Short abstract
Objective
To investigate whether neurofilament light polypeptide (NfL) level in cerebrospinal fluid (CSF), currently a prognostic biomarker of neurodegeneration in patients with multiple sclerosis (MS), may be a potential biomarker of cognitive dysfunction in MS.
Methods
This observational case–control study included patients with MS. CSF levels of NfL were determined using enzyme-linked immunosorbent assay. Cognitive function was measured with the Brief International Cognitive Assessment for MS (BICAMS) battery and Paced Auditory Serial Addition Test (PASAT3), standardized to the Greek population.
Results
Of 39 patients enrolled (aged 42.7 ± 13.6 years), 36% were classified as cognitively impaired according to BICAMS z-scores (–0.34 ± 1.13). Relapsing MS was significantly better than progressive forms regarding BICAMS z-score (mean difference [MD] 1.39; 95% confidence interval [CI] 0.54, 2.24), Symbol Digit Modality Test score (MD 1.73; 95% CI 0.46, 3.0) and Greek Verbal Learning Test (MD 1.77; 95% CI 0.82, 2.72). An inversely proportional association between CSF NfL levels and BICAMS z-scores was found in progressive forms of MS (rp = –0.944).
Conclusions
This study provides preliminary evidence for an association between CSF NfL levels and cognition in progressive forms of MS, which requires validation in larger samples.
Keywords: Biomarkers, multiple sclerosis, neurofilament-light, cognitive dysfunction, BICAMS
Introduction
Cognitive impairment in patients with multiple sclerosis (MS) has received little attention previously, as diagnosis can be challenging in the usual clinical setting.1,2 Around 40–70% of patients with MS have cognitive dysfunction to some degree, even from the earliest stages of the disease.3–6 Cognitive dysfunction in MS has been associated with both white matter (demyelinated lesions, normal appearing white matter) and grey matter (cerebral cortex, deep nuclei), with white matter loss being associated with defects in mental processing speed and working memory, and grey matter atrophy being related to defects in verbal memory.7
Neurofilaments represent important components of the axonal cytoskeleton, maintaining morphological integrity and preserving speed and fidelity of the nerve stimuli. Neurofilament-light (NfL) chains are the ‘backbone’ to which neurofilament heavy (NfH) and neurofilament medium (NfM) chains copolymerise to form neurofilaments. There are a large number of phosphorylation sites for numerous kinases in the tail regions of NfM and NfH. The downregulation of NfL polypeptide-coding mRNA leads to phosphorylation or metabolism defects, common to many neurodegenerative disorders.8,9 The disorganised neurofilaments provoke selective degeneration and interference with axonal transport10 leading to axonal loss and subsequent release of cellular debris in the extracellular space.11,12 To date, cerebrospinal fluid (CSF) NfL levels have been used as a biomarker of neurodegeneration in many conditions8–10 and recent studies suggest significant validity of blood NfL quantification using the Simoa platform (ultrasensitive single-molecule array serum immunoassay).13
Therapeutic interventions for cognitive dysfunction in MS include cognitive-counselling therapy, appropriate drug therapy and cognitive rehabilitation.14,15 Cognitive rehabilitation involves techniques that help the patient to better manage their deficits or to preserve cognitive reserve.16 The fact that there are available treatments underlines the need for clinical vigilance, and thus, adding a cognitive dysfunction biomarker to the clinical follow-up of patients with MS would improve patient care. The present study aimed to highlight the importance of timely recognition of this major cognition issue, that significantly affects the quality of life of patients with MS and their carers.
The primary objective of the present study was to investigate CSF NfL level as a potential biomarker of cognitive decline, detected using the Brief International Cognitive Assessment for MS (BICAMS) battery and the Paced Auditory Serial Addition Test (PASAT3), in patients with MS. The secondary objective was to examine the association of the indicator with clinical parameters such as age, duration of symptoms, Expanded Disability Status Scale (EDSS), relapse and MS type.
