Short abstract
Objective
This study sought to quantify the learning curve for the blind bedside postpyloric placement of a spiral tube in critically ill patients.
Methods
We retrospectively analysed 127 consecutive experiences of three intensivists who performed comparable procedures of blind bedside postpyloric placement of a spiral tube subsequent to failed self-propelled transpyloric migration in a multicentre study. Each intensivist’s cases were divided chronologically into two groups for analysis. The assessment of the learning curve was based on efficiency and safety outcomes.
Results
All intensivists achieved postpyloric placement for over 80% of their patients. The junior intensivist showed major improvement in both efficiency and safety outcomes, and the learning curve for both outcomes was approximately 20 cases. The junior intensivist showed a significant increase in the success rate of proximal jejunum placement and demonstrated a substantial decrease in the major adverse tube-associated events rate. The time to insertion significantly decreased in each intensivist as case experience accumulated.
Conclusions
Blind bedside postpyloric placement of a spiral tube involves a significant learning curve, indicating that this technique could be readily acquired by intensivists with no previous experience using an adequate professional training programme.
Keywords: Learning curve, postpyloric placement, blind bedside, spiral nasojejunal tube, critically ill, intensivist
Introduction
Both European and North American guidelines recommended postpyloric enteral feeding if patients have a risk of aspiration or prepyloric enteral feeding intolerance.1–3 Several blind bedside methods for gaining postpyloric enteral access for transpyloric tube placement have emerged, and acceptable success rates have been demonstrated in several cohorts.4–8 In a multicentre, prospective observational study,9 we recently demonstrated the safety and effectiveness of blind bedside postpyloric placement of a spiral nasoenteric tube (NET) in critically ill adults. This blind bedside method may contribute to the prompt commencement of postpyloric feeding in the intensive care unit (ICU) and increase the number of patients who can tolerate postpyloric spiral NET placement, which may obviate the need for endoscopy or fluoroscopy10,11 and minimize the high risk of timely intrahospital transfer.12–14
Like other ICU operational procedures, blind bedside transpyloric tube placement requires skill in techniques not currently used in training programmes in mainland China,15 making it necessary to perform this technique at highly specialized medical centres. Furthermore, blind bedside technique, although an effective therapy, has potentially serious complications, such as pneumonia, pneumothorax and gastric perforation,16–21 as it is an unguided method. Thus, there is a need to determine the clinical experience required to achieve optimal efficiency and safety outcomes for insertion for blind bedside postpyloric placement of a spiral NET. To test whether there was a significant learning curve for this technique, and to gain insights into the professional experience needed for transpyloric tube insertion, we examined the learning curve for blind bedside postpyloric placement of a spiral NET.
Materials and methods
Study design
Previously, we completed a multicentre, prospective observational study,9 in which critically ill adults were treated using blind bedside postpyloric placement of a spiral NET as a rescue therapy when spontaneous transpyloric migration failed. According to the study protocol, comparable cases were assigned to each intensivist, and placement was consecutively performed by an intensivist affiliated to each centre. This made it possible to assess the learning curve for blind bedside postpyloric placement of a spiral NET. The study was conducted according to the guidelines provided by the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans. The study protocol was approved by the institutional review boards of Guangdong Provincial People’s Hospital and Longgang District Central Hospital. All participants provided their written informed consent before their data were pseudonymously used.
Intensivists, patients and ICUs
This study involved one junior and two senior intensivists responsible for tube insertion from three ICUs with comparable beds in three tertiary hospitals. The intensivists had different work experiences and educational backgrounds. They were asked to provide details of their career background and their caseload of any previous feeding tube placement; that is, nasogastric tube placement, self-advancing spiral NET placement and ultrasound-guided feeding tube placement (Table 1).
Table 1.
Intensivist characteristics.
| Characteristics | Intensivist |
||
|---|---|---|---|
| A | B | C | |
| Primary specialty | Respirology | Surgery | Emergency |
| Academic degree | M.D, PhD | M.D | M.D |
| Senior or junior intensivist | Senior | Junior | Senior |
| Years since graduation | 18 | 6 | 19 |
| Years working in ICU | 10 | 2 | 12 |
| Reported cases of prior nasogastric tube placement | 50 | 50 | 35 |
| Reported cases of prior spontaneous self-advancing spiral tube placement | 210 | 0 | 110 |
| Reported cases of prior ultrasound-guided feeding tube placement | 0 | 3 | 0 |
ICU, intensive care unit
Training programme
First, blind bedside postpyloric placement of a spiral NET was introduced by intensivist A, a senior intensivist, in accordance with the method previously described.4 Then intensivist A established a 60-min training programme, which consisted of a slide presentation of a study protocol and a procedural guide presented in a manual and video. After completing the theoretical training, a junior intensivist (intensivist B) and a senior intensivist (intensivist C) from two other centres were required to watch five tube placements and then perform five procedures supervised by intensivist A.
