Short abstract
Introduction
Primary renal lymphoma is a rare malignant lymphoma that is difficult to differentiate from renal cell carcinoma. Positron emission tomography/computed tomography and image-guided percutaneous biopsy are valuable tools for diagnosis.
Case report
A 64-year-old woman presented with a 2-year history of repeated right waist pain and a 1-month history of nausea, vomiting, and frequent and urgent urination. A computed tomography scan showed a huge mass that was initially considered to be renal cell carcinoma at the upper pole of the right kidney. The mass had invaded the renal pelvis, narrowed the right renal artery, and constricted the inferior vena cava and liver. Postoperative examination of the tumor confirmed lymphoma. We herein present this case and its multidisciplinary team management.
Conclusion
Multidisciplinary team management is efficient for preoperative assessment and surgery in difficult and high-risk cases. Based on our literature review, we suggest biopsy before chemotherapy whenever possible. Chemotherapy can be implemented after surgery for better survival outcomes.
Keywords: Kidney, diffuse large B-cell lymphoma, primary, multidisciplinary teams, computed tomography, renal cell carcinoma
Background
Malignant lymphoma, especially non-Hodgkin lymphoma (NHL), may infiltrate into extranodal tissues such as the kidney. However, NHL primarily arising in extranodal tissue is rare and accounts for only one-third of all cases of NHL.1 NHL arising in the kidney is extremely rare2 and difficult to differentiate from renal cell carcinoma (RCC). Lymphoma that originates from the kidney is called primary renal lymphoma (PRL), and only a few cases have been reported. Clinical information regarding the diagnosis, treatment, and prognosis of PRL is scarce. We herein report a case of enormous primary renal diffuse large B-cell lymphoma (DLBCL) with management by multidisciplinary teams (MDTs) and present a review of the literature.
Case presentation
Ethics approval and consent were not applicable in this case because the case was reported retrospectively without personal information and the patient underwent no non-routine procedures.
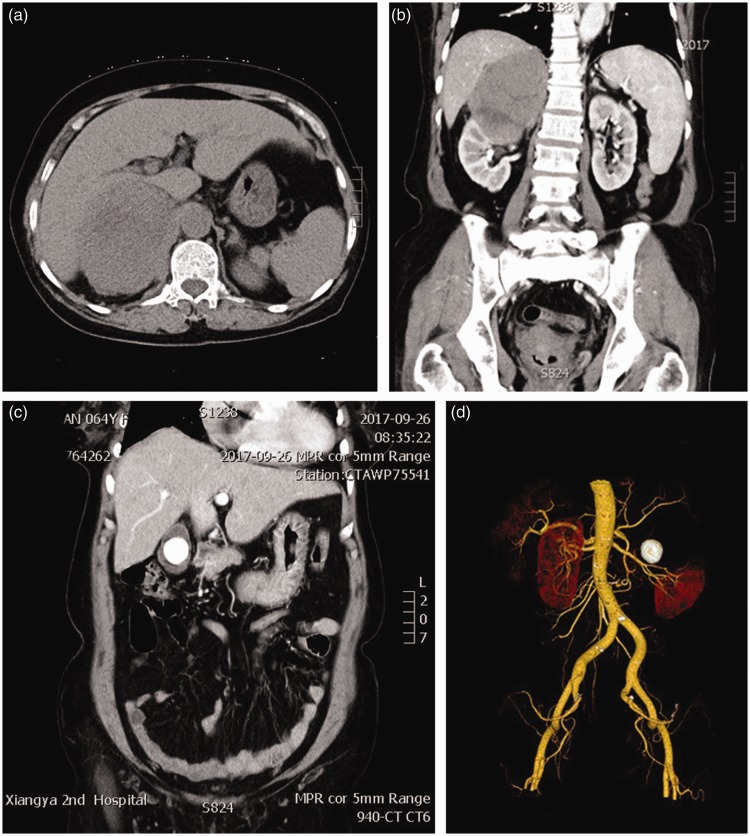
A 64-year-old woman presented with a 2-year history of repeated right waist pain and a 1-month history of nausea, vomiting, and frequent and urgent urination. A previous computed tomography (CT) scan had revealed a huge renal mass. The patient was admitted to the urology department of our hospital for further treatment. Her medical history also included diagnoses of coronary heart disease (cardiac function grade II, high blood pressure (level 3, high-risk group), and calculous cholecystitis. Physical examination showed percussion tenderness over the kidney region but no superficial lymph node enlargement. Laboratory tests revealed the following: red blood cell count, 3.72 × 1012/L; hemoglobin concentration, 102 g/L; white blood cell count, 2.39 × 109/L; generally