the TNM stage, histological grade, presence of peritumor lymphangiovascular invasion (LVI) or neural invasion, presence of circumferential margin (CRM) involvement, and pretreatment carcinoembryonic antigen serum level.21,22 In 2016, Zhu et al.23 evaluated the role of DKI (b = 0, 700, 1400, and 2100 s/mm2) and conventional DWI (b = 0 and 1000 s/mm2) in 56 patients with rectal adenocarcinoma. Kurtosis and diffusivity from DKI and ADC were extracted. Zhu et al evaluated the correlation between kurtosis, diffusivity, ADC, the local pT and pN stages, and the performance of kurtosis, diffusivity, and ADC to differentiate high- and low-grade rectal adenocarcinoma and the correlation between kurtosis, diffusivity, ADC, and histologic grades. Kurtosis was significantly higher in tumors with nodal involvement (pN1–2) than in subgroups without nodal involvement (pN0). In the grading of poorly differentiated clusters (PDCs), significant differences were found in kurtosis, diffusivity, and ADC between lesions with low and high PDC grades. A receiver operating characteristic analysis was performed, and the areas under the curve (AUCs) were 0.905, 0.768, and 0.750 for kurtosis, diffusivity, and ADC, respectively. Kurtosis showed higher sensitivity and specificity than did diffusivity and ADC. According to the World Health Organization (WHO) grading criteria, kurtosis was also significantly higher in high- than low-grade tumors, and its sensitivity and specificity in the identification of high- and low-grade tumors were 92.9% and 50.0%, respectively, with an AUC of 0.696. In contrast, no significant differences in diffusivity or ADC were found between low- and high-grade tumors. Kurtosis showed a higher AUC in differentiating high- from low-grade tumors with PDC grading than with the WHO system. Kurtosis showed a significantly high correlation with the PDC grade, whereas diffusivity and ADC exhibited a negative relationship with the PDC grade. In addition, kurtosis showed a positive correlation with the WHO grade, while diffusivity and ADC showed no relationship with the WHO grade. Therefore, according to Zhu et al.,23 DKI has a greater diagnostic capability in distinguishing high- from low-grade rectal cancer than do diffusivity and ADC, also showing a higher ability to identify patients with and without nodal involvement (Figure 2). Thus, the authors suggested that DKI could serve as an imaging biomarker for grading rectal cancers and predicting lymph node involvement in clinical practice.23 Like Zhu et al.,23 Cui et al.24 also evaluated the relationships between DKI and clinicopathological prognostic factors in patients with LARC. They evaluated 79 patients with rectal cancer with a 3T system. Conventional DWI (b = 0 and 1000 s/mm2) and DKI (b = 0, 700, 1400, and 2100 s/mm2) analyses were performed. The prognostic features that were evaluated were the plasma carcinoembryonic antigen level, pT stage, pN stage, tumor grade, peritumor LVI or neural invasion, and CRM invasion. Cui et al.24 showed that kurtosis was significantly associated with the lymph node status, histological grade, and presence of LVI or CRM involvement. Conversely, ADC and diffusivity were significantly associated with the T and N stages. These finding suggest that DKI should be effective as a noninvasive biomarker of tumor aggressiveness.24
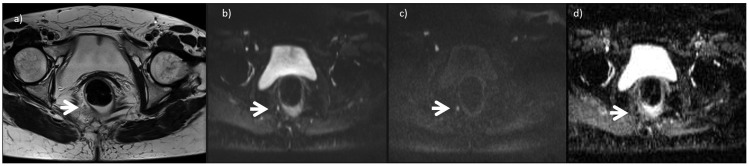
Figure 2.
A 43-year-old woman with rectal cancer. (a) A T2-weighted turbo-spin echo image in the axial plane is shown. The arrow shows a small node with restricted diffusion (b) Diffusion-weighted imaging sequence at b50 (50 s/mm2). (c) Diffusion-weighted imaging sequence at b1500 (1500 s/mm2). (d) Apparent diffusion coefficient map. These findings confirmed that the resected specimen was a neoplastic nodule.