Patients and methods
Study population
This observational case–control study included sequential patients with MS who attended the First Neurology Clinic, AHEPA Hospital, Aristotle University of Thessaloniki between January 2014 and January 2015. CSF samples were collected by lumbar puncture as detailed in the NfL test section below. Inclusion criteria comprised a diagnosis of MS based on the revised McDonald criteria,17 agreement to undergo lumbar puncture and neuropsychological assessment, and age > 18 years. Patients underwent brain and cervical spine magnetic resonance imaging (MRI) scans at baseline or within 3 months pre- or post-CSF sampling. The subgrouping of MS patients followed the 2013 revision of the definitions of MS clinical course by Lublin et al.18 Exclusion criteria were: no consent form, no brain and spine imaging, diagnosis of systemic inflammatory disease, neurological diagnoses other than MS, alcohol or substance misuse, severe psychopathology, and CSF findings not typical of MS. Due to evidence that natalizumab treatment normalises CSF NfL levels,19 patients with previous natalizumab treatment were excluded from the study.
The study was approved by the regional ethics board of Aristotle University of Thessaloniki, Greece, and the study protocol was approved under the umbrella of an ongoing PhD project. Patient samples were collected after written informed consent had been obtained from each participant.
Tests
Despite great difficulty in establishing a brief test that is sufficiently sensitive to the heterogeneous pattern of cognitive difficulties in MS,20,21 the BICAMS battery has been recommended as an adequate, brief and sensitive tool that has been standardised in the Greek population, to detect cognitive dysfunction in MS.21–24
The BICAMS battery includes the California Verbal Learning Test (CVLT) that is mainly used to assess verbal episodic memory and learning curve, the Brief Visuospatial Memory Test (BVMT) that assesses visual spatial episodic memory, and the Symbol Digit Modalities Test (SDMT) that assesses processing speed, attention, and working memory.20 The Greek validated form of the CVLT, namely the GVLT, was used instead of the CVLT in this study. The PASAT3, another screening test for cognitive dysfunction in MS that measures working memory, was used for comparative purposes. Fatigue and depression were controlled by conducting a neuropsychological assessment in all participants, which comprised the Hamilton Depression Rating Scale (HDRS)25 and the Modified Fatigue Impact Scale (MFIS) for fatigue.26 Patients with severe psychopathology (defined as HDRS score > 17) were excluded from analysis. To decrease levels of fatigue, assessments were performed during morning hours, and time was given to patients to rest after hospital arrival. Previously published norms were used to calculate z-scores for each of the BICAMS component tests. Cognitive dysfunction, according to the individual tests (GVLT, BVMT and SDMT), was defined as a score of less than the 5th percentile of the performance of healthy controls, as described previously.24 According to the BICAMS battery, patients were classified as being cognitively impaired when their scores were outside the normal range in any of the three BICAMS tests.24 Specific attention was given to the time that cognitive function was assessed, since relapses and high doses of corticosteroids are indicated to have an adverse effect on attention and memory.27 Thus, it was decided that assessments would be performed simultaneously with CSF sampling in patients without relapse or steroid use, and at 8 weeks following any steroid administration or relapse. The MFIS was self-completed in a quiet waiting area before the patient proceeded to the neuropsychological assessment that included a short 10 min interview and the administration of HDRS, BICAMS and PASAT3 by a trained psychologist (T Koukoulidis). A neurostatus certified neurologist (T Kalatha) rated all patients on the EDSS.28
NFL assay
Cerebrospinal fluid samples were centrifuged for 5 min at 10 000 × g immediately following collection, and the supernatant was collected and stored at –80°C until use. CSF NfL levels were then measured using an NfL sandwich enzyme-linked immunosorbent assay kit29 (NF-light® [Neurofilament light] ELISA; UmanDiagnostics, Umea, Sweden) according to the manufacturer’s instructions. The optical density was read at 450 nm with a STAT FAX ELISA Microplate Reader 2100 (Awareness Technology, Palm City, FL, USA). A 4-parameter logistic regression was performed to provide the best curve fit using Softmax® Pro software (Molecular Devices, San Jose, CA, USA). The investigators who conducted the measurements (EH, TK) were blinded to the clinical data. CSF NfL values obtained in the present study were compared with published values and results from previous studies performed in the neuroimmunology department of the First Neurology clinic of AHEPA Hospital Aristotle University of Thessaloniki.30,31
