Short abstract
Objective
To compare genome-wide DNA methylation between samples of sinonasal inverted papilloma (SNIP) and squamous cell carcinoma (SCC) samples in order to identify aberrantly methylated genes that might be involved in malignant transformation.
Methods
Tissue samples were collected from patients. DNA methylation in C-phosphate-G islands and gene promoters was analysed using a DNA methylation microarray kit. The levels of mRNA or protein from aberrantly methylated genes were measured using real-time polymerase chain reaction or Western blot analysis.
Results
A total of 27 tissue samples were included in this study; 15 SNIP samples and 12 SCCs arising in SNIPs. A total of 11 201 nominally differentially methylated sites were observed between SNIP and SCC arising in SNIPs. Six sites were significantly different at P < 0.01 and contained three genes (MIR661, PLEC and OPA3). These three genes were hypermethylated. In addition, the levels of mature miR-661 mRNA and PLEC protein were significantly upregulated in SCC tissues compared with SNIP samples. The levels of OPA3 protein were downregulated in SCC tissues compared with SNIP samples.
Conclusions
This study demonstrated hypermethylation and abnormal expression of the MIR661, PLEC and OPA3 genes, suggesting a role for their involvement in the malignant transformation of SNIP.
Keywords: Sinonasal inverted papilloma, squamous cell carcinoma, methylation, C-phosphate-G islands, epigenetics
Introduction
Sinonasal tumours are rare malignancies with an annual worldwide incidence of approximately 1 in 100000.1 Histologically, sinonasal tumours are divided into sarcomas, olfactory neuroblastomas, mucosal melanomas and carcinomas. Over 55% of sinonasal tumours are carcinomas, which include squamous cell carcinomas (SCC), adenocarcinomas and adenoid cystic carcinoma.2,3 Inverted papillomas (IP) are common benign tumours of the nasal cavity and paranasal sinuses. The malignant transformation rate of IPs is less than 27%,4 but the histomorphology, immunohistochemistry and biological markers involved in their transformation remain unknown.5 It is therefore important to explore the molecular mechanisms underlying the malignant transformation of IPs, which could help identify biomarkers for improved diagnosis of sinonasal tumours and better targeted therapy.
Epigenetic regulation, via DNA methylation and microRNA (miRNA) regulation, plays a critical role in a variety of cancers including head and neck SCC.6,7 Hypomethylation and hypermethylation are thought to contribute to carcinogenesis by overexpressing tumour oncogenes and downregulating tumour suppressor genes.8 A global methylation study revealed that aberrant hypermethylation of gene promoters is involved in the pathogenesis of sinonasal papilloma.9 A single gene methylation study showed that low DLEC1 gene expression in sinonasal SCC tissues is associated with promoter hypermethylation.10 However, no study has reported the effects of genome methylation on the malignant transformation of sinonasal IP (SNIP). The aim of this current study was to identify the different methylated genes between SNIP tissue samples and samples of SCC arising in SNIPs.
Materials and methods
Study design
This retrospective tissue analysis study compared DNA methylation and gene expression between tissue samples of SNIP and SCC arising in SNIP samples. A comprehensive methylation profiling technique was used to investigate DNA methylation in C-phosphate-G (CpG) islands and gene promoter regions in tissue samples of SNIP and samples of SCC arising in SNIPs. Gene ontology analysis and network analysis were used to analyse gene function and to construct gene networks of aberrantly methylated genes. Expression of these aberrantly methylated genes was further validated using real-time polymerase chain reaction (PCR) or Western blot analysis in tissue samples of SNIP and samples of SCC arising in SNIPs. All of the SCCs arising in SNIP samples showed signs of malignant transformation of the SNIP based on histological analysis.
