Short abstract
Objective
To investigate the effect of ascorbic acid (AA) on hemostatic function during living donor liver transplantation (LDLT).
Methods
Blood samples from 21 LDLT recipients were taken within 30 minutes after induction and at 120 minutes after reperfusion. Rotational thromboelastography (TEG) and western blot analysis were used to analyze for fibrinolysis and functional changes in c-Cbl and Cbl-b, respectively. TEG test samples were prepared as one of three groups: C group (0.36 mL of blood), N group (0.324 mL of blood + 0.036 mL of 0.9% normal saline), and A group (0.324 mL of blood + 0.036 mL of 200 µmol/L-AA dissolved in 0.9% normal saline).
Results
AA decreased fibrinolysis and increased clot rigidity at baseline and 120 minutes after reperfusion. Cbl-b expression was significantly increased at baseline and 120 minutes after reperfusion in the A group compared with the C and N groups. However, c-Cbl phosphorylation was most significantly decreased in the A group at baseline and 120 minutes after reperfusion.
Conclusion
AA can significantly decrease fibrinolysis and improve clot rigidity in LT recipients during LDLT, and functional changes in Cbl-b and c-Cbl might represent the underlying mechanism. AA may be considered for use during LDLT to decrease hyperfibrinolysis.
Keywords: Ascorbic acid, Cbl-b, c-Cbl, E3 ubiquitin ligase, liver transplantation, platelet, thromboelastography
Introduction
Profound oxidative stress can occur during living donor liver transplantation (LDLT). Excessive amounts of reactive oxygen species (ROS) or reactive nitrogen species (RNS) can be produced under various conditions such as preoperative liver disorder, liver removal (anhepatic period), ischemia/reperfusion injury, and graft dysfunction during LDLT.1–3 Excessive ROS or RNS levels can affect diverse biochemical processes at the cellular level, thereby converting physiological processes into pathological conditions.4,5 Additionally, the effects of excessive ROS or RNS can include hemostatic derangement, which can lead to adverse hemorrhagic or thrombotic outcomes in LDLT recipients. The underlying mechanisms of these hemostatic functional changes likely originate from protein hydroperoxide formation, thiol group oxidation, and carbonyl group formation by excessive ROS or RNS production, which leads to the deterioration of normal enzymatic actions of hemostasis.6–8 Therefore, it is important to maintain the oxidant/antioxidant balance and hemostatic defense mechanisms in antioxidant-depleted LDLT recipients by supplying exogenous antioxidants.9–11 In particular, ascorbic acid (AA, vitamin C), a water soluble and essential micronutrient, is a biologically important cofactor for biosynthetic enzymes and a potent antioxidant, which can play an important role in hemostatic defense against oxidative stress.12 AA can have broad effects on the coagulation pathway, from the initiation of coagulation to fibrinolysis. However, the actions of AA on platelet function during LDLT remain poorly understood and controversial.13,14
To date, three mammalian casitas B-lineage lymphoma proto-oncogene (Cbl) E3 ubiquitin ligase proteins have been identified: c-Cbl, Cbl-b, and Cbl-3.15 Among these, c-Cbl and Cbl-b are expressed in anucleate human platelets and they play an important role in the platelets’ hemostatic function.16,17 Ubiquitin, a small 76-amino acid protein, was initially considered to be a modifier protein that contributes to protein degradation via the ubiquitin-proteasome system. However, accumulating evidence shows that ubiquitin controls many intracellular protein actions including receptor downregulation by promoting endocytosis, gene transcription, and enzyme activation or inactivation.18 Although Cbl-b and c-Cbl are mainly known as T-cell anergy factors,19 they also have important roles as positive or negative regulators of platelet activation and aggregation. Daniel et al.17 reported that E3 ubiquitin ligases, Cbl-b, and c-Cbl, which are important regulators of platelet function and clot stability, modify the actions of phospholipase Cγ2 and Bruton’s tyrosine kinase. Therefore, we used thromboelastography to investigate whether AA affects hemostatic function in LDLT recipients. Additionally, because excessive ROS or RNS production can affect intracellular enzymatic actions, we investigated whether AA might mediate functional changes in Cbl-b and c-Cbl during LDLT.
Methods
This prospective study was approved by the Institutional Review Board at our hospital (IRB File No. 2014-06-091-002) and registered at the Korea Clinical Research Information Service (Register # KCT0002503). All of the procedures in this study were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. We obtained written informed consent from each patient before enrollment into the study.