Statistical analyses
Data are presented as mean ± SD, n (%) prevalence or median (interquartile range), and normality of distribution was assessed using Shapiro–Wilk test. Individual z-scores for GVLT, SDMT, BVMT were estimated according to published norms of a Greek population.24 A composite z-score for the BICAMS battery was calculated as the mean of the three individual z-scores.32 Simple correlations were estimated using Pearson’s correlation coefficient (r) and Spearman’s rank correlation coefficient (rho [ρ]). Furthermore, partial correlations between each individual test and BICAMS z-scores, PASAT3, EDSS and NfL were estimated adjusting for age. Differences in BICAMS test performance between the MS subtypes were evaluated using Wilcoxon rank-sum test or Student’s t-test, while between-group differences in categorical variables were evaluated using Fisher’s exact test. Patients were classified as having cognitive dysfunction when they scored less than the 5th percentile of the performance of healthy controls on one or more tests, as described previously.24 Logistic regression was used to test the association between NfL levels and cognitive dysfunction, adjusting for age, where the dependent variable was defined as the categorical outcome of the BICAMS battery (normal or abnormal), and NfL was included as the independent variable. Regression was also used to examine the association between NfL and relapse risk. All statistical analyses were performed using R version 3.4.4. software (Cran R project) and a P value < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the study population are presented in Table 1. A total of 39 patients enrolled into the study, with a mean age of 42.7 ± 13.6 years, and a female majority (31/39; 80%). Mean SDMT, GVLT, BVMT and BICAMS composite z-scores (–0.70 ± 1.63, –0.31 ± 1.31, –0.02 ± 1.02 and –0.34 ± 1.13, respectively) were below average compared with a healthy Greek population24 with 14/39 patients (36%) classified as having cognitive dysfunction according to the BICAMS battery. Twenty-three patients (59%) had completed secondary education and 16 patients (41%) had completed higher education.
Table 1.
Demographic and clinical characteristics in patients with multiple sclerosis.
| Variable | Study population, n = 39 |
|---|---|
| Age, years | 42.7 ± 13.6 |
| Sex, female | 31 (79.5) |
| CIS, | 5 (12.8) |
| RRMS, | 27 (69.2) |
| SPMS, | 2 (5.1) |
| PPMS, | 5 (12.8) |
| SDMT, z-score | –0.70 ± 1.63 |
| GVLT, z-score | –0.31 ± 1.31 |
| BVMT, z-score | –0.02 ± 1.02 |
| BICAMS, z-score | –0.34 ± 1.13 |
| PASAT3, score | 39.2 ± 12.9 |
| EDSS, score | 2.0 (0.0–3.0) |
| NfL, ng/l | 785.0 (410.0–1301.0) |
| SDMT, impaired | 12 (30.8) |
| GVLT, impaired | 7 (17.9) |
| BVMT, impaired | 5 (12.8) |
| BICAMS battery, impaired | 14 (35.9) |
| Disease duration, months | 36.0 (12.0–126.0) |
| Education level | |
| Secondary education | 23 (59) |
| Tertiary education | 16 (41) |
Data presented as mean ± SD, n (%) prevalence or median (interquartile range).
Cognitive impairment was defined as SDMT, GVLT and BVMT test scores below the 5th percentile of published norms, and BICAMS battery impairment was defined as abnormal performance in any of the three individual tests.24
BICAMS, brief international cognitive assessment for multiple sclerosis; BVMT, brief visuospatial memory test; CIS, clinically isolated syndrome; EDSS, expanded disability status scale; GVLT, Greek verbal learning test; NfL, neurofilament light; PASAT3, paced auditory serial addition test; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SDMT, symbol digit modalities test; SPMS, secondary progressive multiple sclerosis.
In patients grouped into relapsing (n = 32) and progressive (n = 7) forms of MS, cognitive function was better in the relapsing group, shown by statistically significant between-group differences in mean z-score values for BICAMS composite (mean difference [MD] 1.39; 95% confidence interval [CI] 0.54, 2.24; P = 0.002), SDMT (MD 1.73; 95% CI 0.46, 3.0; P = 0.009), and GVLT (MD 1.77; 95% CI 0.82, 2.72; P = 0.001), and in PASAT3 performance (P = 0.005). Mean age was significantly lower in patients with relapsing forms (MD –15.8; 95% CI –26.3, –5.4; P = 0.004; Table 2), and EDSS scores were significantly different between the two subgroups (P < 0.001). There were no statistically significant between-group differences in CSF NfL levels (P = 0.143), BVMT z-score (P = 0.115), educational level (P = 0.206) or disease duration (P = 0.390).
Table 2.