Study participants
Tissue samples were collected from surgical patients at Beijing Tongren Hospital, Capital Medical University, Beijing, China between January 2013 and December 2016. None of the patients had received chemotherapy, radiotherapy or biological therapy before sample collection. None of the patients had other types of cancer. Cancer was staged according to the 7th edition of the American Joint Committee on Cancer Staging Manual.11 All of the SCCs were arising in SNIPs and showed malignant progression. All tissues were snap-frozen in liquid nitrogen and stored at –80°C until further use.
Written informed consent was obtained from all patients involved in this study, which was approved by the Ethics Committee of the Beijing Tongren Hospital, Capital Medical University, Beijing, China (no. TRECKY2014-027).
DNA extraction, microarray analysis and analysis of aberrantly methylated genes
Genomic DNA from each cryopreserved tissue was extracted according to the user manual of the QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA). An EZ DNA Methylation™ Kit (ZymoResearch, Orange, CA, USA) was used to bisulfite the purified DNA. The methylation levels of over 485 000 individual cytosines were measured using an Infinium HumanMethylation450K BeadChip kit (Illumina, San Diego, CA, USA). The Infinium HumanMethylation450K BeadChip assay was performed according to the manufacturer’s instructions using an iScan™ SQ System (Illumina). The methylation level for each locus was designated as the β value, which was a quantitative measure of DNA methylation ranging from 0 (no cytosine methylation) to 1 (complete cytosine methylation) using Illumina GenomeStudio V2011.1 Methylation Module 1.9.0 (Illumina). Quality control was conducted by checking and removing the poorly performing probes or samples with the following exclusion criteria: (i) probes with a detection P-value above a certain cut-off (P < 0.05); (ii) samples that failed in any of the array control probes; (iii) probes that had a significant detection P-value in >10–25% of the samples; (iv) probes for sex chromosomes, single nucleotide polymorphism sites and cross-reaction. A differential methylation analysis of the Infinium HumanMethylation450K BeadChip array data was conducted using Illumina GenomeStudio V2011.1 Methylation Module 1.9.0 (Illumina), with the following Deltaβ and DiffScore calculations: Deltaβ = β(scc) – β(snip); and DiffScore = 10 sign (Deltaβ) log10(P). The Illumina DiffScore is a transformation of raw P-values and no multiple adjustment is applied. The differentially methylated site was defined when the DiffScore value was <–20 or >20 (corresponding to a P-value of 0.01), while the Deltaβ value was >0.17 or < –0.17 (corresponding to the degree of methylation of above the mean) between SNIP and SCC. Compared with SNIP, SCC tissues were identified as negative values for hypomethylation and positive values for hypermethylation, respectively.
Gene ontology analysis
The microarray data were subjected to gene ontology (GO) analysis as previously described.12 The over-represented GO terms were tested and the overlapping probabilities of differentially methylated region datasets were calculated as previously described.13,14 The cellular components, molecular function and biological processes of differentially methylated genes were analysed. P-values for GO term enrichment were calculated using Fisher’s exact test for all differentially methylated genes.
Gene network analysis
Qiagen’s ingenuity pathway analysis was used to identify the gene networks. The Kyoto Encyclopedia of Genes and Genomes database was used to build the networks of genes.15 Fisher’s exact test was used to select significant pathways.
Real-time PCR of miR-661 mRNA
Total RNA was extracted using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed using a One Step PrimeScript® miRNA cDNA Synthesis Kit (Takara Bio, Dalian, China). Real-time quantitative PCR was performed using SYBR® Premix Ex Taq™ II (Takara Bio).16 The forward primers used for microRNA 661 (miR-661) mRNA amplification were the sequences of mature miR-661 and the reverse primers were provided by the kit.
