Short abstract
The heart is one of the organs most vulnerable to sepsis. This review describes the general characteristics of sepsis-induced cardiomyopathy and the main pathogenesis of myocardial dysfunction in sepsis. Levosimendan is a novel drug for treatment of sepsis-induced myocardial dysfunction. This review also elaborates on the pathogenesis of levosimendan, including the mechanisms of its anti-inflammatory effects, improvement of myocardial ischaemia, increased synthesis of nitric oxide, vascular endothelial cell protection, increased myocardial contractility, improved diastolic function, and inhibition of hypoxia-inducible factor-1α expression. Many clinical studies have proven that levosimendan effectively prevents myocardial dysfunction in sepsis. In addition to the widespread use of levosimendan in patients with heart failure, the role of levosimendan in the treatment of patients with sepsis-induced cardiomyopathy will be increasingly studied and applied in the future.
Keywords: Sepsis, cardiomyopathy, levosimendan, pathogenesis, ischaemia, nitric oxide, contractility
Introduction
Sepsis is an uncontrolled inflammatory response that is common among patients in the intensive care unit.1 One British study showed that among patients with severe sepsis, 3.7 million people die in hospital each year.2 Mortality in patients with sepsis has not decreased in many countries.3–5
Sepsis is complicated by acute organ dysfunction, which is the main cause of death in patients with sepsis.6,7 One of the main causes of death in patients with myocardial dysfunction is sepsis;8 thus, the effective treatment of myocardial dysfunction in patients with sepsis is a hot topic. Levosimendan is a new inotropic and vasodilator agent that has been widely used in patients with acute heart failure and has provided great benefit for clinicians in the treatment of acute heart failure.9,10 In recent years, clinical studies have resulted in new progress in levosimendan treatment. With increased research and evidence of its clinical effectiveness, the use of levosimendan in the treatment of patients with heart damage and sepsis is a new trend.11,12 Thus, the present review mainly explores the treatment of patients with sepsis-associated heart damage with levosimendan and related research progress.
Presentation of sepsis-induced cardiomyopathy
Organ dysfunction is a well-known presentation during sepsis and septic shock. The heart shows different changes following sepsis based on the duration and severity of the septic event. In the early phase of sepsis, ultrasonic examination reveals a left ventricular ejection fraction (LVEF) of >55%, which may be caused by increased myocardial contractility due to adrenaline. Although the LVEF is high, the stroke volume is concurrently low due to high vessel permeability, decreased vessel tension, and insufficient cardiac preload. Despite the compensated increase in heart rate, the stroke volume is typically insufficient to maintain adequate cardiac output as demonstrated with clinical indexes such as a high lactic acid level and decreased central vein blood saturation. During the development phase of sepsis, the LVEF and systolic and diastolic function of the heart gradually decrease; this is accompanied by low blood pressure, cardiac failure, and arrhythmia. Inflammatory factors directly damage the myocardial cells and affect myocardial mitochondrial function, cardiac adrenergic receptors, myocardial calcium ion transport, myocardial apoptosis, cardiac microcirculation, and other processes that result in cardiac dysfunction. This dysfunction is clinically observed as increases in myocardial enzyme and myocardial necrosis marker levels and changes in the electrocardiogram, including ST-T segment reduction, increased T-wave inversion, and other presentations. Additionally, haemodynamics may change.13,14 A uniform definition of sepsis-induced cardiomyopathy has not been established. The most commonly recognised definition is reversible myocardial dysfunction caused by sepsis, and the three main features are an LVEF of ≤0.50 as the diagnostic criterion, left ventricular dilatation, and a return to a normal clinical condition during the early stage of the disease.15 At present, sepsis-induced cardiomyopathy is mainly treated with early anti-infective agents, fluid resuscitation, continuous blood purification treatment to remove interstitial oedema, improved myocardial tissue microcirculation, and positive inotropic drug therapy. Treating sepsis-induced cardiomyopathy is costly. The previously used positive inotropic drugs increased the myocardial calcium ion concentration and therefore increased myocardial oxygen consumption; they have thus had poor therapeutic effects on sepsis-induced cardiomyopathy.16
Mechanisms of sepsis-induced cardiac dysfunction and mechanism of action of levosimendan in treatment of sepsis-induced cardiomyopathy
Cardiac dysfunction occurs in patients with sepsis and may be characterised by systolic and diastolic dysfunction, a low LVEF, and ultrastructural changes of the myocardium. The main mechanisms of myocardial damage in patients with sepsis and treatment with levosimendan for sepsis-induced cardiomyopathy are as follows.