Between-group differences in cognitive test performance and demographic/clinical characteristics in 39 patients with multiple sclerosis.
| Parameter |
Multiple sclerosis type |
Mean difference(95% CI) | Statistical significance | |
|---|---|---|---|---|
| Relapsing forms(RRMS and CIS)n = 32 | Progressive forms(SPMS and PPMS)n = 7 | |||
| SDMT, z-score | –0.39 ± 1.55 | –2.11 ± 1.26 | 1.73 (0.46, 3.00) | P = 0.009 |
| GVLT, z-score | 0.01 ± 1.10 | –1.76 ±1.25 | 1.77 (0.82, 2.72) | P = 0.001 |
| BVMT, z-score | 1.18 ± 0.95 | 0.51 ± 1.21 | 0.67 (–0.17, 1.51) | NS, P = 0.115 |
| BICAMS, z-score | 0.27 ± 1.00 | –1.12 ± 1.05 | 1.39 (0.54, 2.24) | P = 0.002 |
| PASAT3, score | 41.8 ± 12.1 | 27.1 ± 9.7 | 14.7 (4.8, 24.6) | P = 0.005 |
| EDSS, score | 2.0 (0, 2.5) | 5.5 (4.0, 6.0) | n/a | P < 0.001 |
| NfL, ng/l | 701.5 (384.25, 1121.25) | 1120.0 (834.5, 1402.5) | n/a | NS, P = 0.143 |
| Age, years | 39.9 ±12.1 | 55.7 ± 13.5 | –15.8 (–26.3, –5.4) | P = 0.004 |
| Disease duration, months | 33.0 (11.5, 132.0) | 114.0 (42.0, 120.0) | n/a | NS, P = 0.390 |
| Education, tertiary | 15 (46.9) | 1 (14.3) | n/a | NS, P = 0.206 |
Data presented as mean ± SD, median (interquartile range) or n (%) prevalence, and statistically analysed using Wilcoxon rank-sum test, Student’s t-test, or Fisher’s exact test.
BICAMS, brief international cognitive assessment for multiple sclerosis; BVMT, brief visuospatial memory test; CI, confidence interval; CIS, clinically isolated syndrome; PASAT3, paced auditory serial addition test; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SDMT, symbol digit modalities test; GVLT, Greek verbal learning test; SPMS, secondary progressive multiple sclerosis; n/a, not applicable.
NS, no statistically significant between-group difference (P > 0.05).
Correlation analyses of the overall study population (Table 3) showed that age was inversely associated with GVLT (r = –0.428; P = 0.007), SDMT (r = –0.624; P < 0.001), BVMT (r = –0.538; P < 0.001), and BICAMS (r = –0.625; P < 0.001) z-scores (Table 3 and Figure 1) and with PASAT3 (r = –0.318; P = 0.049), and was positively associated with EDSS scores (ρ = 0.517; P = 0.001). No statistically significant association was observed between age and CSF NfL levels (P = 0.512; Table 3).
Table 3.
Correlations between demographic and clinical characteristics, cognitive test performance and disability scale in 39 patients with multiple sclerosis.
|
Correlation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | Age, years | 42.7 | 13.6 | 1 | ||||||||
| 2 | Disease duration, monthsa | 36.0 | (12.0–126.0) | 0.355* | 1 | |||||||
| 3 | GVLT z-score | –0.31 | 1.31 | –0.428* | –0.190 | 1 | ||||||
| 4 | SDMT z-score | –0.70 | 1.63 | –0.624* | –0.096 | 0.605* | 1 | |||||
| 5 | BVMT z-score | –0.02 | 1.02 | –0.538* | –0.074 | 0.668* | 0.542* | 1 | ||||
| 6 | BICAMS z-score | –0.34 | 1.13 | –0.625* | –0.145 | 0.875* | 0.875* | 0.816* | 1 | |||
| 7 | PASAT3, score | 39.2 | 12.9 | –0.318* | 0.018 | 0.718* | 0.669* | 0.533* | 0.756* | 1 | ||
| 8 | EDSS, scorea | 2.0 | (0.0–3.0) | 0.517* | 0.481* | –0.597* | –0.444* | –0.381* | –0.544* | –0.337* | 1 | |
| 9 | NfL, ng/la | 785.0 | (410.0–1301.0) | 0.108 | –0.243 | –0.064 | –0.104 | –0.318* | –0.115 | –0.168 | –0.001 | 1 |
aData presented as median (interquartile range).