Yu et al.25 recently assessed the diagnostic value of DKI for discriminating between benign and malignant lymph nodes in patients with rectal carcinoma. They evaluated 85 patients with rectal adenocarcinoma who underwent total mesorectal excision. MRI examinations were performed using a 3.0-Tesla MRI scanner; conventional DWI (b = 0 and 1000 s/mm2) and DKI (b = 0, 700, 1400, and 2000 s/mm2) were performed. In total, 273 lymph nodes were harvested and subjected to histological analysis. Quantitative parameters [apparent diffusion parameter of Gaussian distribution (Dapp), apparent kurtosis coefficient (Kapp), and ADC] of lymph nodes were derived from DKI. They showed that the median Dapp and ADC values of metastatic lymph nodes were significantly greater than those of benign lymph nodes, whereas the median Kapp of metastatic lymph nodes was statistically lower than that of normal lymph nodes. Dapp had the highest AUC of 0.774. When 1126.15 × 10−6 mm2 s−1 was used as the Dapp threshold value, the sensitivity and specificity were 96.97% and 41.82%, respectively. Therefore, the authors concluded that the kurtosis model is useful for differentiation between metastatic and benign lymph nodes. Lymph nodes with higher Dapp and ADC values were significantly more likely to be metastatic. The use of DKI in patients with rectal carcinoma may provide a new means of improving the ability to predict the presence of metastatic lymph nodes.25
The results reported by Zhu et al.,23 Cui et al.,24 and Yu et al.25 suggest that DKI has a positive correlation with the nodal status. This element may guide decisions regarding proper therapy considering that patients with a positive nodal status may be subjected to neoadjuvant therapy and extended pelvic surgery and that detection of metastatic lymph nodes remains challenging in current clinical practice.
Another prognostic feature of patients with rectal cancer is the presence of distant metastases, which drastically changes the prognosis and treatment. In 2016, Yu et al.26 evaluated the relationship of DWI and DKI in patients with distant metastases of rectal carcinoma. They evaluated 58 patients (27 with metastasis and 31 without metastasis). The ADC parameter from standard DWI (b values of 0 and 1000 s/mm2) and the Dapp (10−3 mm2/s) and Kapp from DKI (b values of 0, 700, 1400, and 2000 s/mm2) were calculated from the entire volume of the tumors. The researchers assessed the pT stage, pN stage, and tumor grade. They found that Dapp was significantly lower in patients with than without distant metastasis. The ADC 10th values showed a similar trend. However, the authors demonstrated an improved goodness of Dapp for discerning distant metastasis. In fact, the higher AUC and specificity with the DKI model than standard DWI model is due to the limits of conventional DWI based on the fact that water molecules diffuse following a Gaussian model. However, the cellular microstructure is more complex and heterogeneous in high- than low-grade lesions, and the more frequent mitosis and higher proliferative ability in neoplastic tissues can increase the density to affect water diffusion in each voxel.26 The possibility of using DKI as a tool to identify patients at risk of early distant metastasis affects treatment decision-making and allows proper stratification of patients.
The standard of care for patients with LARC is pCRT followed by total mesorectal excision.2–4 Therefore, an essential point during the work-up of patients with rectal cancer is the rapid identification of patients who respond (Figure 3) and do not respond to therapy (Figure 4). Considering that the treatment effectiveness and therefore the severity of necrosis does not always translate into volumetric reduction of the tumor, therapy should be assessed by evaluating the functional changes in the target tissue rather than by the changes in tumor size. Functional data obtained by DWI reflect the tissue viability and allow differentiation of fibrosis from residual tumor tissue.2–4 The imaging modalities that enable assessment of tumor perfusion and diffusion have the capability of improving patient management because the percentage change of perfusion is discriminated with high specificity in responding and non-responding patients.27 Because cellular death and vascular changes can occur before changes in lesion size during treatment, DWI may serve as an early biomarker of treatment effectiveness.27 Only a few