Tube placement
On the confirmation of a failed spontaneous transpyloric migration, this rescue technique was performed to initiate timely nasoenteric feeding in the absence of endoscopy or fluoroscopy. A 145-cm spiral NET composed of radiopaque polyurethane (CH10, Flocare Bengmark, Nutricia, Amsterdam, the Netherlands) used in previous failed spontaneous migration was withdrawn and sterilized before insertion.22 The technique of blind bedside postpyloric placement of a spiral NET was introduced according to the method previously described by Gatt et al.4 Patients were prepared by the administration of an appropriate dose of metoclopramide before placement and were laid in a semi-supine position. This insertion method involved three phases: oesophageal, gastric and postpyloric placement. During each phase, tube position was assessed using the whoosh test,23 the vacuum test24 or the pH test25 where appropriate, and tube coiling was examined by the guide wire withdrawal test.4 Central to the postpyloric NET placement was the determination of the tube tip position at each stage before further advancement. If placement could not be confirmed, the tube was drawn back before a further attempt. All tube tip positions were confirmed radiologically and were reviewed by an independent expert group before feeding.
Database
The analyses data derived from a database used in our previous study, which included 127 consecutive patients who received blind bedside postpyloric placement of a spiral NET as rescue therapy in line with the eligibility and exclusion criteria defined in the previous study.26 All these patients originally underwent spontaneous transpyloric migration that failed despite the use of prokinetic agents, and all still required enteral nutrition for more than 3 days. Patients excluded from data analysis were those with deterioration in medical conditions (e.g. uncontrolled shock, uncontrolled sepsis, uncontrolled gastrointestinal bleeding, emergency surgery) or those transferred out of the ICU.9 The following baseline data were extracted: demographic characteristics, diagnosis, concomitant medication, and severity of illness comprising the Acute Physiology and Chronic Health Evaluation II score, Sequential Organ Failure Assessment score and Acute Gastrointestinal Injury (AGI) grading. Data for the following efficacy variables were also extracted: the success rate of postpyloric spiral NET placement, success rate of spiral NET placement at the third portion of duodenum (D3) or beyond, success rate of placement at the proximal jejunum, time to insertion, length of insertion and number of attempts (oesophageal, gastric, postpyloric). Major adverse tube-associated events (MATEs) served as safety outcomes; these included vital signs alert events, the requirement of sedatives or analgesics during the procedure, nausea, nasal mucosa bleeding, lung insertion, pneumothorax and others. Data on vital signs, including heart rate (HR), respiratory rate (RR), mean arterial pressure (MAP) and pulse oxygen saturation (SpO2), which were recorded every 5 minutes from the beginning to 30 minutes after the procedure, were detailed in the database. A vital signs event was defined as HR, RR or MAP that fluctuated beyond the range of ±15%, or SpO2 declining to less than 90%.
The learning curve
We assigned patient sequence number as a continuous variable for analysis. The assessment of the learning curve was based on the efficiency and safety outcomes among intensivists (group A, B, and C). To further analyse the evolution of the learning curve, we divided each intensivist’s cases in chronological order into two groups (group A1, B1, and C1 as the first 20 cases of each intensivist; group A2, B2, and C2 as the remaining cases of each intensivist).
Statistical considerations
Continuous variables were presented as mean ± standard deviation or median (interquartile ranges) where appropriate, and categorical variables were presented as frequencies and percentages. One-way analysis of variance was used to compare clinical and demographic characteristics among different groups of patients. Continuous variables were compared between groups using the unpaired t-test and discrete variables were compared between groups using the unpaired rank sum test. Categorical variables were compared using the χ2 test or Fisher exact test. To examine the relationship between procedural time and accumulated cases, a simple linear regression was conducted. A P value of less than 0.05 was considered significant for all tests.