Western blot analysis of OPA3 and PLEC
Protein extracts were prepared from tissue samples using ice-cold lysis buffer (50 mM Tris-HCl, pH 6.8, 32 mM 2-mercaptoethanol, 2% w/v sodium dodecyl sulphate [SDS], 10% glycerol) supplemented with ethylenediaminetetra-acetic acid-free complete protease inhibitors (Roche, Penzberg, Germany). Following lysis, samples were centrifuged at 12830 g for 20 min at 4°C and the supernatant was collected. Proteins were separated using SDS-polyacrylamide gel electrophoresis by adding 30 µg total extract to 12% SDS-polyacrylamide gels for OPA3 and by adding 30 µg total extract to 5% SDS-polyacrylamide gels for PLEC. Separated proteins were then blotted onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dried milk at room temperature for 1 h, then incubated with 1:2500 dilution goat antihuman OPA3 primary antibody or 1:5000 dilution goat antihuman plectin-1 primary antibody or 1:500 dilution goat antihuman β-actin primary antibody (all antibodies from Abcam, Cambridge, MA, USA) overnight at 4°C. Membranes were then washed three times with Tris-buffered saline Tween 20 (pH 8.0), followed by incubation with the secondary horseradish peroxidase-conjugated antigoat antibody (1:5000 dilution; Arigo, Taiwan, China) for 1 h at room temperature. Finally, the membranes were washed four times with Tris-buffered saline Tween 20 (pH 8.0). β-actin was used as the internal reference for relative quantification. Immunodetection was conducted with enhanced chemiluminescence (ECL; Engreen, Beijing, China) and exposed on X-radiography film. Immunoblots scanned by the densitometer were subjected to a grey value analysis using Quantity One software, version 4.6.2 (Bio-Rad, Hercules, CA, USA).
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Windows®. The data on gene expression are presented as mean ± SE. Statistical significance between groups were evaluated using Student's t-test or one-way analysis of variance. A P-value < 0.05 was considered statistically significant.
Results
This study analysed 27 tissue samples that were collected from surgical patients: 15 SNIP samples and 12 SCCs arising in SNIPs. The 15 SNIPs were collected from nine males and six females (age range, 35–61 years). The 12 SCCs were collected from eight males and four females (age range, 39–65 years).
The methylated DNAs from six SNIP samples and seven SCC samples were profiled using the Infinium HumanMethylation450K BeadChip array. During the process, two SCC samples failed during analysis. Thus, six SNIP and five SCC samples were included in the final analysis. The differentially methylated sites were determined by the DiffScore value. A total of 42 9607 methylation sites were measured and 11 201 methylation sites showed 2-fold differences between SNIP and SCC tissues after log transformation (r2 = 0.9606, r2sel = 0.6311). Among the 11 201 differentially methylated sites, six sites were significantly different between the SNIP and SCC samples at P < 0.01. The six sites contained three genes: outer mitochondrial membrane lipid metabolism regulator (OPA3), plectin (PLEC) and microRNA 661 (MIR661). All three abnormally methylated genes were hypermethylated in SCCs and not in the SNIPs (Table 1).
Table 1.
Six differentially methylated sites identified in six samples of sinonasal inverted papilloma (SNIP) and five samples of sinonasal squamous cell carcinoma arising in SNIPs.
| Target identification | Differential scorea | UCSC reference gene name | UCSC reference gene location | Island name | C-phosphate-G | Adjusted P-values |
|---|---|---|---|---|---|---|
| cg03537810 | 21.9 | PLEC | Body | chr8:145018815-145019214 | N_Shore | P = 0.006418433 |
| cg04018533 | 22.6 | PLEC; MIR661 | Body | chr8:145017868-145018444 | S_Shore | P = 0.005434417 |
| cg09235308 | 20.8 | PLEC; MIR661 | Body | chr8:145017868-145018444 | S_Shore | P = 0.008295069 |
| cg10451724 | 21.9 | PLEC; MIR661 | Body | chr8:145017868-145018444 | S_Shore | P = 0.005418433 |
| cg11956819 | 21.9 | PLEC | Body | chr8:145018815-145019214 | Island | P = 0.006418433 |
| cg27391679 | 20.4 | OPA3 | Body | chr19:46056783-46057149 | Island | P = 0.009097289 |
A differential score equal to 20, 30 or 40 corresponds to a significant P-value of 0.01, 0.001 or 0.0001, respectively.