*Statistically significant correlation (P < 0.05).
BICAMS, brief international cognitive assessment for multiple sclerosis; BVMT, brief visuospatial memory test; PASAT3, paced auditory serial addition test; SDMT, symbol digit modalities test; GVLT, Greek verbal learning test; EDSS, expanded disability status scale; NfL, neurofilament light.
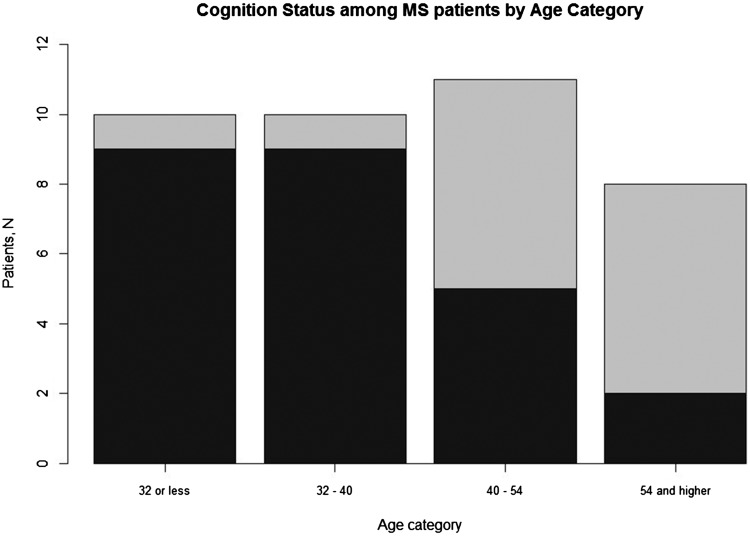
Figure 1.
Association between age and overall cognitive dysfunction (normal or impaired), classified using the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) composite z-scores, in 39 patients with multiple sclerosis (MS) grouped into different age categories: Dark shade indicates normal cognitive function, light grey shade indicates cognitive impairment. P = 0.005 between the different age groups.
Duration of disease was positively associated with age (P = 0.03; Table 3) but did not correlate with cognitive dysfunction test results, or other factors (P = 0.223–0.370) except EDSS (ρ = 0.481, P < 0.05). After adjusting for age, the PASAT3 score was associated with individual cognitive z-scores (P < 0.01) and the BICAMS z-score (rp = 0.754; P < 0.001), an association which remained significant in the relapsing (rp = 0.708; P < 0.001) and the progressive (rp = 0.922; P = 0.009) subgroups (data not shown). EDSS score was associated with the BICAMS z-score in the total study sample (rp = –0.327; P = 0.045), a trend that was not reproduced within the relapsing or progressive subgroups (P = 0.153 and 0.819, respectively).
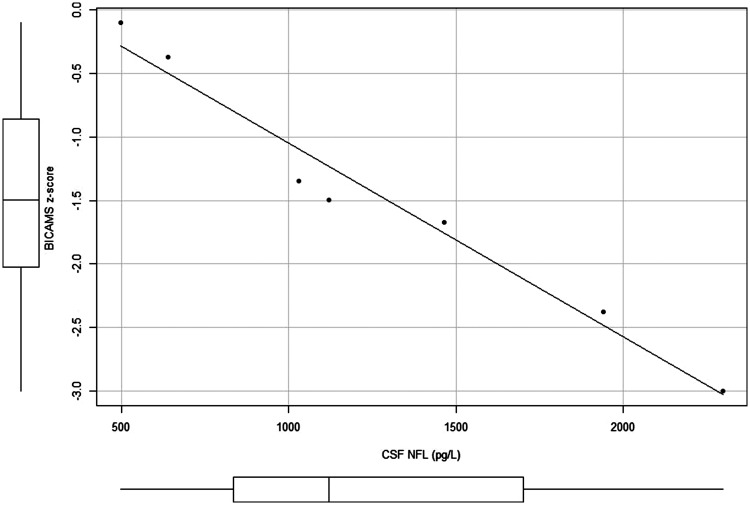
Subgroup analyses, adjusting for age, showed a significant inverse association between CSF NfL levels and the BICAMS composite z-score in patients with progressive forms of MS (n = 7) (rp = –0.944; P = 0.005; Figure 2). After adjusting for age, there was no statistically significant association between CSF NfL levels and BICAMS composite z-scores in patients with relapsing forms of MS (n = 32) (rp = –0.110; P = 0.554), and this was the same for the total study sample (rp = –0.142; P = 0.394). Regression analysis revealed only a trend between CSF NfL levels and cognitive dysfunction risk according to BVMT scores (Odds ratio [OR] 1.08; 95% CI 1.00, 1.17; per 100 ng/l increase in CSF NfL levels) but not according to the BICAMS battery (OR 1.02; 95% CI 0.98, 1.06; per 100 ng/l increase in NfL), in the total study sample. Cognitive dysfunction risk was not examined by regression in the progressive subgroup due to the limited number of patients.