Myocardial ischaemia
The development of myocardial ischaemia is the early theoretical basis of myocardial dysfunction in sepsis. When sepsis developed in response to hypovolemic shock in a mouse model, the myocardial tissue was damaged and both the myocardial cell submicrostructure and early growth of cardiomyocyte mitochondria were altered. The structure gradually changed, including the vasculature, and the myofilaments gradually caused damage to the myocardial fibre structure.15,17 During severe sepsis, cardiac output is normal or increased, peripheral vascular resistance is decreased, and coronary artery blood flow is increased. Additional abnormalities may include increased lactic acid and free fatty acids, abnormal glucose utilisation, hypoxic coronary sinuses, and caecal ligation- and puncture-induced sepsis as seen in mouse models of high-power sepsis (i.e., high kinetic metabolism, increased cardiac output, decreased peripheral vascular resistance, and increased heart rate in the early stage of sepsis). The heart function of mice in such models is inhibited, and glycogen is present in the myocardial blood circulation with coronary artery calcium deposition. In high-power sepsis, the coronary blood flow and myocardial metabolism change during the pathogenesis of sepsis-induced cardiomyopathy. With the development of sepsis, patients gradually transition to a “low-power period” characterised by decreased cardiac output and increased peripheral vascular resistance. During this period, levosimendan can induce peripheral vasodilatation and reduce the preload and postload of the heart by activating adenosine triphosphate (ATP)-sensitive potassium channels. Additionally, levosimendan can dilate the pulmonary arteries and decrease pulmonary artery pressure, thereby reducing the right ventricular postload, dilating the coronary arteries, and reducing the peripheral blood resistance during the “low-power period” of sepsis. Moreover, levosimendan reduces the plasma levels of soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) during this period. Therefore, levosimendan can protect against myocardial damage caused by myocardial ischaemia during sepsis.18 In recent animal studies of myocardial ischaemia, levosimendan had an anti-ischaemic effect in isolated rat hearts after arterial ligation and continuous infusion of levosimendan.19,20 A dose-dependent increase in coronary flow was observed within 30 to 120 minutes of infusion, and the ischaemic area was reduced after 60 to 120 minutes of infusion. Therefore, levosimendan can improve myocardial ischaemia caused by sepsis.19,20
Myocardial depression factor
Bacterial toxins stimulate mononuclear macrophages, lymphocytes, and other immune cells and induce an immune reaction. The macrophages secrete interleukin (IL)-1, tumour necrosis factor α (TNF-α), IL-6, and IL-8 during pathogen phagocytosis when the bacteria invade the body. These factors directly or indirectly cause myocardial damage. TNF-α and IL-1 decrease the calcium concentration in the sarcoplasmic reticulum of myocardial cells during systole and decrease the peak value of L-form calcium, causing myocardial dysfunction. Toll-like receptors are the portal proteins for inflammation signal transduction and play an important role in the immune response of patients with sepsis. Toll-like receptors stimulate TNF-α and IL-1 release by activating NF-κB to translocate into the cell nucleus, affecting calcium, which is important for myocardial contraction, and then damaging the myocardium.21 Levosimendan plays an important role in inhibiting inflammatory cytokines and exerts an anti-inflammatory effect by decreasing the expression of IL-1β, IL-6, IL-8, TNF-α, ICAM-1, and E-selectin. Thus, levosimendan can block the direct or indirect myocardial damage caused by the above factors. In a series of small randomised trials in patients with acute decompensated heart failure, levosimendan reduced hypersensitive C-reactive protein and the levels of all of the above-mentioned inflammatory cytokines, especially when administered at a conventional therapeutic dose as a single 24-hour intravenous infusion; additionally, the anti-inflammatory effect lasted up to 30 days.22 Therefore, levosimendan reduces the myocardial inhibitory factors involved in myocardial injury in sepsis and protects the heart. In patients with congestive heart failure, pharmacological treatment with levosimendan and placebo altered pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10), and the relationships of these cytokines with haemodynamic parameters were studied. The results showed that levosimendan inhibits cardiomyocyte cytokine production and downregulates the TNF-α receptor superfamily and Fas/Fas ligand apoptotic signalling pathway.23,24