UCSC, University of California, Santa Cruz; PLEC, plectin; MIR661, microRNA 661; OPA3, outer mitochondrial membrane lipid metabolism regulator.
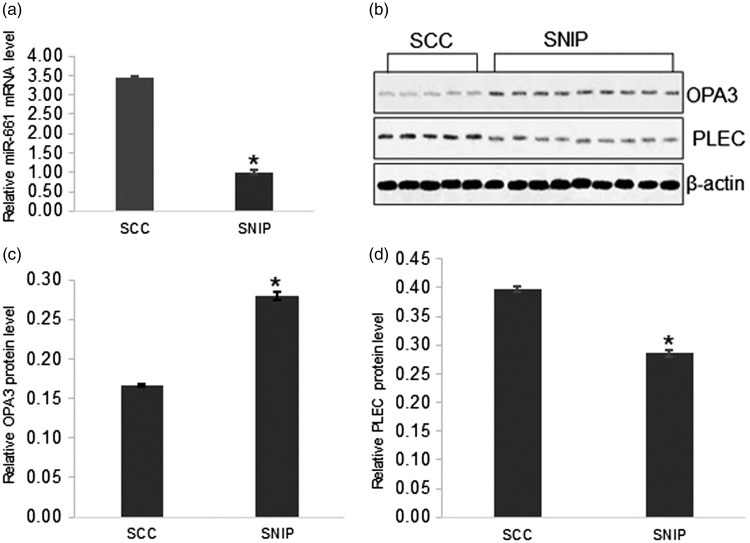
The expression levels of the aberrantly methylated genes were further validated using either real-time PCR to measure miR-661 mRNA levels or Western blot analysis to measure OPA3 and PLEC protein levels in nine SNIP samples and five SCC arising in SNIP samples. Real-time PCR confirmed that miR-661 mRNA levels were significantly higher in SCCs compared with SNIPs (P < 0.001) (Figure 1a). Western blot analysis demonstrated that the protein levels of OPA3 were downregulated in SCCs compared with SNIPs (Figures 1b and 1c) (P < 0.005), but the PLEC protein levels were significantly higher in SCCs compared with SNIPs (Figures 1b and 1d) (P < 0.005).
Figure 1.
Comparison of the expression levels of the aberrantly methylated genes as determined using either real-time polymerase chain reaction (PCR) or Western blot analysis in nine samples of sinonasal inverted papilloma (SNIP) and five samples of sinonasal squamous cell carcinomas (SCC) arising in SNIPs. (a) Real-time PCR of microRNA 661 (miR-661) mRNA levels in six SNIP samples and seven SCC samples. (b) Western blot analysis of outer mitochondrial membrane lipid metabolism regulator (OPA3) and plectin (PLEC) protein levels. β-actin was used as the internal control. (c) Relative OPA3 protein levels in Western blot analysis. (d) Relative PLEC protein levels in Western blot analysis. Data presented as mean ± SE; *P < 0.001 compared with SCC group; Student's t-test or one-way analysis of variance.
Discussion
Even though the malignant transformation of SNIP into SCC is documented in the literature, the molecular mechanisms responsible for this transformation have not been elucidated. Aberrant methylation is an important mechanism that regulates the expression of tumour suppressive genes or oncogenes and is subsequently involved in the carcinogenesis and development of cancers.7 The role of methylation in the malignant transformation of SNIP into SCC has not been currently addressed. In this current study, global DNA methylation in CpG islands and the promoter regions of genes was compared between SNIPs and SCCs arising in SNIPs using a comprehensive methylation profiling technique. This current study found six methylation sites that were significantly different between the two groups at P < 0.01. These six sites contained three hypermethylated genes. The levels of miR-661 mRNA and PLEC protein were significantly higher in SCC arising in SNIP compared with SNIPs; and the levels of OPA3 protein were significantly downregulated in SCCs compared with SNIPs. To the best of our knowledge, this is the first study to compare global DNA methylation between SNIPs and SCCs arising in SNIPs. These current findings suggest that the three genes that exhibited hypermethylation and abnormal levels of expression in SCC arising in SNIPs could be candidate biomarkers for the diagnosis of the malignant transformation of SNIP. These three genes might also serve as potential targets for developing treatment strategies in the future.