Figure 2.
Partial correlation between cerebrospinal fluid (CSF) neurofilament-light (NFL) polypeptide levels and Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) composite z-scores in patients with the progressive forms of multiple sclerosis (secondary progressive multiple sclerosis and primary progressive multiple sclerosis; n = 7), controlled for age: Box-whisker plots show median (central line), 25th and 75th percentiles (box extremities) and range (error bars).
As noted above, there were no statistically significant differences in CSF NfL levels between patients with relapsing versus progressive forms of MS (P = 0.143; Table 2). Patients with relapse did not demonstrate significantly different levels of CSF NfL compared with patients not on relapse at the time of sampling (data not shown).
Discussion
Cognitive dysfunction in MS is considered to be driven by grey matter damage with trans-synaptic axonal degeneration playing a complementary role.21,33,34 Studies in animal models have shown that autoimmunity to NfL polypeptide is associated with axonal loss and grey matter pathology in contrast to the lesions associated with experimental myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis that involve less grey matter.35 Synapse loss contributes to increased NfL levels, as it is an important component of the synapses.36 In the present study sample, cognitive dysfunction, measured using BICAMS, was found to correlate with raised CSF NfL levels in the progressive forms of MS only. Apart from axonal remodelling, these elevated NfL levels in the progressive forms of MS may reflect grey matter degeneration, which is known to be more prominent than inflammation in the progressive forms of the disease.
The age-related increase in CSF NfL that has been reported previously37 was not observed in the present study. Khademi et al.38 found that low or high NfL levels in patients aged >54 years do not follow the strong correlation with CXCL13 (a B-cell attracting chemokine) demonstrated in younger aged patients, possibly reflecting a different pathophysiological mechanism than inflammation induced axonal degeneration in the older age group. Contrary to the present findings, the same study38 demonstrated lower NfL levels in patients with progressive MS versus those with relapsing-remitting MS, but used an age cut-off of 54 years unlike the mean age of 55.7 ± 13.5 years in the present patients with progressive MS. This difference may in part reflect the phenomenon of immunosenescence, with an age-related decline in T-cell function that, in age groups above 54 years, is substituted by a more pronounced neurodegenerative profile.39
The present study did not verify the presence of higher NfL levels in patients with relapse compared with patients in remission.37,40–42 This may be attributed to the fact that CSF sampling in patients with relapse was usually performed within days of the presentation of symptoms and in the vast majority within one week, so the relapse period was not extended to three months, as in other studies.43 In the present study, CSF NfL levels were not found to correlate with disability progression, measured using EDSS score, in keeping with previous studies.40 The EDSS scores were correlated with composite BICAMS in the overall study population, in keeping with previous studies that showed poorer performance associated with physical disability, rather than disease subtype.44 Poorer cognitive performance in patients with progressive versus relapsing MS, particularly in higher order working memory tests (SDMT) or tests that require high speed of information processing (PASAT3) has been previously noted.45 Worse performances in BVLT showed a modest correlation with increasing NfL levels in the whole sample and may represent the cognitive sub-territories that are first affected in patients with MS, when neurodegeneration increases. Age correlation with cognitive dysfunction in the present study population concurs with other studies that found advanced age to be a risk factor for cognitive dysfunction in patients with MS.44,46
The present results may be limited by several factors. First, the sample size was relatively small and thus the power of the analysis, and the ability to control for a number of potential covariates, including sex, education, depression, and anxiety, were limited. Secondly, the exact years in education of the participants were not recorded in the present study, and only data regarding education completion level was available. This hindered verification of the known profile of cognitive dysfunction in MS that is subject to education and professional attributes,2,47 as well as the effect of cognitive reserve in the prevention of cognitive dysfunction in MS,48 as it has been shown that the impact of pathology on phenotype can be influenced by lifetime intellectual enrichment (cognitive reserve) and lifestyle variability (cognitive leisure).49 Thirdly, only clinical parameters of the disease were investigated as confounders of early cognitive dysfunction, and not predisposing factors, as this was not the primary goal of the present study. Nonetheless, the present patients were screened for depression and fatigue, and patients with severe psychopathology were excluded. Patients who were included willingly underwent screening tests and no difficulties in concentration were noted, which precludes severe depression: lesser degrees of depression are considered to alter self-report of cognitive problems rather than the actual performance in cognitive tests.50 Fourthly, the cross-sectional nature of the study may also limit the results, because NfL levels have been shown to vary over time and/or according to medication/treatment,51 thus, future research should use longitudinal designs with focus on the predictive validity of CSF NfL measurements. Lastly, radiological investigations were not performed in a manner that allowed quantitative measures to be extracted and calculated uniformly, thus MRI data could not be combined in the analyses. Future studies should aim to include quantitative MRI measurements in order to link immunological responses to imaging measures.