Mitochondria damage and nitric oxide
In sepsis, the respiratory function of myocardial mitochondria is disrupted and oxidative phosphorylation is damaged, leading to decreased ATP synthesis and increased reactive oxygen species (ROS) production. This damages the myocardial mitochondria by calcium overload, decreasing myocardial systolic function and damaging the myocardium. The produced ROS can change the protein, cell membrane, and DNA functional structures of myocardial cells and finally result in myocardial necrocytosis. In addition, the ROS produced in the mitochondria can alter cell signal transduction pathways, activate NF-κB via translocation to the cell nucleus from the cytoplasm by degrading IκBα, and regulate the release of the inflammatory marker TNF-α, thus decreasing the myocardial systolic function.25 One study showed that nitric oxide (NO) plays an important role in sepsis-induced cardiomyopathy.26 NO plays an important role in maintaining cardiovascular system stability and killing microorganisms. In sepsis, NO production reduces ions that disturb cardiomyocytes. Metabolism affects the respiratory function of the mitochondria, which affects myocardial contractile function. NO is produced by NO synthase (NOS). Inhibiting cytokines in the myocardium can be reversed by NOS blockers.26
Levosimendan is modulated by the activation of extracellular signal-regulated kinase, Akt (a serine/threonine-specific protein kinase, also known as protein kinase B), and p38 mitogen-activated protein kinase and is increased by epithelial NOS expression to increase NO synthesis. This prevents sepsis-induced cardiomyopathy due to decreased NO synthesis, which leads to myocardial systolic dysfunction. Uberti et al.27 found that levosimendan exerts its cardioprotective effect through mitochondrial K(ATP), a multi-protein mitochondrial ATP-sensitive potassium channel. Both inhibition of the mitochondrial K(ATP) channel and blocking of NOS attenuate the cardioprotection elicited by levosimendan. The mitochondrial K(ATP) channel and NO may be involved in such cardioprotection by interfering with mitochondrial function. Das and Sarkar28 found that pharmacological preconditioning with levosimendan was mediated by inducible NOS and mitochondrial K(ATP) channel activation in an in vivo anaesthetised rabbit heart model. In their model of ischaemia–reperfusion in the hearts of adult male rabbits, pretreatment with specific preparations was used. The ischaemia–reperfusion injury of the rabbit model was protected through the activation of mitochondrial K(ATP) channels and NO in cardiomyocytes.28
Cell apoptosis
In sepsis, the Akt/glycogen synthase kinase-3p (Akt/GSK3p) pathway will be affected or activated, increasing the BAX/Bcl-2 ratio (an apoptosis factor), caspase-3 release, and induction of platelet-derived growth factor (PDGF). Together, these changes damage vascular endothelial cells and lead to cardiomyocyte apoptosis.29 Levosimendan has been studied as a treatment for right heart failure in clinical pulmonary hypertension. Levosimendan can reduce the increased wall thickness of the pulmonary vasculature. Hence, levosimendan significantly reduces the proliferation of pulmonary artery smooth muscle cells. In cell culture, levosimendan directly inhibits PDGF-induced proliferation of pulmonary artery smooth muscle cells, weakens vascular remodelling, and protects vascular endothelial cell function.30
Autophagy