Previous studies have shown that miR-661 functions as a tumour suppressor in malignancy and acts as a tumour suppressor in breast cancer where it inhibits cell proliferation, cell motility and cell invasion.17 There are some conflicting findings in breast cancer studies.17,18 A recent study showed that low miR-661 expression was correlated with a poor outcome in patients with breast cancers expressing wild-type p53, whereas high miR-661expression promoted invasion of tumour cells harbouring p53 mutations.19 Thus, miR-661 may either suppress or promote cancer aggressiveness in the same type of tumour depending upon the status of p53 expression.19 In glioma cells, miR-661 levels were downregulated and inhibited cancer cell proliferation, migration and invasion.20 In this study, miR-661 could silence human telomerase reverse transcriptase (hTERT) by recognizing and specifically binding to the predicted site of the hTERT mRNA 3' untranslated region.20 Another study indicated that hsa-circ-0012129 might act as a natural miR-661 sponge and expression of circ-0012129 could be suppressed by miR-661.21 Several recent studies have shown that miR-661 may participate in the regulation of occurrence and progression in non-small cell lung cancer by directly targeting runt related transcription factor 322 or RB transcriptional corepressor 123 and interacting with adenocarcinoma predictive long intergenic non-coding RNA.24 The expression of the MIR661 gene and its role in sinonasal tumours remains unclear. In the current study, miR-661 mRNA levels were upregulated in SCCs compared with SNIPs. Although the targets of miR-661 in sinonasal carcinomas have not been identified, the upregulation of miR-661 mRNA in SCC suggests a role in the malignant transformation of SNIP.
The OPA3 protein is an integral component of the mitochondrial outer membrane and mutations in the OPA3 gene are associated with hereditary optic neuropathies.25 A recent study in a mouse model showed that OPA3 is a novel regulator of mitochondrial function and controls thermogenesis and abdominal fat mass.26 However, its role in tumour cells has not been reported. In this current study, the levels of OPA3 protein were significantly downregulated in SCCs compared with SNIPs, suggesting that OPA3 may be involved in the malignant transformation of SNIP into SCC.
Plectin is a giant multifunctional cytokine protein that helps stabilize and orchestrate the intermediate filament network in cells.27 Mutations in the human PLEC gene result in epidermolysis bullosa simplex associated with muscular dystrophy.28 Only a few studies have explored the role of plectin in tumour cells. For example, a study screening potential biomarkers in oesophageal SCC detected upregulated plectin levels.29 Another study suggested that plectin is either a biomarker for invasive and metastatic pancreatic ductal adenocarcinomas or serves as a marker for preinvasive precursor (PanIN III) lesions.30 In addition, an earlier study demonstrated that stimulatory ascites affected the expression of the PLEC gene in epithelial ovarian cancer.31 The expression of the PLEC gene in sinonasal tumours has not been reported. In this current study, PLEC protein levels were significantly upregulated in SCCs compared with SNIPs, suggesting that PLEC may be a marker for the malignant transformation of SNIP.
Recently, gene body methylation (GbM) was found to frequently occur in the transcribed regions of many oncogenic genes and to be actively involved in multiple regulatory processes.32,33 More detailed genome-wide studies have demonstrated that GbM may alter gene expression by silencing alternative promoters, affecting transcription elongation and regulating splicing.34–36 Therefore, GbM could be serve as a novel biomarker or therapeutic target for human cancers.37 In the current study, all hypermethylation occurred in the gene body and the expression of these three genes were different between SNIPs and SCCs, indicating that GbM might play a role in the malignant transformation of SNIP.