Study subjects and collection of blood samples
Recipients who underwent elective LDLT at our university-affiliated hospital were enrolled into this study. To minimize the factors that could affect platelet function during liver transplantation, we excluded patients who had previously received liver transplantation, because of the possibility of large amounts of bleeding and transfusions; patients whose blood platelet concentrations were lower than 30,000/µL; those who had received any type of blood component within 1 week before surgery; and patients who received platelet concentrates before the blood sampling time point at 120 minutes after reperfusion.
Rotational thromboelastography (ROTEM®)
We initially performed the in vitro study using rotational thromboelastography. Blood samples were taken from the LDLT recipients, and by adding AA to the blood sample, we aimed to investigate whether AA affects platelet function during liver transplantation.
Blood samples were taken from an arterial catheter placed in the radial artery at the following two time points: within 30 minutes after induction of general anesthesia (before the surgical incision was made), and at 120 minutes after reperfusion. We performed rotational thromboelastography tests three times with each blood sample, which was prepared under three different conditions as follows: a control group (C group; 0.36 mL of native blood only), a normal saline group (N group; 0.324 mL of blood +0.036 mL of 0.9% normal saline), and an AA group (A group; 0.324 mL of blood sample + 0.036 mL of 200 µmol of L-AA dissolved in 0.9% normal saline).
Rotational thromboelastography with the native blood only was performed as a control (C group). To prepare the blood sample for A group rotational thromboelastography, we first dissolved the L-AA in 0.9% normal saline to a concentration of 5.55 mmol/L, and then added 0.036 mL of the AA aliquot to 0.324 mL of the native blood sample. We added 20 µmol/L of the L-AA aliquot to the native whole blood, with a 10% hemodilution effect. Therefore, to assess the effect of hemodilution and to differentiate it from the effect of AA, we performed another rotational thromboelastography test using 0.036 mL of normal saline that was added to the blood sample (0.324 mL; N group). We believed that this was necessary because previous studies indicated that hypercoagulability can occur with an acute hemodilution with 0.9% normal saline up to 30% of estimated blood volume.20,21
The rotational thromboelastography parameters were as follows: clotting time (CT, time to reach an amplitude of 2 mm from the start of the text), clot formation time (CFT, time between 2 mm and 20 mm amplitudes), amplitude 10 minutes (A10) and 20 minutes (A20) after CFT, maximum clot firmness (MCF, maximum amplitude reached during the test), maximum lysis (ML, maximum lysis during the run time), lysis index at time 60 minutes (LI60), time to maximum velocity of clot formation (MAXV-t), MCF-time (MCF-t), area under the first derivative curve from the start of the derivative curve until MCF is reached (AUC), and clot formation rate (CFR).
Washed platelet preparation
At the corresponding times, fresh whole blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes and divided into three samples, in the same manner as those used in the rotational thromboelastometry study, as follows: the C group contained 4 mL of fresh blood, the N group contained 3.6 mL of fresh blood + 0.4 mL of 0.9% normal saline, and the A group contained 3.6 mL of fresh blood + 0.4 mL of 200 µmol of L-AA dissolved in 0.9% normal saline.
The tubes were gently mixed on a shaker for 30 minutes at room temperature, and the platelets were isolated in accordance with the method described by Cazenabe et al.22 Briefly, we obtained platelet-rich plasma by centrifuging the samples at 180 g for 15 minutes. To isolate the platelets, we centrifuged the platelet-rich plasma again at 2200 g for 10 minutes. The platelet pellet was then gently resuspended in Ca2+-free Tyrode’s albumin solution (136 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgSO4, 5 mM glucose, and 0.2% bovine serum albumin, pH 7.35) containing 10 U of heparin and 0.5 µM of prostaglandin I2 (Sigma-Aldrich Co., St. Louis, MO, USA), and centrifuged at 1900 g for 8 minutes. The platelets were washed again with Ca2+-free Tyrode’s albumin solution containing 0.5 M of prostaglandin I2. All centrifuge procedures were performed at room temperature.