Autophagy is an important process and mechanism of cell death. In recent years, the role of autophagy in the pathogenesis of myocardial injury has become a hot research topic. Myocardial ischaemia–reperfusion injury and hypoxia can induce myocardial cell autophagy. In sepsis-induced cardiomyopathy, myocardial ischaemic–reperfusion injury and hypoxia are also important mechanisms of myocardial dysfunction. Long noncoding RNA (lncRNA) is an intracellular source of RNA molecules that regulates gene expression at multiple levels of epigenetic regulation, transcriptional regulation, and post-transcriptional regulation. Recent studies have shown that lncRNA plays an important role in myocardial cell hypertrophy, fibrosis, and myocardial ischaemia–reperfusion injury.31 Hypoxia-inducible factor-1α (HIF-1α) is a key molecule responsible for the biological response of cells to the level of oxygen. HIF-1α is a DNA-binding protein that is induced by hypoxia. In many human tissues, ischaemia and hypoxia can induce HIF-1α expression. In the pathogenesis of sepsis-induced cardiomyopathy, HIF-1α can induce autophagy and programmed cell death of cardiomyocytes. HIF-1α is an lncRNA that is located on human chromosome 29 and is effective against smooth muscle cell apoptosis. Cell proliferation and apoptosis have regulatory roles. Ischaemia–reperfusion myocardial injury and hypoxia models have shown that the expression of autophagy-related protein Beclin-1 may be increased in cardiomyocytes and that the level of HIF-1α in cardiomyocytes may be inhibited, decreasing the expression of autophagy-related protein Beclin-1 and reducing the level of autophagy in cardiomyocytes. Mitochondrial autophagy may be an important mechanism of mitochondrial dysfunction in sepsis-induced cardiomyocyte injury.32,33 In a study of the effect of levosimendan on HIF-1α activation, Li Yongwang found that the HIF-1α activation level in the observation group improved more significantly than that in the control group, indicating that levosimendan can significantly inhibit HIF-1α activation.34 Jun et al.35 performed a study of the effect of levosimendan combined with dobutamine on N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and HIF-1α in patients with obstinate heart failure. The authors found that levosimendan improved heart failure and decreased the role of NT-proBNP compared with the conventional cardiac drug dobutamine, and HIF-1α levels in vivo were even more pronounced in response to levosimendan.35,36
Effects of levosimendan on the heart
To summarise the pathogenesis of sepsis-induced cardiomyopathy and the molecular biological mechanism of levosimendan on cardiac function, the main effects of levosimendan on cardiac function in sepsis are described as follows.
Increased myocardial contractility
Levosimendan increases cardiomyocyte calcium sensitivity by altering the configuration of troponin C. This alteration increases cardiac myocyte contractility without increasing the intracellular calcium concentration, and it does not evoke significant changes in oxygen requirements. Clinical experimental studies have shown that in patients with heart failure, intravenous levosimendan increases cardiac output and the cardiac index and decreases the ventricular filling pressure, pulmonary vascular resistance, and systemic vascular resistance.37 Levosimendan is a new calcium sensitiser that enhances myocardial contractility without increasing the intracellular calcium load or intracellular cyclic adenosine monophosphate level; at the therapeutic dose, levosimendan does not cause ventricular arrhythmias and improves myocardial injury in sepsis due to mitochondrial damage, apoptosis-inducing factor, calcium overload, and other factors, leading to decreased myocardial contractility.38 Adanir et al.39 found that after the application of levosimendan, the clinical symptoms of patients with acute heart failure due to septic shock disappeared. Cunha-Goncalves40 investigated the effects of levosimendan on clinically relevant plasma concentrations during the first 6 h of endotoxaemia in a model of experimental sepsis. In the levosimendan treatment group, cardiac output and the LVEF increased, and right ventriculovascular coupling and mechanical efficiency tended to improve. This study showed that early treatment with levosimendan during resuscitated sepsis can increase cardiac output and improve right ventricular contractility at a low energy cost. Rincón et al.41 conducted a study in which levosimendan was used in patients with septic shock with myocardial dysfunction. The study showed that levosimendan significantly improved the LVEF; significantly improved the pH, lactate, and base excess; and decreased the systemic vascular resistance without affecting the mean arterial pressure or increasing the cardiac index, and all of these changes occurred without a significant decrease in the left and right atrial pressures. In this study of septic shock in patients with myocardial dysfunction, levosimendan was effective in reversing low cardiac output syndrome without increasing catecholamine requirements.41