studies have been performed to evaluate the role of DKI in assessing the treatment response in patients undergoing neoadjuvant CRT for LARC.28,29 Yu et al.28 evaluated the feasibility and clinical value of DKI in the assessment of neoadjuvant treatment for LARC. In their study, 41 patients underwent MRI before and 7 weeks after neoadjuvant CRT. DWI was evaluated using b values of 0, 700, 1400, and 2000 s/mm2. Histogram analysis of Dapp, Kapp, and ADC were calculated. The researchers evaluated the mean, median, and 10th and 90th percentiles. According to the method described by Mandard et al.,30 patients with pTRG-1 or pTRG-2 were considered good responders and patients with pTRG-3 to 5 were considered poor responders (Figure 4). The results reported by Yu et al.28 suggest that the percentage change in Dapp has higher diagnostic performance for evaluating therapy. Among the pre-CRT data, the histogram indices (medians and 10th percentiles of the pre-Dapp) were associated with the pTRG. In the identification of prognostic features, the advantage of DKI over standard DWI is that after CRT, the tissue microstructure is more complex and heterogeneous, and the spread of water molecules thus follows a non-Gaussian model.13 The opportunity to identify a good responder to neoadjuvant therapy allows the multidisciplinary team to personalize patient treatment: a patient with a complete response could be subject to a “wait-and-see” approach with the possibility of “true” organ-sparing treatment.5 Hu et al.29 evaluated the role of DKI in assessing a pathological complete response in patients with LARC compared with conventional DWI. Fifty-six patients underwent MRI using a 3.0-T scanner (DWI sequence with b values of 0, 700, 1400, and 2100 s/mm2) before and after CRT. The ADC map, MD (mean diffusion) map, and MK (mean kurtosis) map were calculated. They showed that the MKpre and MKpost values were much lower for responder than non-responder patients. The ADCpost and the rate of change in the ADC were significantly higher for the responder patients. The MDpost and the rate of change in the MD also increased, whereas no significant difference was noted in the ADCpre, MDpre, or rate of change in the MK value between responder and non-responder patients. The MKpost had higher sensitivity and specificity than other data. Therefore, Hu et al.29 demonstrated that both DKI and conventional DWI could predict the response to treatment and that MKpost has higher specificity than DWI data for assessing responders patients. Fusco et al.31 assessed the preoperative short-course radiotherapy tumor response in patients with LARC by means of the standardized index of shape using dynamic contrast-enhanced MRI and by means of the ADC, IVIM, and DKI parameters derived from diffusion-weighted MRI. The standardized index of shape, ADC, IVIM parameters [tissue diffusion (Dt), pseudo-diffusion (Dp), and perfusion fraction (fp)], and DKI parameters (MD and MK) were calculated for each patient. The IVIM parameters were estimated using two methods: conventional bi-exponential fitting and variable projection (VARPRO). The mean and standard deviation of the pretreatment Dp obtained by conventional bi-exponential fitting and the mean pretreatment Dp obtained by VARPRO showed statistically significant differences in the prediction of a pathological complete response (p value by Mann–Whitney test = 0.05, 0.03, and 0.008, respectively). The best results for prediction of a pathological complete response were obtained by the mean pretreatment VARPRO Fp value with an AUC of 0.84, sensitivity of 96.4%, specificity of 71.4%, positive predictive value of 92.9%, negative predictive value of 83.3%, and accuracy of 91.2%. Promising initial results were obtained using a decision tree tested with all ADC, IVIM, and DKI extracted parameters: high accuracy for assessment of a pathological complete response was reached after short-course radiotherapy in patients with LARC (accuracy of 85.3% to assess a pathological complete response after short-course radiotherapy using the post-treatment VARPRO Dp mean value, pretreatment ADC standard deviation value, and post-treatment MD standard deviation value). Therefore, the pretreatment VARPRO Fp mean value and a decision tree composed of diffusion parameters derived by DWI and DKI played an important prognostic role in assessing a pathological complete response.
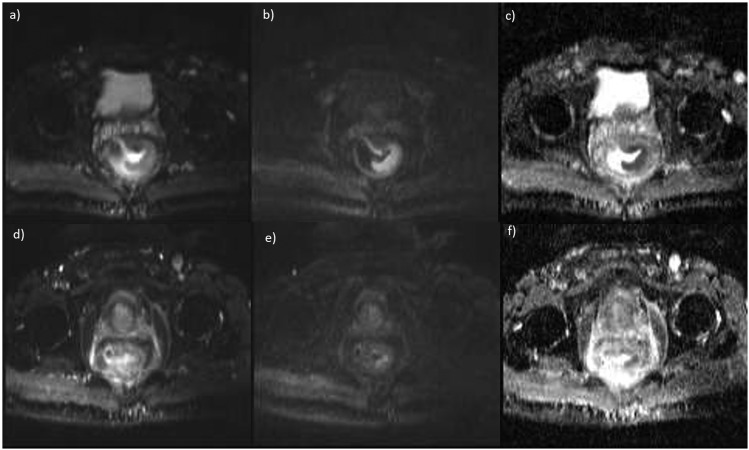
Figure 3.