normal coagulation function, liver and renal function, electrolyte levels, and brain natriuretic peptide concentration; 24-h urine free cortisol level, 827.3 nmol/24 h; supine position plasma renin activity, 30 ng/L; supine position angiotensin II, 367 ng/L; supine position aldosterone, 192 ng/L; standing position plasma renin activity, 202 ng/L; standing position angiotensin II, 91 ng/L; standing position aldosterone, 297 ng/L; high aldosterone-to-renin ratio; and neuron-specific enolase, 62.57 ng/mL. A CT scan of the urinary system and CT angiography of the renal vasculature revealed a huge mass that was primarily considered to be RCC at the upper pole of the right kidney. The mass invaded the renal pelvis, narrowed the right renal artery, and constricted the inferior vena cava and liver (Figure 1(a)–(c)). Additionally, the presence of a large gallbladder stone was suspected (Figure 1(d)). A comprehensive evaluation with MDT consultation (including radiologists, vascular surgeons, anesthesiologists, general surgeons, urologists, and intensive care unit doctors) was completed. This evaluation revealed that the tumor was closely related to the inferior vena cava, liver, gallbladder, and other surrounding tissues and that careful preparation was needed for repair of the damaged inferior vena cava. Because the tumor was next to the gallbladder stone, cholecystectomy during the surgery was suggested. The MDT concluded that the patient should be sent to the intensive care unit after the surgery for intensive nursing. Generally, the operation was considered difficult and high-risk.
Figure 1.
Abdominal computed tomography scan. (a) Transverse section. (b) Coronal section. (c) Three-dimensional reconstruction. (d) Gallbladder stone.
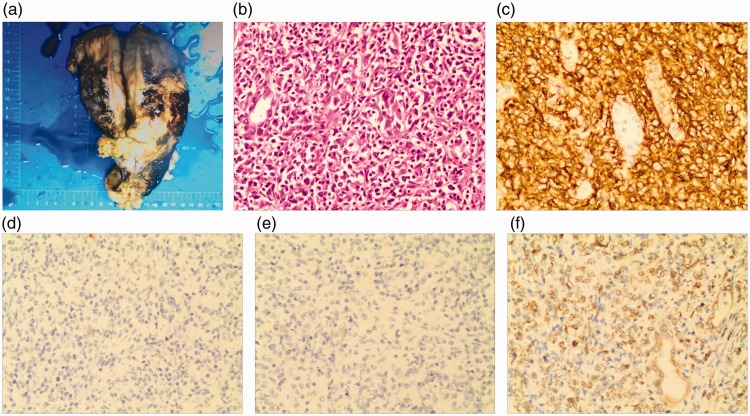
The urologists, vascular surgeons, hepatobiliary surgeons, and cardiothoracic surgeons all played an important part in the collective effort of radical nephrectomy, cholecystectomy, inferior vena cava repair, diaphragm repair, and regional lymph node dissection. During the surgical exploration, the tumor was measured as 10 × 9 × 6 cm and was located close to inferior vena cava, liver, diaphragm, and surrounding structures. The tumor had an incomplete capsule and brittle quality, and some fish-meat-like sections were present. The pathology specimens were evaluated in our hospital (Figure 2(a)–(f)), and the examination findings confirmed right renal DLBCL (non-germinal center type). Immunohistochemical analysis showed that the tumor cells were positive for vimentin, CD20, bcl-2, and PAX-5; negative for CK, CD23, CD21, CD5, bcl-6, CD10, cxcl-13, Mum-1, cyclin D1, CD30, TdT, EMA, and EBER; and equivocal for CD3 and C-myc. The Ki67 labeling index was 70%. The four removed lymph nodes were negative. After the surgery, the patient received the first course of chemotherapy comprising cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP regimen), and she was undergoing follow-up at the time of this writing.