Results
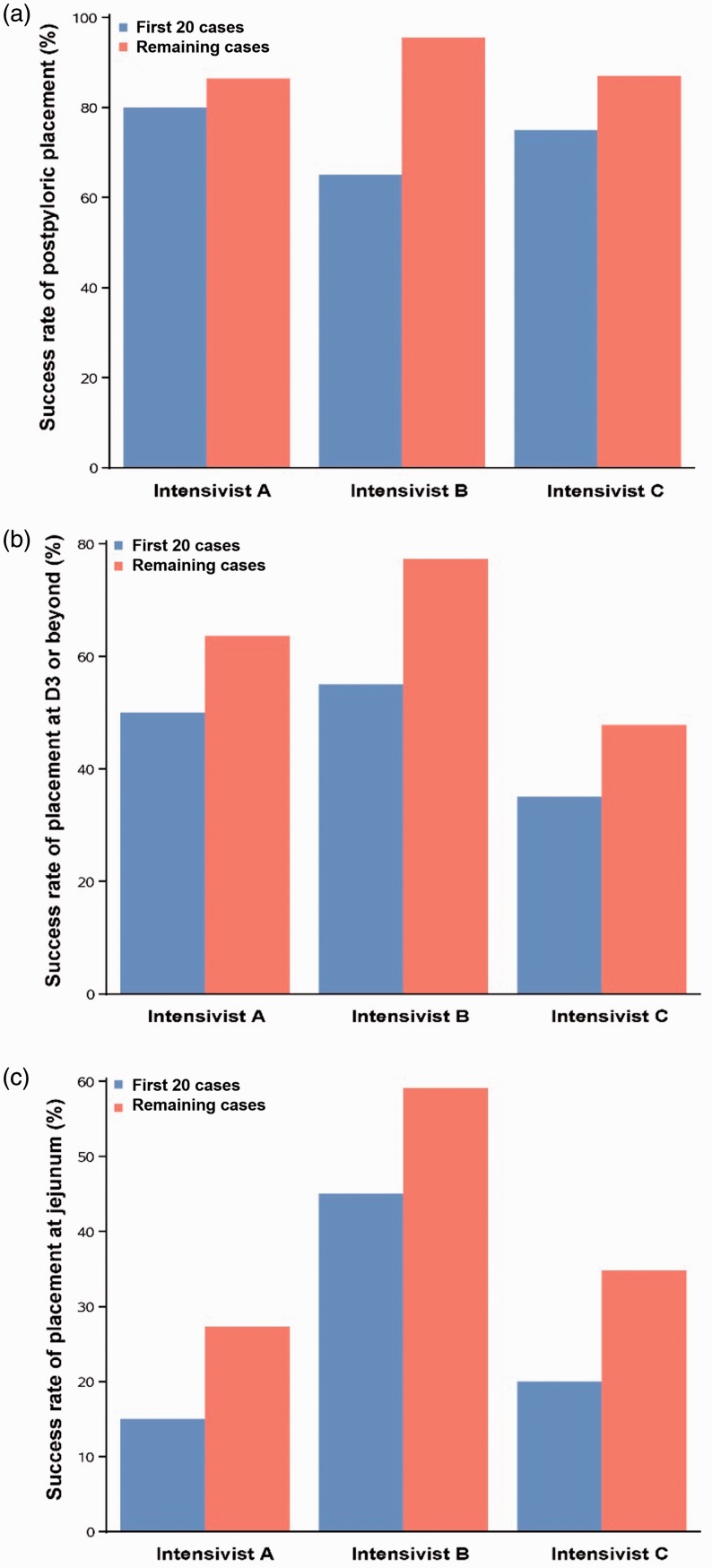
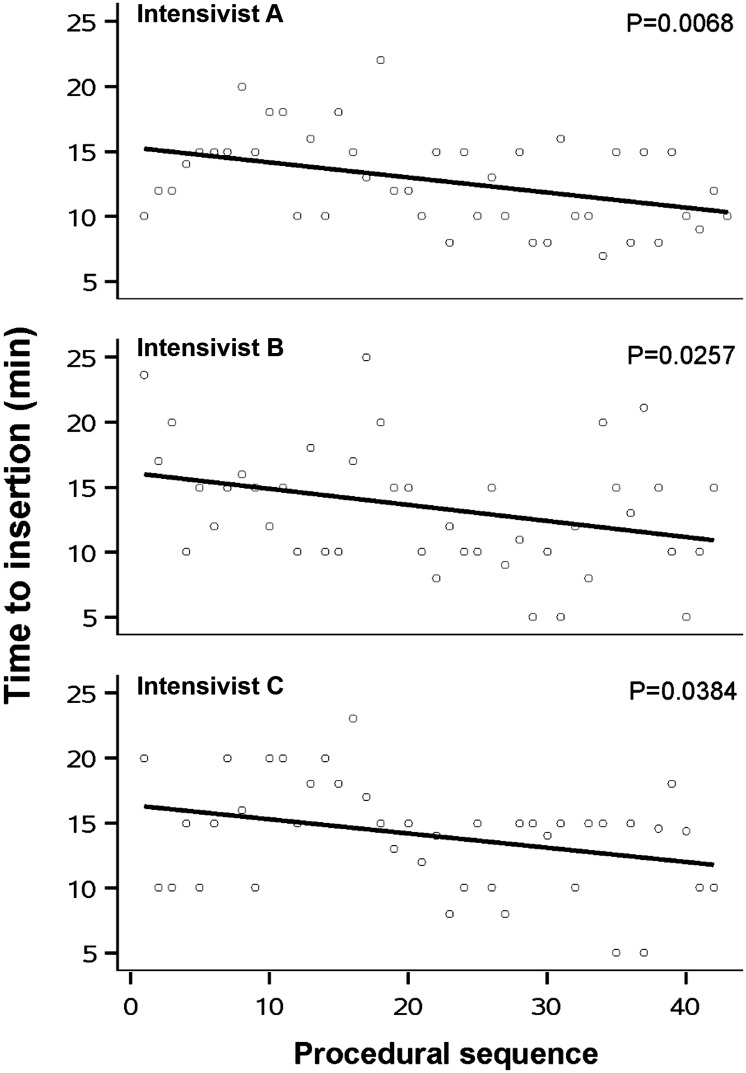
The clinical and demographic data showed no statistically significant differences among the three patient groups, as shown in Table 2. As illustrated in Figure 1, efficiency outcomes for each intensivist displayed a visible improvement trend. The overall success rate of postpyloric placement for all intensivists exceeded 80%. Assessment of the learning curve showed that the success rate of postpyloric placement significantly improved for the junior intensivist (P = 0.0182), but did not significantly improve for the two senior intensivists. The success rate of placement at D3 or beyond was comparable among the three intensivists. With accumulated case experience, time to insertion significantly decreased in all intensivists (Intensivist A: P = 0.0068; Intensivist B: P = 0.0257; Intensivist C: P = 0.0384) (Figure 2) and the success rate of proximal jejunum placement increased dramatically in the junior intensivist (P = 0.0498) (Table 3). Interestingly, the junior intensivist also showed improvement in the length of insertion (P = 0.0077) and number of postpyloric attempts after 20 cases (P = 0.0476) (Table 3). Regarding efficiency outcomes, this technique appeared to demonstrate a learning curve of approximately 20 cases.
Table 2.
Clinical and demographic data grouped by intensivist.
| Variables | Groupa |
P value | ||
|---|---|---|---|---|
| A (n = 43) | B (n = 42) | C (n = 42) | ||