Diffusion-weighted magnetic resonance images and apparent diffusion coefficient (ADC) map for a complete responder (a–c) before and (d–f) after treatment. Tumor regression grade = 1 and ΔADC = 56%.
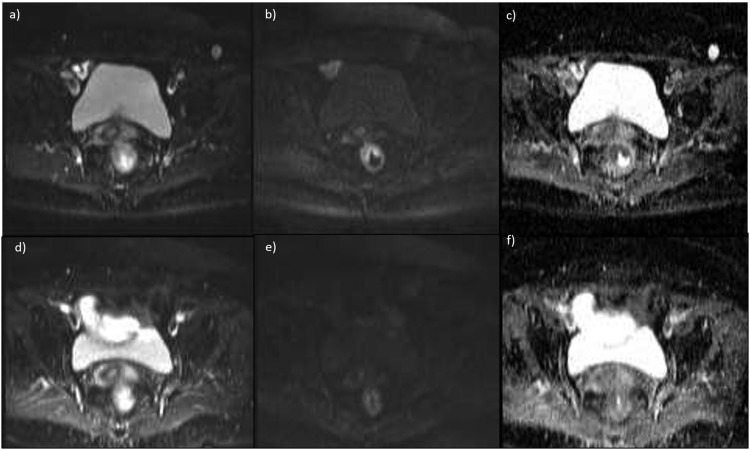
Figure 4.
Diffusion-weighted magnetic resonance images and apparent diffusion coefficient (ADC) map for a partial responder (a–c) before and (d–f) after treatment. Tumor regression grade = 4 and ΔADC = 18%.
DKI is a useful tool in the assessment of patients with rectal cancer; however, it is essential that DKI is a reproducible evaluation method. Therefore, standardization of the sequence is mandatory; standardization of kinetic model application and analysis methodology is also mandatory to calculate derivate quantitative parameters.
Conclusions
DKI is a useful tool in identifying tumor aggressiveness, the nodal status, and patients at risk for early metastases as well as predicting the response to neoadjuvant CRT. The findings of DKI should be considered in treatment decision-making during the work-up of patients with rectal cancer. However, DKI standardization is required to establish the hardware, software, models, and analysis methodology to calculate quantitative parameters.
Acknowledgments
The authors are grateful to Alessandra Trocino, librarian at the National Cancer Institute of Naples, Italy. The authors are also grateful to Rita Guarino and Assunta Zazzaro for their collaboration.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Petrillo A, Fusco R, Granata V, et al. MR imaging perfusion and diffusion analysis to assess preoperative Short Course Radiotherapy response in locally advanced rectal cancer: standardized Index of Shape by DCE-MRI and intravoxel incoherent motion-derived parameters by DW-MRI. Med Oncol 2017; 34: 198. doi: 10.1007/s12032-017-1059-2 [DOI] [PubMed] [Google Scholar]
- 3.Fusco R, Petrillo M, Granata V, et al. Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: a systematic review. Radiol Oncol 2017; 51: 252–262. doi: 10.1515/raon-2017-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrillo A, Fusco R, Petrillo M, et al. Standardized index of shape (DCE-MRI) and standardized uptake value (PET/CT): two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017; 8: 8143–8153. doi: 10.18632/oncotarget.14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gani C, Bonomo P, Zwirner K, et al. Organ preservation in rectal cancer - challenges and future strategies. Clin Transl Radiat Oncol 2017; 3: 9–15. doi: 10.1016/j.ctro.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delli Pizzi A, Basilico R, Cianci R, et al. Rectal cancer MRI: protocols, signs and future perspectives radiologists should consider in everyday clinical practice. Insights Imaging 2018; 9: 405–412. doi: 10.1007/s13244-018-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fusco R, Sansone M, Petrillo A. A comparison of fitting algorithms for diffusion-weighted MRI data analysis using an intravoxel incoherent motion model. MAGMA 2017; 30: 113–120. doi: 10.1007/s10334-016-0591-y [DOI] [PubMed] [Google Scholar]
- 8.Petrillo M, Fusco R, Catalano O, et al. MRI for assessing response to neoadjuvant therapy in locally advanced rectal cancer using DCE-MR and DW-MR data sets: a preliminary report. Biomed Res Int 2015; 2015: 514740. doi: 10.1155/2015/514740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MJ, Kim SH, Lee SJ, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 2011; 260: 771–780. doi: 10.1148/radiol.11102135 [DOI] [PubMed] [Google Scholar]
- 10.Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011; 260: 734–743. doi: 10.1148/radiol.11102467 [DOI] [PubMed] [Google Scholar]
- 11.Feng Q, Yan YQ, Zhu J, et al. T staging of rectal cancer: accuracy of diffusion-weighted imaging compared with T2-weighted imaging on 3.0 tesla MRI. J Dig Dis 2014; 15: 188–194. doi: 10.1111/1751-2980.12124 [DOI] [PubMed] [Google Scholar]