Improvements in diastolic function
Levosimendan has improved cardiac diastolic function in studies of patients with cardiac dysfunction. However, animal studies have confirmed that levosimendan improves ventricular diastolic function by improving the diastolic velocity ratios, shortening diastole, and improving diastolic filling. Compared with the traditional positive inotropic drugs, levosimendan does not cause diastolic dysfunction due to calcium overload; it also improves diastolic speed and function. Levosimendan is advantageous for myocardial injury caused by diastolic dysfunction in sepsis. Branzi et al.42 assessed the inodilator properties of levosimendan in patients with chronic heart failure and severe functional mitral regurgitation. The effective regurgitation area of the mitral valve was significantly reduced, and the displacement velocity of the mitral annulus of E/E' waves had a significantly weaker influence on Doppler and tissue Doppler. In addition, levosimendan can improve the contraction and diastolic function of the heart in patients with chronic heart failure. These changes are associated with acute modulation of neurohormonal and myocardial inhibitor control, as shown by the reduction in the BNP level and the haemodynamic improvement induced by the drug.42,43 Additionally, a significant correlation was shown between the effective regurgitant orifice area and indexes of diastolic dysfunction, and the subsequent reduction in the effective regurgitant orifice area was related to the degree of improvement in diastolic function.42 Barraud et al.44 found that levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits, and their research indicates that treatment with levosimendan as a calcium sensitiser improves both systolic and diastolic cardiac function in septic animals. In contrast, the cyclic adenosine monophosphate-dependent inotropes milrinone and dobutamine only improved systolic function.44
Effects on haemodynamics
To study the effect of levosimendan on circulatory function in patients with sepsis-induced cardiomyopathy, Morelli et al.2 compared the effect of levosimendan injection and dobutamine injection on the microcirculatory blood flow in patients with septic shock. Their results showed that compared with dobutamine, levosimendan improved sublingual microcirculatory blood flow in patients with septic shock as reflected by changes in the microcirculatory flow indexes of small and medium vessels. Meng et al.45 investigated the effect of levosimendan on biomarkers of myocardial injury and systemic haemodynamics in patients with septic shock. This study showed that compared with dobutamine, levosimendan reduces biomarkers of myocardial injury, improves systemic haemodynamics in patients with septic shock, and reduces the time on mechanical ventilation and the duration of the intensive care unit stay.
Effects on prognosis and outcome of patients
Zangrillo et al.46 performed a meta-analysis of randomised trials of levosimendan and traditional positive inotropic drugs for sepsis and septic shock and found that levosimendan was associated with a significant reduction in mortality compared with standard inotropic therapy. However, a small-scale study by Vaitsis et al.47 did not show that levosimendan could significantly reduce the mortality of patients with sepsis. Because these results may be related to many factors (e.g., basic diseases, infection control), they require large-scale clinical experimental studies for further verification.46,47
Based on the above studies, although the ability of levosimendan to reduce the mortality rate of patients with sepsis is controversial and further research is needed, the cardiac function and haemodynamic indexes of patients with sepsis can be significantly improved by levosimendan.