Figure 2.
Histological findings of the resected right renal kidney. (a) Gross specimen (10 × 9 × 6 cm). (b) Hematoxylin and eosin staining (200×). (c) CD20 staining (200×). (d) CD10 staining (200×). (e) Negative for bcl-6 (200×). (f) Positive for bcl-2 (200×).
Discussion
The prevalence of MDTs for management of complex cases has been increasing globally. There is evidence showing the benefits of MDTs for both patients and health-care professionals.3 Our MDTs provide a means of effective group consultation for the management of complex cases. MDTs collaborate in the decision-making process in a highly time-efficient manner. Although the establishment of MDTs has financial implications, it provides patients numerous benefits.
Primary renal DLBCL constitutes a majority of cases of PRL; however, only a few cases have been reported.4 In 2000, Stallone et al.5 reviewed the available literature and reported only 29 cases of PRL fulfilling 3 diagnostic criteria: lymphomatous renal infiltration, nonobstructive unilateral or bilateral kidney enlargement, and no extrarenal localization at the time of diagnosis. In the present case, CT showed a single occupying lesion of the right renal parenchyma that had not invaded the surrounding tissues, nor had it invaded other organs of the body. According to the available clinical data, it was reasonable to diagnose the patient with primary renal DLBCL.
Yang et al.6 reviewed the clinical data of 24 cases of renal DLBCL (including primary DLBCL) and concluded that primary renal DLBCL mostly existed in people of advanced age who experienced abdominal or low back pain, abdominal distension, and palpation of a renal mass. Clinicobiological features and pathological characteristics showed a very aggressive malignancy and poor prognosis. The clinical manifestations in the present case were generally consistent with the previous reports except that the patient developed nausea, vomiting, and frequent and urgent urination for 2 years. In addition, the enormous tumor was closely associated with the inferior vena cava, liver, gallbladder, and surrounding tissues, which increased the difficulty and risk of surgery.
We thoroughly reviewed all 54 cases of PRL reported in the literature since 2002 (Table 1).4,7–49 DLBCL was found to be the most common pathological type, and more male than female patients (64.8%) were reported. Additionally, PRL was generally located in the bilateral kidneys in younger patients (<30 years old) and in a unilateral kidney in older patients, indicating that the site of PRL is age-related. Of the 40 patients whose laboratory results were reported, 25 (62.5%) developed renal impairment. In addition, bilateral PRL and younger age seem to be associated with a shorter survival time according to the limited follow-up data. Unexpectedly, however, the review showed no significant relationship between pathological type and survival. Moreover, chemotherapy was the main treatment for PRL, and R-CHOP was the most common chemotherapeutic regimen. Patients treated with surgery plus chemotherapy had a longer survival time when treated with single-agent chemotherapy; combined treatments appeared to result in slower disease progression. However, the data were obtained from different cases, and significant bias might therefore be present.
Table 1.
Literature review of the 54 cases of primary renal lymphoma reported in the literature since 2002.