This current study had a number of limitations. First, the small sample size may lead to insufficient statistical power. The study only identified three genes with abnormal methylation at P < 0.01. Secondly, although the levels of mRNA and proteins from these three genes were validated in both the SCCs arising in SNIPs and SNIPs, their biological activities in sinonasal tumour cells were not studied further. Thirdly, the clinical significance of these three aberrantly methylated genes has not been analysed due to the small sample size. Finally, since these current results are very preliminary due to the small sample size, whether or not DNA methylation plays a crucial role in the malignant transformation of SNIP needs to be validated in future studies with larger sample sizes.
In conclusion, this current study identified three methylated genes that were differently expressed between SNIPs and SCCs arising in SNIPs. Although the roles of these three genes have not been previously shown to participate in tumorigenesis and progression, this study is the first to highlight their potential involvement in the malignant transformation of SNIP. This current study also demonstrates the ability of genome-wide epigenetic studies to identify potential biomarkers and thus, provide new insights into their involvement in tumorigenesis. Future research will focus on the association of the methylation landscape with clinical outcome of patients with SNIP.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by grants from the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (no. XMLX201507), the National Natural Science Foundation of China (no. 81670946), the Beijing Key Laboratory of Nasal Diseases, Capital Development Fund for Medical Research (no. 2011-2005-06), the Priming Scientific Research Foundation for Senior Researchers in Beijing Tongren Hospital, Capital Medical University (no. 2016-YJJ-GGL-001) and 2018-1-2052 Capital's Funds for Health Improvement and Research (CFH2018-1-2052).
References
- 1.Llorente JL, López F, Suárez C, et al. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014; 11: 460–472. [DOI] [PubMed] [Google Scholar]
- 2.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer 2009; 115: 2701–2709. [DOI] [PubMed] [Google Scholar]
- 3.Das S, Kirsch CF. Imaging of lumps and bumps in the nose: a review of sinonasal tumours. Cancer Imaging 2005; 5: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol 2014; 8: 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrjänen S. Human papillomavirus infections and oral tumors. Med Microbiol Immunol 2003; 192: 123–128. [DOI] [PubMed] [Google Scholar]
- 6.Degli Esposti D, Sklias A, Lima SC, et al. Unique DNA methylation signature in HPV-positive head and neck squamous cell carcinomas. Genome Med 2017; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrer A, Wellen KE. Metabolism and epigenetics: a link cancer cells exploit. Curr Opin Biotechnol 2015; 34: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci 2015; 16: 2472–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephen JK, Vaught LE, Chen KM, et al. Epigenetic events underlie the pathogenesis of sinonasal papillomas. Mod Pathol 2007; 20: 1019–1027. [DOI] [PubMed] [Google Scholar]
- 10.Chang PH, Huang CC, Lee TJ, et al. Downregulation of DLEC1 in sinonasal inverted papilloma and squamous cell carcinoma. J Otolaryngol Head Neck Surg 2012; 41: 94–101. [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 12.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 2007; 23: 257–258. [DOI] [PubMed] [Google Scholar]
- 13.Fury W, Batliwalla F, Gregersen PK, et al. Overlapping probabilities of top ranking gene lists, hypergeometric distribution, and stringency of gene selection criterion. Conf Proc IEEE Eng Med Biol Soc 2006; 1: 5531–5534. [DOI] [PubMed] [Google Scholar]
- 14.Rodenhiser DI, Andrews J, Kennette W, et al. Epigenetic mapping and functional analysis in a breast cancer metastasis model using whole-genome promoter tiling microarrays. Breast Cancer Res 2008; 10: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JD, Wiemann S. KEGGgraph: a graph approach to KEGG PATHWAY in R and bioconductor. Bioinformatics 2009; 25: 1470–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponsuksili S, Brunner RM, Goldammer T, et al. Bovine NALP5, NALP8, and NALP9 genes: assignment to a QTL region and the expression in adult tissues, oocytes, and preimplantation embryos. Biol Reprod 2006; 74: 577–584. [DOI] [PubMed] [Google Scholar]