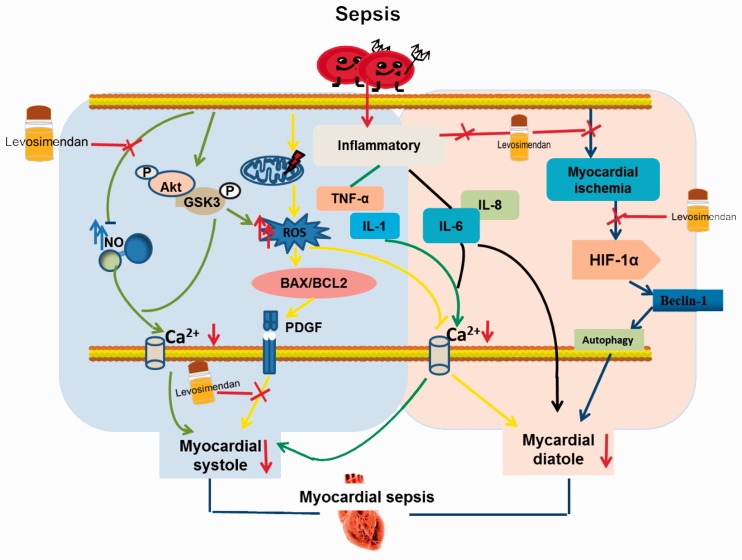
Figure 1 illustrates the main molecular mechanism leading to the occurrence of sepsis-induced cardiomyopathy and the main mechanism of action of levosimendan in the treatment of sepsis-induced cardiomyopathy. First, sepsis decreases the production of NO in the body, which decreases calcium influx and myocardial contractile function. In contrast, levosimendan increases NO synthesis, which increases myocardial contractile function. In patients with sepsis, activation of the Akt/GSK3p pathway leads to an increase in ROS, which leads to myocardial cell mitochondrial damage and calcium overload; these changes then decrease myocardial diastolic function. However, this process increases the BAX/Bcl2 ratio and promotes increased PDGF, which causes systolic dysfunction. Within this molecular mechanism, levosimendan improves myocardial contractility by inhibiting PDGF production. Patients with sepsis who exhibit increased inflammatory factor TNF-α and IL-1 production exhibit a decrease in calcium influx, leading to systolic dysfunction in addition to direct damage to the myocardium by increasing IL-6 and IL-8 production; these changes result in systolic and diastolic dysfunction in patients with sepsis. However, levosimendan improves cardiac contraction and diastolic function in patients with sepsis by inhibiting the production of the above four inflammatory factors. Additionally, patients with sepsis develop myocardial cell ischaemia, hypoxia-damaged myocardial cells, and ischaemia-induced or hypoxia-induced HIF-1α expression in the myocardium. These changes increase the expression level of autophagy protein Beclin-1, leading to myocardial cell autophagy and myocardial necrosis, which cause diastolic dysfunction; levosimendan then activates ATP-sensitive potassium channels, which cause cardiac vasodilation and reduce plasma levels of sICAM-1 and sVCAM-1. This vasodilation increases coronary blood flow and improves myocardial ischaemia. In addition, levosimendan can significantly inhibit the activation of HIF-1α and the self-inhibition of cardiomyocytes. Depletion improves the diastolic function of the patient’s heart.
Figure 1.
Mechanism of levosimendan in patients with sepsis and myocardial dysfunction.
Summary
Based on the above-described pathogenesis of sepsis-induced cardiomyopathy and levosimendan treatment, levosimendan is effective for the treatment of sepsis-induced cardiomyopathy. Small-scale research has shown that levosimendan can improve cardiac function and improve the prognosis of myocardial dysfunction in patients with sepsis. Thus, levosimendan can be used not only for the treatment of patients with acute and chronic heart failure and cardiac surgery but also in patients with sepsis-induced cardiomyopathy. Based on the current clinical studies, patients with sepsis-induced cardiomyopathy should undergo treatment with levosimendan as soon as possible.
A hot research topic in the pathogenesis of sepsis-induced cardiomyopathy is myocardial cell autophagy. Research on myocardial cell autophagy has shown that levosimendan can reduce myocardial ischaemia. The level of HIF-1α induced by perfusion and hypoxia induces the expression of the autophagy protein Beclin-1 in cardiomyocytes, but in the autophagy mechanism of myocardial cell mitochondria, levosimendan can reduce sepsis-induced cardiomyopathy. The expression of mitochondrial autophagy proteins LC3, PINK1, and parkin in patients with sepsis-induced cardiomyopathy has not yet been studied. Therefore, further research is required. In addition, ROS play an important role in the pathogenesis of sepsis-induced cardiomyopathy; whether levosimendan can inhibit ROS production and the mechanism by which it inhibits ROS production also require further study.