| No. | Sex | Age (years) | Site | Renal impairment | Treatment | Chemotherapeutic agents | Histology | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 62 | Bilateral | Yes | Chemotherapy | CHOP | B-cell lymphoma, follicular type | Died at 2 months |
| 2 | Male | 45 | Right | Yes | Surgery + chemotherapy | B-ALL | B-cell lymphoma, Burkitt-like type | Alive at 47 months |
| 3 | Male | 14 | Bilateral | Yes | Chemotherapy | CCG-5942 | Diffuse large B-cell lymphoma | Alive at 2 weeks |
| 4 | Male | 79 | Left | Yes | Surgery | None | Marginal-zone B-cell lymphoma | Alive at 2 months |
| 5 | Male | 43 | Right | Unknown | Surgery | None | B-cell lymphoma of MALT | Alive at 28 months |
| 6 | Male | 46 | Bilateral | Yes | Surgery + chemotherapy | Pro-MECE-Cyta, BOM + Flu-Ctx-Idec | Diffuse large B-cell lymphoma | Alive at 67 months |
| 7 | Female | 70 | Right | No | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 8 months |
| 8 | Female | 65 | Left | Unknown | Surgery + chemotherapy + radiation | R-CHOP | Diffuse large B-cell lymphoma | Alive at 18 months |
| 9 | Female | 68 | Bilateral | Yes | Unknown | Unknown | Large B-cell lymphoma | Died at 10 days |
| 10 | Male | 2 | Bilateral | Yes | Chemotherapy | cpa + L-asp + vcr + prednisolone | T-cell lymphoma | Unknown |
| 11 | Female | 71 | Left | No | Surgery + chemotherapy | CHOP | B-cell lymphoma | Died at 4 months |
| 12 | Male | 50 | Right | No | Surgery + chemotherapy | CHOP | Diffuse large B-cell lymphoma | Alive at 1 month |
| 13 | Male | 62 | Left | No | Surgery + chemotherapy+ interferon | R-CHOP | Diffuse B-cell lymphoma | Alive at 5 years |
| 14 | Male | 84 | Left | Yes | Surgery + chemotherapy + interferon | COP | B-cell lymphoma | Alive at 5 years |
| 15 | Male | 58 | Right | Unknown | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Unknown |
| 16 | Female | 21 | Bilateral | Yes | Chemotherapy | VACOP-B | Diffuse large B-cell lymphoma | Unknown |
| 17 | Male | 5 | Bilateral | Yes | Chemotherapy | CCG-1961 | T-cell lymphoblastic lymphoma | Died at 2 months |
| 18 | Male | 57 | Bilateral | Yes | Chemotherapy + autologous stem cell transplantation | R-CHOP | Unknown | Unknown |
| 19 | Male | 62 | Right | Unknown | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 1 year |
| 20 | Female | 77 | Left | Yes | Surgery + chemotherapy | CVP | Diffuse large B-cell lymphoma | Alive at 15 months |
| 21 | Male | 46 | Right | Unknown | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 7 months |
| 22 | Male | 47 | Renal graft | Unknown | Surgery | None | B-cell lymphoma | Alive at 6.5 years |
| 23 | Male | 74 | Left | Unknown | Surgery + chemotherapy | Unknown | Diffuse small B-cell lymphoma | Died after chemotherapy course 2 |
| 24 | Male | 71 | Right | Unknown | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 2 years |
| 25 | Female | 75 | Left | Unknown | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 1 year |
| 26 | Male | 81 | Right | Unknown | Surgery + chemotherapy | Unknown | Small B-cell lymphoma | Unknown |
| 27 | Female | 52 | Bilateral | Yes | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 2 years |
| 28 | Male | 3 | Bilateral | No | Chemotherapy | BFM-90 | B-cell lymphoma | Died after chemotherapy course 5 |
| 29 | Male | 60 | Right | No | Surgery + chemotherapy | CHOP | Follicular non-Hodgkin lymphoma | Unknown |
| 30 | Male | 70 | Right | Unknown | Surgery | None | Diffuse large B-cell lymphoma | Unknown |
| 31 | Male | 32 | Left | No | Surgery + chemotherapy | CHOP | B-cell lymphoma | Died at 2 months |
| 32 | Male | 72 | Left | Yes | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 15 months |
| 33 | Female | 7 | Bilateral | No | Chemotherapy | CHOP | Unknown | Unknown |
| 34 | Female | 67 | Bilateral | Yes | Chemotherapy | R-CHOP | Large B-cell lymphoma | Alive at 4 weeks |
| 35 | Female | 77 | Left | Yes | Surgery + chemotherapy | CVP + R | Diffuse large B-cell lymphoma | Alive at 5.5 years |
| 36 | Male | 46 | Left | Yes | Surgery + chemotherapy+ radiation | R-CHOP | Diffuse large B-cell lymphoma | Alive at 5 years |
| 37 | Male | 73 | Right | Yes | Surgery | None | Large B-cell lymphoma | Unknown |
| 38 | Female | 82 | Right | Yes | Chemotherapy | R-CHOP | B-cell lymphoma | Unknown |