- 12.Hausmann D, Liu J, Budjan J, et al. Image quality assessment of 2D versus 3D T2WI and evaluation of ultra-high b-value (b=2,000 mm/s(2)) DWI for response assessment in rectal cancer. Anticancer Res 2018; 38: 969–978. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010; 23: 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K, Chen X, Chai W, et al. Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 2015; 277: 46–55. [DOI] [PubMed] [Google Scholar]
- 15.Suo S, Chen X, Wu L, et al. Non-Gaussian water diffusion kurtosis imaging of prostate cancer. Magn Reson Imaging 2014; 32: 421–427. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira L, Brandão S, Matos E, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol 2014; 24: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Sigmund EE, Winnick A, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging 2012; 30: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 18.Van Cauter S, Veraart J, Sijbers J, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology 2012; 263: 492–501. [DOI] [PubMed] [Google Scholar]
- 19.Raab P, Hattingen E, Franz K, et al. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology 2010; 254: 876–881. [DOI] [PubMed] [Google Scholar]
- 20.Rosenkrantz AB, Sigmund EE, Johnson G, et al. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 2012; 264: 126–135. [DOI] [PubMed] [Google Scholar]
- 21.Cienfuegos JA, Rotellar F, Baixauli J, et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol 2015; 22: 916–923. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Jang HS, Kim JG, et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol 2012; 19: 1213–1221. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Pan Z, Ma Q, et al. Diffusion kurtosis imaging study of rectal adenocarcinoma associated with histopathologic prognostic factors: preliminary findings. Radiology 2017; 284: 66–76. doi: 10.1148/radiol.2016160094 [DOI] [PubMed] [Google Scholar]
- 24.Cui Y, Yang X, Du X, et al. Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: correlation with clinical pathologic prognostic factors. Eur Radiol 2018; 28: 1485–1494. doi: 10.1007/s00330-017-5094-3 [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Dai X, Zou HH, et al. Diffusion kurtosis imaging in identifying the malignancy of lymph nodes during the primary staging of rectal cancer. Colorectal Dis 2018; 20: 116–125. doi: 10.1111/codi.13835 [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Huang DY, Li Y, et al. Correlation of standard diffusion-weighted imaging and diffusion kurtosis imaging with distant metastases of rectal carcinoma. J Magn Reson Imaging 2016; 44: 221–229. doi: 10.1002/jmri.25137 [DOI] [PubMed] [Google Scholar]
- 27.Granata V, Fusco R, Catalano O, et al. Early assessment of colorectal cancer patients with liver metastases treated with antiangiogenic drugs: the role of intravoxel incoherent motion in diffusion-weighted imaging. PLoS One 2015; 10: e0142876. doi: 10.1371/journal.pone.0142876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Xu Q, Song JC, et al. The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 2017; 27: 1848–1857. doi: 10.1007/s00330-016-4529-6 [DOI] [PubMed] [Google Scholar]
- 29.Hu F, Tang W, Sun Y, et al. The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget 2017; 8: 75597–75606. doi: 10.18632/oncotarget.17491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–2686. [DOI] [PubMed] [Google Scholar]
- 31.Fusco R, Sansone M, Granata V, et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: a comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom Radiol (NY) 2018. doi: 10.1007/s00261-018-1801-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]