Acknowledgement
We are grateful to Li Jiao Zhao, PhD for her guidance on Figure 1 in this paper.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics
This clinical research topic (application of levosimendan in acute organ dysfunction in sepsis) has been reviewed by the Chifeng City Hospital Ethics Committee. Because this article is a review and does not involve human or animal testing, no formal ethical review was required.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morelli A, Donati A, Ertmer C, et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: a randomized controlled study. Crit Care 2010; 14: R232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore JX, Donnelly JP, Griffin R, et al. Defining sepsis mortality clusters in the United States. Crit Care Med 2016; 44: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoller J, Halpin L, Weis M, et al. Epidemiology of severe sepsis: 2008–2012. J Crit Care 2016; 31: 58–62. [DOI] [PubMed] [Google Scholar]
- 6.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med 2007; 35: 2408–2416. [DOI] [PubMed] [Google Scholar]
- 7.Semeraro N, Ammollo CT, Semeraro F, et al. Sepsis, thrombosis and organ dysfunction. Thromb Res 2012; 129: 290–295. [DOI] [PubMed] [Google Scholar]
- 8.Flierl MA, Rittirsch D, Huber-Lang MS, et al. Molecular events in the cardiomyopathy of sepsis. Mol Med 2008; 14: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong B, Li Z, Yat Wong PC. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2015; 29: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 10.Silvetti S, Nieminen MS. Repeated or intermittent levosimendan treatment in advanced heart failure: an updated meta-analysis. Int J Cardiol 2016; 202: 138–143. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med 2016; 375: 1638–1648. [DOI] [PubMed] [Google Scholar]
- 12.Putzu A, Belletti A, Zangrillo A. Levosimendan in sepsis. N Engl J Med 2017; 376: 798–799. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, et al. Sepsis-induced cardiomyopathy. Curr Cardiol Rev 2011; 7: 163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin L, Derwall M, Thiemermann C, et al. [Heart in sepsis: molecular mechanisms, diagnosis and therapy of septic cardiomyopathy]. Anaesthesist 2017; 66: 479–490 [in German]. doi: 10.1007/s00101-017-0329-x [DOI] [PubMed] [Google Scholar]
- 15.McDonough KH, Virag JI. Sepsis-induced myocardial dysfunction and myocardial protection from ischemia/reperfusion injury. Front Biosci 2006; 11: 23–32. [DOI] [PubMed] [Google Scholar]
- 16.Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care 2015; 3: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kan H, Failinger CF, Fang Q, et al. Reversible myocardial dysfunction in sepsis and ischemia. Crit Care Med 2005; 33: 2845–2847. [DOI] [PubMed] [Google Scholar]
- 18.Husebye T, Eritsland J, Arnesen H, et al. Association of interleukin 8 and myocardial recovery in patients with ST-elevation myocardial infarction complicated by acute heart failure. PLoS One 2014; 9: e112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiraz HA, Poyraz F, Kip G, et al. The effect of levosimendan on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats. Libyan J Med 2015; 10: 29269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che X, Wang X, Zhang J, et al. Vitexin exerts cardioprotective effect on chronic myocardial ischemia/reperfusion injury in rats via inhibiting myocardial apoptosis and lipid peroxidation. Am J Transl Res 2016; 8: 3319–3328. [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parissis JT, Adamopoulos S, Antoniades C, et al. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol 2004; 93: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 23.Krychtiuk KA, Watzke L, Kaun C, et al. Levosimendan exerts anti-inflammatory effects on cardiac myocytes and endothelial cells in vitro. Thromb Haemost 2015; 113: 350–362. [DOI] [PubMed] [Google Scholar]
- 24.Adam M, Meyer S, Knors H, et al. Levosimendan displays anti-inflammatory effects and decreases MPO bioavailability in patients with severe heart failure. Sci Rep 2015; 5: 9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrabou G, Moren C, Lopez S, et al. The effects of sepsis on mitochondria. J Infect Dis 2012; 205: 392–400. [DOI] [PubMed] [Google Scholar]
- 26.Dal-secco D, Dalbó S, Lautherbach NES, et al. Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of G protein receptor kinase. Am J Physiol Heart Circ Physiol 2017; 313: H149–H163. [DOI] [PubMed] [Google Scholar]