| 39 | Female | 27 | Bilateral | Yes | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Unknown |
| 40 | Male | 77 | Left | No | Radiation therapy | None | Marginal zone B-cell lymphoma | Alive at 3 years |
| 41 | Female | 12 | Right | No | Surgery + chemotherapy | vcr + dex + cpa + mtx + ara-c + other drugs | Diffuse large B-cell lymphoma | Alive at 3 years 2 months |
| 42 | N/A | 8 | Bilateral | Yes | Chemotherapy | R-CHOP | B-cell lymphoma | Alive at 1 year |
| 43 | Male | 49 | Right | Unknown | Surgery | None | B-cell lymphoma | Alive at 1 year |
| 44 | Male | 42 | Left | Yes | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 28 months |
| 45 | Male | 52 | Bilateral | Yes | Chemotherapy | R-CHOP | Intravascular large B-cell lymphoma | Alive at 26 months |
| 46 | Male | 50 | Left | Unknown | Surgery + chemotherapy | CHOP | Diffuse large B-cell lymphoma | Died at 5 months |
| 47 | Male | 56 | Right | No | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 14 months |
| 48 | Male | 84 | Left | No | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Recurrence at 58 days |
| 49 | Male | 22 | Right | No | Chemotherapy | EPOCH | Diffuse large B-cell lymphoma | Alive at 8 weeks |
| 50 | Male | 50 | Left | Unknown | Chemotherapy | CHOP | B-cell lymphoma | Died after chemotherapy course 3 |
| 51 | Female | 52 | Bilateral | No | Chemotherapy | R-CHOP | Intravascular large B-cell lymphoma | Alive at 26 months |
| 52 | Female | 54 | Right | Yes | Surgery + chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Unknown |
| 53 | Female | 64 | Right | Unknown | Chemotherapy | R-CHOP | Diffuse large B-cell lymphoma | Alive at 2 months |
| 54 | Female | 70 | Right | No | Surgery + chemotherapy | CHOP | Diffuse large B-cell lymphoma | Alive at 2 months |
MALT, mucosa-associated lymphoid tissue.
Chemotherapeutic agents: C/ctx/cpa, cyclophosphamide; H, hydroxydaunorubicin; O, Oncovin; vcr, vincristine; P, prednisone; R, rituximab; M, methotrexate; B, bleomycin; D/dex, dexamethasone; Flu, fludarabine; L-asp, L-asparaginase; mtx, methotrexate; ara-c, cytarabine.
CHOP, R-CHOP, B-ALL, LSA2-L2, CCG5942, Pro-MECE-CytaBOM, Flu-Ctx-Idec, VACOP-B, CCG-1961, CVP, and BFM-90 are combinations of chemotherapeutic agents used to treat lymphoma.
PRL has frequently been misdiagnosed as RCC, although they can coexist with each other.50 Supplementary examinations of PRL include three steps: imaging, minimally invasive biopsy, and surgical exploration. PRL frequently produces complex CT and magnetic resonance images,51 which can show single or multiple focal lesions or diffuse renal enlargement. A main difference between PRL and RCC is that PRL often lacks a blood supply and rarely invades the inferior vena cava as shown by CT, while RCC is rich in blood vessels and invades the inferior vena cava. In addition, the center of the PRL tumor is outside the renal collection system, which helps to differentiate PRL from other urothelial tumors. A benign hyperdense cyst would measure ≥70 HU on unenhanced CT images, whereas a PRL would measure 30 to 50 HU on unenhanced CT images and would be of lower density than other benign renal tumors.52 CT is the imaging modality most commonly used to evaluate renal lymphoma. However, magnetic resonance imaging may also be useful in selected patients and usually shows PRL with low to intermediate signal intensity on T1- and T2-weighted sequences.51 In 2010, Ye et al.53 revealed that positron emission tomography/CT appeared to be useful in the differential diagnosis of PRL because RCC, including the papillary and chromophobe subtypes, is not as intensely fluorodeoxyglucose-avid as PRL.54 Positron emission tomography/CT also helps to assess the response to therapy.51 Hagihara et al.2 suggested that imaging-guided percutaneous biopsy could be of high sensitivity and specificity for the diagnosis of PRL. Previous studies indicated that patients diagnosed with PRL receiving chemotherapy alone can also achieve a good treatment response and avoid radical nephrectomy.33,40,55 Nevertheless, the sensitivity of needle biopsy is 70% to 92%, which is well below 100%, and has risks of adjacent organ and vessel damage. Therefore, the gold standard diagnostic technique is still surgery and pathological examination.