| Age, years | 61 (45–69) | 60 (48–72) | 62 (55–73) | 0.4333 |
| Gender (male), n (%) | 26 (60.5) | 31 (73.8) | 29 (69.1) | 0.4103 |
| Pre-existing diseases, n (%) | 0.0630 | |||
| Hypertension | 4 (9.1) | 10 (22.7) | 13 (28.9) | |
| Diabetes mellitus | 2 (4.6) | 4 (9.1) | 3 (6.7) | |
| Previous gastrointestinal surgery | 0 (0) | 3 (6.8) | 1 (2.2) | |
| Primary diagnosis, n (%) | 0.1242 | |||
| Neurological | 25 (58.1) | 14 (33.3) | 14 (33.3) | |
| Respiratory | 8 (18.6) | 12 (28.6) | 16 (38.1) | |
| Cardiovascular | 6 (14.0) | 4 (9.5) | 5 (11.9) | |
| Multitrauma | 3 (7.0) | 8 (19.1) | 3 (7.1) | |
| Sepsis | 0 (0) | 4 (4.8) | 3 (7.1) | |
| Gastrointestinal | 0 (1.6) | 1 (2.4) | 1 (2.4) | |
| Others | 1 (2.3) | 1 (2.4) | 0 (0) | |
| Use of sedatives or analgesics, n (%) | 6 (13.9) | 4 (9.5) | 4 (9.5) | 0.8240 |
| Use of vasopressors, n (%) | 6 (14.0) | 1 (2.4) | 3 (7.1) | 0.1557 |
| Mechanical ventilation, n (%) | 25 (58.1) | 24 (57.1) | 17 (40.5) | 0.2063 |
| APACHE II score | 19 (16–23) | 14 (11–23) | 19 (15–26) | 0.0656 |
| SOFA score | 10 (8–12) | 10 (8–17) | 9 (7–11) | 0.0547 |
| AGI grade, n (%) | 0.6200 | |||
| Without AGI | 3 (7.0) | 2 (4.8) | 0 (0) | |
| I | 5 (11.6) | 2 (4.8) | 4 (9.5) | |
| II | 30 (69.8) | 31 (73.8) | 31 (73.8) | |
| III | 5 (11.6) | 7 (16.6) | 7 (16.7) | |
Data are presented as n (%) or median with interquartile range. AGI, Acute Gastrointestinal Injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
aThe subjects of intensivists A, B and C were divided into groups A, B and C, respectively.
Figure 1.