- 27.Uberti F, Caimmi PP, Molinari C, et al. Levosimendan modulates programmed forms of cell death through K(ATP) channels and nitric oxide. J Cardiovasc Pharmacol 2011; 57: 246–258. [DOI] [PubMed] [Google Scholar]
- 28.Das B, Sarkar C. Pharmacological preconditioning by levosimendan is mediated by inducible nitric oxide synthase and mitochondrial K ATP channel activation in the in vivo anesthetized rabbit heart model. Vascul Pharmacol 2007; 47: 248–256. [DOI] [PubMed] [Google Scholar]
- 29.Dong M, Hu N, Hua Y, et al. Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta 2013; 1832: 848–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossini E, Molinari C, Caimmi PP, et al. Levosimendan induces NO production through p38 MAPK, ERK and Akt in porcine coronary endothelial cells: role for mitochondrial K ATP, channel. Br J Pharmacol 2009; 156: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godar RJ, Ma X, Liu H, et al. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy 2015; 11: 1537–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi H, Merceron C, Mangiavini L, et al. Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling. Autophagy 2016; 12: 1631–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciarretta S, Zhai P, Shao D, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 2012; 125: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yongwang. Levosimendan effect on hemodynamics and hypoxia inducible factor-1α activation in patients with coronary heart disease and heart failure [J]. Chinese Journal of Primary Medicine and Pharmacy 2017; 24: 2796. [Google Scholar]
- 35.Jun W. Effects of levosimendan combined with dobutamine treatment on NT-proBNP and HIF-1α in patients with refractory heart failure. Chinese Health Nutrition 2017; 27. [in Chinese] [Google Scholar]
- 36.Zhibin F, Zhong S, Zhu T. Levosimendan improves hemodynamics and reduces the activation of HIF-1α in CHD patients with heart failure . Medical Journal of West China 2015. [in Chinese] [Google Scholar]
- 37.Chang W, Xie JF, Xu JY, et al. Effect of levosimendan on mortality in severe sepsis and septic shock: a meta-analysis of randomised trials. BMJ Open 2018; 8: e019338. doi:10.1136/bmjopen-2017-019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolseth SM, Rolim NP, Salvesen O, et al. Levosimendan improves contractility in vivo and in vitro in a rodent model of post-myocardial infarction heart failure. Acta Physiol (Oxf) 2014; 210: 865–874. [DOI] [PubMed] [Google Scholar]
- 39.Adanir T, Karahan N, Yetkin U, et al. The role of calcium desensitization in the pathophysiology of septic myocardial depression and effects of levosimendan: results of fifteen septic shock patients. Internet Journal of Thoracic & Cardiovascular Surgery 2007. http://ispub.com/IJTCVS/10/2/10310
- 40.Cunha-Goncalves D, Perez-de-Sa V, Larsson A, et al. Inotropic support during experimental endotoxemic shock: part II. A comparison of levosimendan with dobutamine. Anesth Analg 2009; 109: 1576–1583. [DOI] [PubMed] [Google Scholar]
- 41.Rincón VJA, Martínez MJ, Monteón BI, et al. Levosimendan treatment of myocardial depression in sepsis: a case series and review of the literature. Medicina Interna De Mexico 2010; 26: 324–336. [Google Scholar]
- 42.Branzi G, Malfatto G, Villani A, et al. Acute effects of levosimendan on mitral regurgitation and diastolic function in patients with advanced chronic heart failure. J Cardiovasc Med (Hagerstown) 2010; 11: 662–668. [DOI] [PubMed] [Google Scholar]
- 43.Parissis JT, Panou F, Farmakis D, et al. Effects of levosimendan on markers of left ventricular diastolic function and neurohormonal activation in patients with advanced heart failure. Am J Cardiol 2005; 96: 423–426. [DOI] [PubMed] [Google Scholar]
- 44.Barraud D, Faivre V, Damy T, et al. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med 2007; 35: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 45.Meng JB, Hu MH, Lai ZZ, et al. Levosimendan versus dobutamine in myocardial injury patients with septic shock: a randomized controlled trial. Med Sci Monit 2016; 22: 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zangrillo A, Putzu A, Monaco F, et al. Levosimendan reduces mortality in patients with severe sepsis and septic shock: a meta-analysis of randomized trials. J Crit Care 2015; 30: 908–913. [DOI] [PubMed] [Google Scholar]
- 47.Vaitsis J, Michalopoulou H, Thomopoulos C, et al. Use of levosimendan in myocardial dysfunction due to sepsis. Crit Care Med 2009; 13(Suppl 1): 165. [Google Scholar]