In addition to surgery, chemotherapy is also an important part of integrated therapy and may achieve a satisfactory curative effect. One study showed that rituximab combined with high-dose chemotherapy (R-CHOP regimen) may improve progression-free survival and that chemotherapy combined with hematopoietic stem cell transplantation may further improve the prognosis and reduce recurrence.6 The application of CHOP after surgery plays an important part in the patient’s MDT treatment.
Acknowledgment
We thank the staff of the Department of Pathology at The Second Xiangya Hospital, especially Dr. Daiqiang Li, for providing support.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Airaghi L, Greco I, Carrabba M, et al. Unusual presentation of large B cell lymphoma: a case report and review of literature. Clin Lab Haematol 2006; 28: 338–342. [DOI] [PubMed] [Google Scholar]
- 2.Hagihara M, Hua J, Iwaki Y, et al. Primary renal lymphoma: a case report and literature review. Intern Med 2015; 54: 2655–2659. [DOI] [PubMed] [Google Scholar]
- 3.Lamb BW, Jalil RT, Sevdalis N, et al. Strategies to improve the efficiency and utility of multidisciplinary team meetings in urology cancer care: a survey study. BMC Health Serv Res 2014; 14: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupisti A, Riccioni R, Carulli G, et al. Bilateral primary renal lymphoma treated by surgery and chemotherapy. Nephrol Dial Transplant 2004; 19: 1629–1633. [DOI] [PubMed] [Google Scholar]
- 5.Stallone G, Infante B, Manno C, et al. Primary renal lymphoma does exist: case report and review of the literature . J Nephrol 2000; 13: 367–372. [PubMed] [Google Scholar]
- 6.Yang P, Jing HM, Zhao W, et al. Analysis of clinical feature and treatment outcome of patients with renal diffuse large B cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016; 24: 1737–1742 [in Chinese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 7.Gellrich J, Hakenberg OW, Naumann R, et al. Primary renal non-Hodgkin's lymphoma-a difficult differential diagnosis. Onkologie 2002; 25: 273–277. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad AH, Maclennan GT, Listinsky C. Primary renal lymphoma: a rare neoplasm that may present as a primary renal mass. J Urol 2005; 173: 239. [DOI] [PubMed] [Google Scholar]
- 9.Levendoglu-Tugal O, Kroop S, Rozenblit GN, et al. Primary renal lymphoma and hypercalcemia in a child. Leuk Lymphoma 2002; 43: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 10.Olusanya AA, Huff G, Adeleye O, et al. Primary renal non-Hodgkins lymphoma presenting with acute renal failure. J Natl Med Assoc 2003; 95: 220–224. [PMC free article] [PubMed] [Google Scholar]
- 11.Tuzel E, Mungan MU, Yorukoglu K, et al. Primary renal lymphoma of mucosa-associated lymphoid tissue. Urology 2003; 61: 463. [DOI] [PubMed] [Google Scholar]
- 12.Zomas A, Leivada A, Gortzolidis G, et al. Primary renal lymphoma presenting with chronic low-grade fever. Int J Hematol 2004; 79: 361–363. [DOI] [PubMed] [Google Scholar]
- 13.Kaya A, Kanbay M, Bayrak O, et al. Primary renal lymphoma associated with hepatitis C virus infection. Leuk Lymphoma 2006; 47: 1976–1978. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SB, Debnath PR, Tripathi R. Primary renal lymphoma in a child. Indian J Pediatr 2006; 73: 947. [DOI] [PubMed] [Google Scholar]
- 15.Ladha A, Haider G. Primary renal lymphoma. J Coll Physicians Surg Pak 2008; 18: 584–585. [PubMed] [Google Scholar]
- 16.Tefekli A, Baykal M, Binbay M, et al. Lymphoma of the kidney: primary or initial manifestation of rapidly progressive systemic disease? Int Urol Nephrol 2006; 38: 775–778. [DOI] [PubMed] [Google Scholar]