Success rate of (A) postpyloric placement, (B) placement at D3, (C) placement at jejunum for the first 20 cases and the remaining cases of the three intensivists.
Figure 2.
Procedure time (y-axis) according to procedural sequence (x-axis). With increased case experience, there was a significant reduction in the time required to complete blind bedside postpyloric placement of a spiral tube in the three intensivists.
Table 3.
Learning data for blind bedside postpyloric placement of spiral tube.
| Variables | Group A |
Group B |
Group C |
P value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A(1–43) | A1 (1–20) | A2 (21–43) | B (1–42) | B1 (1–20) | B2 (21–42) | C (1–42) | C1 (1–20) | C2 (21–42) | A vs. B vs. C | A1 vs. A2 | B1 vs. B2 | C1 vs. C2 | |
| Success rate of placement, n (%) | |||||||||||||
| Postpyloric | 35 (81.4) | 15 (75.0) | 20 (87.0) | 34 (81.0) | 13 (65.0) | 21 (95.5) | 35 (83.3) | 16 (80.0) | 19 (86.4) | 0.9555 | 0.4396 | 0.0182 | 0.6909 |
| D3 or beyond | 18 (41.9) | 7 (35.0) | 11 (47.8) | 28 (66.7) | 11 (55.0) | 17 (77.3) | 24 (57.1) | 10 (50.0) | 14 (63.6) | 0.1091 | 0.5670 | 0.0513 | 0.7578 |
| Proximal jejunum | 12 (27.9) | 4 (20.0) | 8 (34.8) | 22 (52.4) | 9 (45.0) | 13 (59.1) | 9 (21.4) | 3 (15.0) | 6 (27.3) | 0.0177 | 0.4641 | 0.0498 | 0.6786 |
| Time to insertion, min | 12 (10–15) | 15 (12–17) | 10 (8–15) | 13 (10–15) | 15 (12–18) | 10 (9–15) | 15 (10–16) | 15 (14–20) | 14 (10–15) | 0.3224 | 0.0022 | 0.0030 | 0.0029 |
| Length of insertion, cm | 94.5 ± 9.9 | 92.3 ± 9.7 | 96.5 ± 9.9 | 97.5 ± 9.2 | 93.3 ± 9.9 | 101.4 ± 6.6 | 94.9 ± 8.7 | 93.3 ± 7.9 | 95.9 ± 9.3 | 0.2359 | 0.1456 | 0.0077 | 0.4342 |
| Number of oesophageal attempts | 1.1 (0.2) | 1.1 (0.2) | 1.0 (0.2) | 1.1 (0.4) | 1.2 (0.4) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.2) | 1.1 (0.3) | 0.2652 | 0.9203 | 0.3188 | 0.6115 |
| Number of gastric attempts | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0) | 0.3635 | 1.0 | 1.0 | 0.2943 |
| Number of postpyloric attempts | 1.3 (0.5) | 1.5 (0.5) | 1.22 (0.4) | 1.5 (0.6) | 1.7 (0.7) | 1.3 (0.5) | 1.5 (0.6) | 1.6 (07) | 1.4 (0.6) | 0.4220 | 0.1086 | 0.0476 | 0.3542 |
| MATEs, n (%) | 13 (30.2) | 8 (45.0) | 5 (21.7) | 10 (23.8) | 8 (40.0) | 2 (9.1) | 6 (14.3) | 5 (25.0) | 1 (4.6) | 0.2122 | 0.3184 | 0.0296 | 0.0866 |
| Requirement of sedatives or analgesics during procedure | 6 (14.0) | 4 (20.0) | 2 (8.7) | 4 (9.5) | 3 (15.0) | 1 (4.6) | 4 (9.5) | 3 (15.0) | 1 (4.6) | 0.8240 | 0.3929 | 0.3327 | 0.3327 |
| Vital signs alert ratea | 8 (18.6) | 4 (25.0) | 4 (17.4) | 6 (14.3) | 5 (25.0) | 1 (4.6) | 1 (2.4) | 1 (5.0) | 0 (0) | 0.0466 | 1.0 | 0.0866 | 0.4762 |
Quantitative data are presented as mean ± standard deviation or median (interquartile range) as appropriate, qualitative data are presented as n (%)
D3, the third portion of the duodenum; MATEs, major adverse tube-associated events.
aA vital signs event was defined as heart rate, respiratory rate or mean arterial pressure fluctuating beyond the range of ±15%, or pulse oxygen saturation declining to less than 90%.
The subjects of intensivists A, B and C were divided into groups A, B and C, respectively. Groups A1, B1 and C1 represent the first 20 cases of groups A, B and C; and A2, B2 and C2 represent the remaining cases of groups A, B and C.