In conclusion, the present analysis provides preliminary evidence for a possible association between CSF NfL levels and cognition in the progressive forms of MS. These results should be validated in larger samples, as such an indicator may be useful in marking a subgroup of patients that should be followed closely from the perspective of cognition. Follow-up should include frequent assessment with a brief and adequate tool such as BICAMS in patients with increased CSF NfL, to enable neurocognitive rehabilitation strategies to be included in the multidisciplinary management of MS.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Carone DA, Benedict RH, Munschauer FE, 3rd, et al. Interpreting patient/informant discrepancies of reported cognitive symptoms in MS. J Int Neuropsychol Soc 2005; 11: 574–583. [DOI] [PubMed] [Google Scholar]
- 2.Romero K, Shammi P, Feinstein A. Neurologists׳ accuracy in predicting cognitive dysfunction in multiple sclerosis. Mult Scler Relat Disord 2015; 4: 291–295. [DOI] [PubMed] [Google Scholar]
- 3.Chairavalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 4.Schulz D, Kopp B, Kunkel A, et al. Cognition in the early stage of multiple sclerosis. J Neurol 2006; 253: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 5.Glanz BI, Holland CM, Gauthier SA, et al. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult Scler 2007; 13: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 6.Feuillet L, Reuter F, Audoin B, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 2007; 13: 124–127. [DOI] [PubMed] [Google Scholar]
- 7.Sanfilipo MP, Benedict RH, Weinstock-Guttman B, et al. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 2006; 66: 685–692. [DOI] [PubMed] [Google Scholar]
- 8.Perrot R, Eyer J. Neuronal intermediate filaments and neurodegenerative disorders. Brain Res Bull 2009; 80: 282–295. [DOI] [PubMed] [Google Scholar]
- 9.Abdo WF, van de Warrenburg BP, Kremer HP, et al. CSF biomarker profiles do not differentiate between the cerebellar and parkinsonian subtypes of multiple system atrophy. Parkinsonism Relat Disord 2007; 13: 480–482. [DOI] [PubMed] [Google Scholar]
- 10.Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol 1998; 61: 1–23. [DOI] [PubMed] [Google Scholar]
- 11.Malmestrom C, Haghighi S, Rosengren L, et al. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003; 61: 1720–1725. [DOI] [PubMed] [Google Scholar]
- 12.Singh P, Yan P, Hull R, et al. Levels of phosphorylated axonal neurofilament subunit H (pNfH) are increased in acute ischemic stroke. J Neurol Sci 2011; 304: 117–121. [DOI] [PubMed] [Google Scholar]
- 13.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prosperini L, Piattella MC, Gianni et al. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast 2015; 2015: 481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savulich G, Piercy T, Fox C, et al. Cognitive training using a novel memory game on an iPad in patients with Amnestic Mild Cognitive dysfunction (aMCI). Int J Neuropsychopharmacol 2017; 20: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plohmann AM, Kappos L, Ammann W, et al. Computer assisted retraining of attentional impairments in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 2011; 69: 83–89. [DOI] [PubMed] [Google Scholar]
- 20.Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler 2007; 13: 52–57. [DOI] [PubMed] [Google Scholar]
- 21.Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 2015; 14: 302–317. [DOI] [PubMed] [Google Scholar]
- 22.Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler 2012; 18: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedict RH, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. MBC Neurol 2012; 12: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polychroniadou E, Bakirtzis C, Langdon D, et al. Validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in Greek population with multiple sclerosis. Mult Scler Relat Disord 2016; 9: 68–72. [DOI] [PubMed] [Google Scholar]