- 17.Fang FS, Zhu HL, Song ZG, et al. Three cases of primary renal lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007; 15: 1107–1111 [in Chinese, English Abstract]. [PubMed] [Google Scholar]
- 18.Rajappa S, Digumarti R, Immaneni SR, et al. Primary renal lymphoma presenting with paraneoplastic limbic encephalitis. J Clin Oncol 2007; 25: 3783–3785. [DOI] [PubMed] [Google Scholar]
- 19.Omer HA, Hussein MR. Primary renal lymphoma. Nephrology (Carlton) 2007; 12: 314–315. [DOI] [PubMed] [Google Scholar]
- 20.Becker AM, Bowers DC, Margraf LR, et al. Primary renal lymphoma presenting with hypertension. Pediatr Blood Cancer 2007; 48: 711–713. [DOI] [PubMed] [Google Scholar]
- 21.James TC, Shaikh H, Escuadro L, et al. Bilateral primary renal lymphoma. Br J Haematol 2008; 143: 1. [DOI] [PubMed] [Google Scholar]
- 22.Vázquez Alonso F, Sánchez Ramos C, Vicente Prados FJ, et al. Primary renal lymphoma: report of three new cases and literature review. Arch Esp Urol 2009; 62: 461–465 [in Spanish, English Abstract]. [PubMed] [Google Scholar]
- 23.Kose F, Sakalli H, Mertsoylu H, et al. Primary renal lymphoma: report of four cases. Onkologie 2009; 32: 200–202. [DOI] [PubMed] [Google Scholar]
- 24.Belbaraka R, Elyoubi MB, Boutayeb S, et al. Primary renal non-Hodgkin lymphoma: an unusual diagnosis for a renal mass. Indian J Cancer 2011; 48: 255–256. [DOI] [PubMed] [Google Scholar]
- 25.Al-Salam S, Shaaban A, Alketbi M, et al. Acute kidney injury secondary to renal large B-cell lymphoma: role of early renal biopsy. Int Urol Nephrol 2011; 43: 237–240. [DOI] [PubMed] [Google Scholar]
- 26.Pinggera GM, Peschel R, Buttazzoni A, et al. A possible case of primary renal lymphoma: a case report. Cases J 2009; 2: 6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuter S, Rahbar K, Busch V, et al. Acute renal failure due to primary bilateral renal large B-cell lymphoma: diagnostics and follow-up by FDG-PET/CT. Clin Nucl Med 2009; 34: 722–724. [DOI] [PubMed] [Google Scholar]
- 28.Jindal B, Agarwala S, Bakhshi S, et al. Bilateral primary renal lymphoma with orbital metastasis in a child. Pediatr Blood Cancer 2009; 52: 539–541. [DOI] [PubMed] [Google Scholar]
- 29.Chatzipantelis P, Mastorakis E, Tzortzakakis D, et al. Fine needle aspiration cytology diagnosis of primary renal lymphoma involving the pleura: a case report. Acta Cytol 2010; 54: 71–74. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Bhatt A, Khaira A, et al. Primary renal lymphoma: a differential diagnosis of renal mass in a young male. Saudi J Kidney Dis Transpl 2010; 21: 544–545. [PubMed] [Google Scholar]
- 31.Dash SC, Purohit K, Mohanty SK, et al. An unusual case of bilateral renal enlargement due to primary renal lymphoma. Indian J Nephrol 2011; 21: 56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vázquez-Alonso F, Puche-Sanz I, Sánchez-Ramos C, et al. Primary renal lymphoma: long-term results of two patients treated with a chemotherapy+rituximab protocol. Case Rep Oncol Med 2012; 2012: 726424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brancato T, Alvaro R, Paulis G, et al. Primary lymphoma of the kidney: case report and review of literature. Case Rep Oncol Med 2012; 10: 60–62. [DOI] [PubMed] [Google Scholar]
- 34.Hart S, Ellimoottil C, Shafer D, et al. A case of primary renal lymphoma. Urology 2012; 80: 763–765. [DOI] [PubMed] [Google Scholar]