- 17.Reddy SD, Pakala SB, Ohshiro K, et al. MicroRNA-661, a c/EBPalpha target, inhibits metastatic tumor antigen 1 and regulates its functions. Cancer Res 2009; 69: 5639–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vetter G, Saumet A, Moes M, et al. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene 2010; 29: 4436–4448. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Hoffman Y, Bublik DR, Pilpel Y, et al. miR-661 downregulates both Mdm2 and Mdm4 to activate p53. Cell Death Differ 2014; 21: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Liu YH, Diao HY, et al. MiR-661 inhibits glioma cell proliferation, migration and invasion by targeting hTERT. Biochem Biophys Res Commun 2015; 468: 870–876. [DOI] [PubMed] [Google Scholar]
- 21.Xie G. Circular RNA hsa-circ-0012129 promotes cell proliferation and invasion in 30 cases of human glioma and human glioma cell lines U373, A172, and SHG44, by targeting microRNA-661 (miR-661). Med Sci Monit 2018; 24: 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Li Y, Wu B, et al. MicroRNA-661 promotes non-small cell lung cancer progression by directly targeting RUNX3. Mol Med Rep 2017; 16: 2113–2120. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Cai Y, Rong X, et al. MiR-661 promotes tumor invasion and metastasis by directly inhibiting RB1 in non small cell lung cancer. Mol Cancer 2017; 16: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu H, Chen J, Song Y, et al. Gastric adenocarcinoma predictive long intergenic non-coding RNA promotes tumor occurrence and progression in non-small cell lung cancer via regulation of the miR-661/eEF2K signaling pathway. Cell Physiol Biochem 2018; 51: 2136–2147. [DOI] [PubMed] [Google Scholar]
- 25.Ryu SW, Jeong HJ, Choi M, et al. Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cell Mol Life Sci 2010; 67: 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells T, Davies JR, Guschina IA, et al. Opa3, a novel regulator of mitochondrial function, controls thermogenesis and abdominal fat mass in a mouse model for Costeff syndrome. Hum Mol Genet 2012; 21: 4836–4844. [DOI] [PubMed] [Google Scholar]
- 27.Wiche G. Plectin: general overview and appraisal of its potential role as a subunit protein of the cytomatrix. Crit Rev Biochem Mol Biol 1989; 24: 41–67. [DOI] [PubMed] [Google Scholar]
- 28.Winter L, Wiche G. The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 2013; 125: 77–93. [DOI] [PubMed] [Google Scholar]
- 29.Pawar H, Kashyap MK, Sahasrabuddhe NA, et al. Quantitative tissue proteomics of esophageal squamous cell carcinoma for novel biomarker discovery. Cancer Biol Ther 2011; 12: 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bausch D, Thomas S, Mino-Kenudson M, et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res 2011; 17: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puiffe ML, Le Page C, Filali-Mouhim A, et al. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007; 9: 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singer M, Kosti I, Pachter L, et al. A diverse epigenetic landscape at human exons with implication for expression. Nucleic Acids Res 2015; 43: 3498–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jjingo D, Conley AB, Yi SV, et al. On the presence and role of human gene-body DNA methylation. Oncotarget 2012; 3: 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 2010; 466: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maunakea AK, Chepelev I, Cui K, et al. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res 2013; 23: 1256–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neri F, Rapelli S, Krepelova A, et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature 2017; 543: 72–77. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Han H, De Carvalho DD, et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 2014; 26: 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]