- 25.Ferentinos PP, Kontaxakis VP, Havaki-Kontaxaki BJ, et al. Fatigue and somatic anxiety in patients with major depression. Psychiatriki 2009; 20: 312–318. [PubMed] [Google Scholar]
- 26.Bakalidou D, Voumvourakis K, Tsourti Z, et al. Validity and reliability if the Greek version of the modified fatigue impact scale in multiple sclerosis patients. Int J Rehabil Res 2014; 37: 271–276. [DOI] [PubMed] [Google Scholar]
- 27.Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991; 41: 685–691. [DOI] [PubMed] [Google Scholar]
- 28.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 29.Norgren N, Karlsson JE, Rosengren L, et al. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics 2002; 21: 53–59. [DOI] [PubMed] [Google Scholar]
- 30.Petzold A, Altintas A, Andreoni L, et al. Neurofilament ELISA validation. J Immunol Methods 2010; 352: 23–31. [DOI] [PubMed] [Google Scholar]
- 31.Koutsouraki E, Kalatha T, Kalathas T, et al. Biomarkers in cerebrospinal fluid (Tau, phospho-Tau (181) and neurofilament light) in clinically isolated syndrome patients and healthy controls. In: Joint ACTRIMS/ECTRIMS Meeting, Boston, USA, 10–13 September 2014, paper no. 63966, https://onlinelibrary.ectrims-congress.eu/ectrims/2014/ACTRIMS-ECTRIMS2014/63966 (2014, accessed December 2018).
- 32.Petracca M, Sumowski J, Fabian M, et al. Looking into cognitive impairment in primary-progressive multiple sclerosis. Eur J Neurol 2018; 25: 192–195. [DOI] [PubMed] [Google Scholar]
- 33.Calabrese M, Rinaldi F, Grossi P, et al. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother 2011; 11: 425–432. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006; 21: 38–47. [DOI] [PubMed] [Google Scholar]
- 35.Huizinga R, Gerritsen W, Heijmans N, et al. Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol Dis 2008; 32: 461–470. [DOI] [PubMed] [Google Scholar]
- 36.Yuan A, Sershen H, Veeranna et al. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry 2015; 20: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagberg M, Norgren N, Dring A, et al. Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS One 2015; 10: e0135886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khademi M, Dring AM, Gilthorpe JD, et al. Intense inflammation and nerve damage in early multiple sclerosis subsides at older age: a reflection by cerebrospinal fluid biomarkers. PLoS One 2013; 8: e63172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- 40.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009; 73: 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res 2003; 987: 25–31. [DOI] [PubMed] [Google Scholar]
- 42.Norgren N, Sundstrom P, Svenningsson A, et al. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 2004; 63: 1586–1590. [DOI] [PubMed] [Google Scholar]
- 43.Martínez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 2015; 21: 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler J 2017; 23: 1258–1267. [DOI] [PubMed]
- 45.Huijbregts SC, Kalkers NF, de Sonneville LM, et al. Cognitive impairment and decline in different MS subtypes. J Neurol Sci 2006; 245: 187–194. [DOI] [PubMed] [Google Scholar]
- 46.Amato MP, Ponziani G, Siracusa G. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol 2001; 58: 1602–1606. [DOI] [PubMed] [Google Scholar]
- 47.Haase CG, Tinnefeld M, Lienemann M, et al. Depression and cognitive impairment in disability- free early multiple sclerosis. Behav Neurol 2003; 14: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Mult Scler 2013; 19: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 49.Sumowski JF, Wylie GR, Gonnella A, et al. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology 2010; 75: 1428–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benedict RH, Cox D, Thompson LL, et al. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler 2004; 10: 675–678. [DOI] [PubMed] [Google Scholar]
- 51.Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018; 24: 1046–1054. [DOI] [PubMed] [Google Scholar]