- 35.Hu R, Zhang R, Miao M, et al. Central nervous system involvement of primary renal lymphoma with diffuse large B-cell type lymphoma. Am J Case Rep 2013; 14: 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dedekam E, Graham J, Strenge K, et al. Primary renal lymphoma mimicking a subcapsular hematoma: a case report. J Radiol Case Rep 2013; 7: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhull VS, Mukherjee A, Karunanithi S, et al. Bilateral primary renal lymphoma in a pediatric patient: staging and response evaluation with 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol 2015; 34: 49–52. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa A, Shimotake N, Kubokawa I, et al. Primary pediatric stage III renal diffuse large B-cell lymphoma. Am J Case Rep 2013; 14: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naveen Kumar BJ, Barman P, Chowdhury N, et al. Primary renal lymphoma: an unusual presentation of non-Hodgkin's lymphoma. Indian J Cancer 2014; 51: 370–371. [DOI] [PubMed] [Google Scholar]
- 40.Geetha N, Shahid A, Rajan V, et al. Primary renal lymphoma - a case report. E cancer medical science 2014; 8: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das RN, Dasgupta S, Mondal S, et al. Diffuse large B-cell lymphoma of the kidney: a rare neoplasm. Indian J Pathol Microbiol 2013; 56: 449–452. [DOI] [PubMed] [Google Scholar]
- 42.Desclaux A, Lazaro E, Pinaquy J, et al. Renal intravascular large B-cell lymphoma: a case report and review of the literature. Intern Med 2017; 56: 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Wang W, Li Y, et al. [Primary renal lymphoma with the initial symptom of nephrotic syndrome: a case report]. Zhonghua Xue Ye Xue Za Zhi 2016; 37: 277 [in Chinese, English Abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niitsu N, Okamura D, Takahashi N, et al. Renal intravascular large B-cell lymphoma with early diagnosis by renal biopsy: a case report and review of the literature. Leuk Res 2009; 33: 728–730. [DOI] [PubMed] [Google Scholar]
- 45.Rissman CM, Dagrosa LM, Pettus JR, et al. Primary renal lymphoma: an unusual finding following radical nephrectomy. Clin Nephrol Case Stud 2017; 5: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shetty S, Singh AC, Babu V, et al. Primary renal lymphoma - a case report and review of literature. J Clin Diagn Res 2016; 10: Xd05–Xd07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thawani R, Amar A, Patowary J, et al. Primary renal cell lymphoma: case report, diagnosis, and management. Indian J Med Paediatr Oncol 2017; 38: 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Guo S. Primary renal diffuse large B-cell lymphoma with central nervous system involvement: a rare case report and literature review. Int J Clin Exp Pathol 2015; 8: 7045–7049. [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Hu D, Fang L, et al. Primary renal lymphoma: a case report and literature review. Oncol Lett 2016; 12: 4001–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz H, Namuslu M, Bilgic M, et al. The coexistence of renal cell carcinoma and diffuse large B-cell lymphoma with hypercalcemic crisis as the initial presentation. Endocr Regul 2014; 48: 113–119. [DOI] [PubMed] [Google Scholar]
- 51.Ganeshan D, Iyer R, Devine C, et al. Imaging of primary and secondary renal lymphoma . AJR Am J Roentgenol 2013; 201: W712–W719. [DOI] [PubMed] [Google Scholar]
- 52.Heiken JP, Gold RP, Schnur MJ, et al. Computed tomography of renal lymphoma with ultrasound correlation . J Comput Assist Tomogr 1983; 7: 245–250. [DOI] [PubMed] [Google Scholar]
- 53.Ye XH, Chen LH, Wu HB, et al. 18F-FDG PET/CT evaluation of lymphoma with renal involvement: comparison with renal carcinoma . South Med J 2010; 103: 642–649. [DOI] [PubMed] [Google Scholar]
- 54.Zukotynski K, Lewis A, O’ Regan K, et al. PET/CT and renal pathology: a blind spot for radiologists? Part 2. Lymphoma, leukemia, and metastatic disease . AJR Am J Roentgenol 2012; 199: W168–W174. [DOI] [PubMed] [Google Scholar]
- 55.Pahwa M, Gupta N, Tyagi V, et al. Primary renal lymphoma: is prognosis really that bad? Saudi J Kidney Dis Transpl 2013; 24: 816–817. [DOI] [PubMed] [Google Scholar]