The rates of MATEs varied from 4.6% to 30.2% and were comparable among the three intensivists. The requirement of sedatives or analgesics during the procedure was also consistent across the three groups. There were significant differences among the three groups in vital signs alert rate (18.6% for intensivist A, 14.3% for intensivist B and 2.4% for intensivist C, respectively; P = 0.0466). With increased case experience, MATEs significantly decreased in intensivist B (a junior, P = 0.0296). Regarding safety outcomes, this technique showed a learning curve of approximately 20 cases.
Discussion
The study findings demonstrate that there is a substantial learning curve for blind bedside postpyloric placement of a spiral NET. The success rate of postpyloric and proximal jejunum placement significantly increased, and adverse events significantly decreased, for the junior intensivist. Procedural time significantly decreased in all intensivists as case experience accumulated. Interestingly, the junior intensivist also showed improvement in length of insertion and number of postpyloric attempts after 20 cases.
We found that operational effectiveness improved significantly in all intensivists as experience increased, a finding reflected in declining procedural time. However, different aspects of the learning curve were observed in the three intensivists. Although the success rate of postpyloric placement exceeded 80% for all intensivists, only intensivist B (a junior intensivist) showed significant improvement as experience accumulated. This could be explained by the sharing mechanism underlying skill acquisition of tube insertion. The sharing mechanism was involved as the intensivists learnt a new skill that shared similar features with previously acquired skills. This may have helped the two senior intensivists, who had experienced hundreds of spontaneous self-advancing spiral NET placements, to learn more quickly than the junior intensivist. However, the improvement in performance in the senior intensivists may have been less significant than in the junior intensivist because the latter had only performed six procedures of ultrasound-guided feeding tube placement. However, even less significant improvements are valuable: transpyloric blind bedside NET placement could act as a learning model, as it might share the same mechanism as other tube insertion techniques in the critical illness setting (e.g. endoscopic, fluoroscopic and electromagnetic-guided tube placement). In addition, blind bedside procedures are often quite challenging. Thus it is reasonable to suppose that a junior intensivist must accumulate experience with a procedure before being able to perform it optimally. As such, the learning curve is a universal concept among intensivists.
It is well recognized that transpyloric blind bedside tube placement is not an easy procedure, particularly in the critical illness setting. Feeding tube insertion is also related to unique and complex adverse events.16–20,27 The therapy requires expertise in unguided placement techniques. In our study, the MATEs were relatively high, partly owing to the limited experience in a newly introduced technique. With increased case experience, the adverse events significantly decreased in the junior intensivist. Regarding safety outcomes, this technique appears to have a learning curve of approximately 20 cases, which indicates that the development of the necessary skills is cost-effective and could minimize the risk of complications.
It is worth noting that we observed this learning curve in the setting of a multicentre group of three dedicated intensivists. These intensivists have collaborated closely and frequently to upgrade techniques to facilitate the placement skills. The team cooperates on postpyloric tube insertions, which have been increasing during the practice. This cooperative effort has resulted in substantial procedural improvements that should be used in developing a training course in the future.
Blind bedside postpyloric placement of intestinal feeding tubes has emerged as a promising procedure for postpyloric feeding access. There is extensive interest in the expansion of operational programmes to meet the requirement for operators training in this field of expertise. To optimize the safety and effectiveness of tube placement, it is prudent to characterize the learning curve for blind bedside postpyloric placement of a spiral NET and to design flexible training curricula before its widespread application. In the ICU setting, interest in portraying learning curves is currently limited to ultrasound-guided jugular central venous catheter placement28 and endotracheal intubation using direct laryngoscopy.29 To our knowledge, there are no studies investigating the learning curve for this procedure. The present study highlighted the requirement for specialized training in postpyloric tube placement owing to a rapid growth in nutritional requirements for critically ill patients; however, there are no data on the professional experience required to optimize clinical nutrition supports.6 It is notable that the blind bedside technique is not yet routinely taught in current ICU training programmes in mainland China.15 Therefore, as demonstrated in this study, the nature of the learning curve associated with this technique has implications for professional training. The inclusion of this rescue therapy in standardized teaching programmes would publicize its benefits and its utility in substantially improving the relatively low success rate of spontaneous transpyloric spiral NET placement despite the use of prokinetic drugs.26,30–32 Elucidation of the learning curve could help intensivists to learn this rescue technique. It could also benefit patients; approximately 90% of patients in this cohort were AGI grade II or III, for whom the guidelines33 recommend that initiation of postpyloric feeding should be considered when prokinetic medication is inadequate.
The study had several limitations, which may limit the generalizability of the results to other medical environments. One limitation was that the learning curve was only assessed with 127 cases of three intensivists. Limited by the small samples, we arbitrary chose a cutoff of 20 attempts for statistical analysis. Furthermore, the present study was a retrospective analysis with known inherent limitations, particularly the potential for referral bias. Additionally, the number of subjects was relatively small for learning curve research, though hundreds of cases were recruited. Thus, the validity of the learning curve needs to be examined in a large prospective cohort.
Conclusions
Blind bedside postpyloric placement of a spiral tube involves a significant learning curve, indicating that this technique could be readily acquired by intensivists with no previous experience using an adequate professional training programme.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
Chunbo Chen was funded by a grant (#2014001) from the Guangdong Province Hospital Association Scientific Research Foundation and a grant (#201343) from the Guangdong Hospital Scientific Research Foundation. Cheng Sun was funded by a grant (#2014A020209051) from the Science and Technology Planning Project of Guangdong Province, China. Bo Lv was funded by a grant (#2013B021800158) from the Science and Technology Planning Project of Guangdong Province, China. Bei Hu was funded by a grant (#2014A020212236) from the Science and Technology Planning Project of Guangdong Province, China, a grant (#20181003) from the Administration of Traditional Chinese Medicine of Guangdong Province, China, and a grant (#A2018034) from the Guangdong Medical Scientific Research Foundation.
References
- 1.Kreymann KG, Berger MM, Deutz NE, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006; 25: 210–223. doi: 10.1016/j.clnu.2006.01.021 [published Online First: 2006/05/16] [DOI] [PubMed] [Google Scholar]
- 2.Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016; 44: 390–438. doi: 10.1097/CCM.0000000000001525 [DOI] [PubMed] [Google Scholar]
- 3.Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017; 43: 380–398. doi: 10.1007/s00134-016-4665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatt M, MacFie J. Bedside postpyloric feeding tube placement: a pilot series to validate this novel technique. Crit Care Med 2009; 37: 523–527. doi: 10.1097/CCM.0b013e3181959836 [DOI] [PubMed] [Google Scholar]
- 5.Kohata H, Okuda N, Nakataki E, et al. A novel method of post-pyloric feeding tube placement at bedside. J Crit Care 2013; 28: 1039–1041. doi: 10.1016/j.jcrc.2013.06.018 [published Online First: 2013/09/11] [DOI] [PubMed] [Google Scholar]
- 6.Rollins CM. Blind bedside placement of postpyloric feeding tubes by registered dietitians: success rates, outcomes, and cost effectiveness. Nutr Clin Pract 2013; 28: 506–509. doi: 10.1177/0884533613486932 [DOI] [PubMed] [Google Scholar]
- 7.Lee AJ, Eve R, Bennett MJ. Evaluation of a technique for blind placement of post-pyloric feeding tubes in intensive care: application in patients with gastric ileus. Intensive Care Med 2006; 32: 553–556. doi: 10.1007/s00134-006-0095-8 [DOI] [PubMed] [Google Scholar]
- 8.Spalding HK, Sullivan KJ, Soremi O, et al. Bedside placement of transpyloric feeding tubes in the pediatric intensive care unit using gastric insufflation. Crit Care Med 2000; 28: 2041–2044. [published Online First: 2000/07/13] [DOI] [PubMed] [Google Scholar]
- 9.Lv B, Hu L, Chen L, et al. Blind bedside postpyloric placement of spiral tube as rescue therapy in critically ill patients: a prospective, tricentric, observational study. Crit Care 2017; 21: 248. doi: 10.1186/s13054-017-1839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foote JA, Kemmeter PR, Prichard PA, et al. A randomized trial of endoscopic and fluoroscopic placement of postpyloric feeding tubes in critically ill patients. JPEN J Parenter Enteral Nutr 2004; 28: 154–157. [published Online First: 2004/05/15] [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Yin H, Zhang R, et al. Endoscopy versus fluoroscopy for the placement of postpyloric nasoenteric tubes in critically ill patients: a meta-analysis of randomized controlled trials. J Crit Care 2016; 33: 207–212. doi: 10.1016/j.jcrc.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Wang H, Gao Y, et al. High incidence of adverse events during intra-hospital transport of critically ill patients and new related risk factors: a prospective, multicenter study in China. Crit Care 2016; 20: 12. doi: 10.1186/s13054-016-1183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckmann U, Gillies DM, Berenholtz SM, et al. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med 2004; 30: 1579–1585. doi: 10.1007/s00134-004-2177-9 [DOI] [PubMed] [Google Scholar]
- 14.Waydhas C. Intrahospital transport of critically ill patients. Crit Care 1999; 3: R83–R89. doi: 10.1186/cc362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Xi X, Ma P, et al. Consensus development of core competencies in intensive and critical care medicine training in China. Crit Care 2016; 20: 330. doi: 10.1186/s13054-016-1514-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stayner JL, Bhatnagar A, McGinn AN, et al. Feeding tube placement: errors and complications. Nutr Clin Pract 2012; 27: 738–748. doi: 10.1177/0884533612462239 [DOI] [PubMed] [Google Scholar]
- 17.Halloran O, Grecu B, Sinha A. Methods and complications of nasoenteral intubation. JPEN J Parenter Enteral Nutr 2011; 35: 61–66. doi: 10.1177/0148607110370976 [DOI] [PubMed] [Google Scholar]
- 18.Freeberg SY, Carrigan TP, Culver DA, et al. Case series: tension pneumothorax complicating narrow-bore enteral feeding tube placement. J Intensive Care Med 2010; 25: 281–285. doi: 10.1177/0885066610371185 [DOI] [PubMed] [Google Scholar]
- 19.Creel AM, Winkler MK. Oral and nasal enteral tube placement errors and complications in a pediatric intensive care unit. Pediatr Crit Care Med 2007; 8: 161–164. doi: 10.1097/01.PCC.0000257035.54831.26 [DOI] [PubMed] [Google Scholar]
- 20.Metheny NA, Meert KL, Clouse RE. Complications related to feeding tube placement. Curr Opin Gastroenterol 2007; 23: 178–182. doi: 10.1097/MOG.0b013e3280287a0f [DOI] [PubMed] [Google Scholar]
- 21.Aronchick JM, Epstein DM, Gefter WB, et al. Pneumothorax as a complication of placement of a nasoenteric tube. JAMA 1984; 252: 3287–3288. [PubMed] [Google Scholar]
- 22.Hu B, Lv B, Chen C. The choice of a postpyloric tube and the patient’s position in our procedure: a response. Crit Care 2018; 22: 127. doi: 10.1186/s13054-018-2036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson J. Nasogastric tube incidents and the use of the 'whoosh test'. Crit Care 2007; 11: 419. doi: 10.1186/cc6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch SK, Hanlon MD, Waits M, et al. Comparison of four bedside indicators used to predict duodenal feeding tube placement with radiography. JPEN J Parenter Enteral Nutr 1994; 18: 525–530. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SJ, Clemente R. Confirmation of nasogastric tube position by pH testing. J Hum Nutr Diet 2005; 18: 371–375. doi: 10.1111/j.1365-277X.2005.00635.x [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Ye H, Sun C, et al. Metoclopramide or domperidone improves post-pyloric placement of spiral nasojejunal tubes in critically ill patients: a prospective, multicenter, open-label, randomized, controlled clinical trial. Crit Care 2015; 19: 61. doi: 10.1186/s13054-015-0784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veltcamp Helbach M, Savelkoul C, Festen-Spanjer B, et al. Catastrophic complication of an electromagnetic placed postpyloric feeding tube. BMJ Case Rep 2016; 2016: pii: bcr2016216738. doi: 10.1136/bcr-2016-216738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen BV, Prat G, Vincent JL, et al. Determination of the learning curve for ultrasound-guided jugular central venous catheter placement. Intensive Care Med 2014; 40: 66–73. doi: 10.1007/s00134-013-3069-7 [DOI] [PubMed] [Google Scholar]
- 29.Buis ML, Maissan IM, Hoeks SE, et al. Defining the learning curve for endotracheal intubation using direct laryngoscopy: a systematic review. Resuscitation 2016; 99: 63–71. doi: 10.1016/j.resuscitation.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Sun C, Wei R, et al. Establishing decision trees for predicting successful postpyloric nasoenteric tube placement in critically ill patients. JPEN J Parenter Enteral Nutr 2018; 42: 132–138. doi: 10.1177/0148607116667282 [published Online First: 2018/03/06] [DOI] [PubMed] [Google Scholar]
- 31.Hu B, Ouyang X, Lei L, et al. Erythromycin versus metoclopramide for post-pyloric spiral nasoenteric tube placement: a randomized non-inferiority trial. Intensive Care Med 2018; 44: 2174–2182. doi: 10.1007/s00134-018-5466-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L, Nie Z, Zhang Y, et al. Development and validation of a nomogram for predicting self-propelled postpyloric placement of spiral nasoenteric tube in the critically ill: mixed retrospective and prospective cohort study. Clin Nutr 2018; doi: 10.1016/j.clnu.2018.12.008 [published Online First: 2018/12/11] [DOI] [PubMed] [Google Scholar]
- 33.Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012; 38: 384–394. doi: 10.1007/s00134-011-2459-y [DOI] [PMC free article] [PubMed] [Google Scholar]