Immunoblotting
Platelets were lysed in lysis buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 1 µg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10% NP-40, protease inhibitor cocktail [Sigma-Aldrich Co.], and phosphatase inhibitor mixture [Phosphostop™, Sigma-Aldrich Co.]) for 15 minutes on ice. The protein content of the lysates was obtained by centrifugation at 16000 g for 15 minutes at 4°C. Quantification of the extracted proteins was performed using the Bradford method. Protein extracts (40 µg) from the three groups were loaded onto a 4–15% gradient gel (Bio-Rad, Hercules, CA, USA), subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene difluoride filter membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% skim milk dissolved in Tris-buffered saline (TBS) and incubated overnight (14 h) at 4°C with the following primary antibodies: polyclonal mouse anti-Cbl-b (1:1000; Cat# sc-376409, Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal mouse anti-c-Cbl (1:1000; Cat# 610442, BD Biosciences, Franklin Lakes, NJ, USA), and monoclonal mouse anti-c-Cbl (pY700) (1:1000; Cat# 612305, BD Biosciences). Thereafter, the membranes were washed three times in TBS with 0.5% Tween (TBS-T) and re-incubated with the indicated horseradish peroxidase-linked secondary antibody for 90 minutes at room temperature. The antibody-probed bands were detected on X-ray film (Agfa Healthcare, Canton, MA, USA) using an enhanced chemiluminescence reagent (Promega, Madison, WI, USA). We performed western blotting analysis in triplicate to confirm the results.
Statistical analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). To determine whether there were significant differences among the data obtained at each rotational thromboelastographic measurement from the three groups, we analyzed the data using the two-way analysis of variance test (parametric distribution) or the Friedman test (non-parametric distribution) followed by Tukey’s test. Sample size was determined based on our pilot study. Assuming that the mean value difference in CT between the N and A groups measured at baseline would be considered significant under a correlation coefficient 0.39, the power analysis revealed that 21 patients were required to satisfy α = .05 and β = .8. A P value < .05 was considered to be statistically significant.
Results
The patients’ characteristics are shown in Table 1. Preoperative hemoglobin, hematocrit, coagulation profiles, and the amount of transfused blood product during LDLT are presented in Tables 2 and 3. From January 2014 to February 2015, 21 patients were enrolled in this study; three patients were excluded because they received platelet concentrates before blood collection at 120 minutes after reperfusion. One patient who had a positive venereal disease test result was excluded for the protection of the researcher.
Table 1.
Demographic characteristics of patients.
| Age (years) | 52.1 ± 6.9 years |
| Sex (M/F) | 12/9 |
| Weight (kg) | 62.5 ± 10.8 kg |
| Height (cm) | 166.5 ± 9.4 cm |
| BMI (kg/m2) | 22.5 ± 3.2 kg/m2 |
| MELD score | 18 (12, 23) |
| Anesthetic duration (minutes) | 662.8 ± 90.4 minutes |
| Operation time (minutes) | 585.0 minutes (535.0, 613.0 minutes) |
| Anhepatic time (minutes) | 111.0 minutes (92.0, 130.0 minutes) |
| Operation time until reperfusion (minutes) | 319.0 minutes (283.0, 326.0 minutes) |
| Cold ischemic time (minutes) | 74.0 minutes (64.0, 98.0 minutes) |
| Warm ischemic time (minutes) | 28.0 minutes (23.0, 37.0 minutes) |
| Reason for liver transplantation (n) | |
| Hepatocellular carcinoma | 13 patients |
| Liver cirrhosis | 7 patients |
| Toxic hepatitis | 1 patient |
Data are presented as the mean ± standard deviation (SD; parametric distribution), or the median (25% percentile, 75% percentile; nonparametric distribution). MELD score, model for end-stage liver disease score.
Table 2.
Preoperative hemoglobin, hematocrit, and coagulation profiles.
| Hemoglobin | 10.7 ± 2.2 g/dL |
| Hematocrit | 31.8 ± 6.4% |
| Platelets | 63.0 ×103/µL (47.0 ×103/µL, 72.0 ×103/µL) |
| Prothrombin time | 17.3 (15.2, 22.5) s |
| Prothrombin time (international normalized ratio [INR]) | 1.45 (1.21, 1.88) |
| Activated partial thromboplastin time | 48.6 ± 12.3 s |
| Fibrinogen | 172.0 (152.0, 212.0) mg/dL |
Data are presented as the mean ± SD (parametric distribution), or median (25% percentile, 75% percentile; nonparametric distribution).
Table 3.
Amounts of transfused blood products during living donor liver transplantation.
| Packed red blood cell (packed) | 2 (0, 4) units |
| Fresh frozen plasma (units) | 2 (0, 5) units |
| Cryoprecipitates (units) | 3 (0, 6) units |
| Platelet concentrates (units) | 0 (0, 6) units |
Data are presented as the median (25% percentile, 75% percentile).
Rotational thromboelastographic analysis
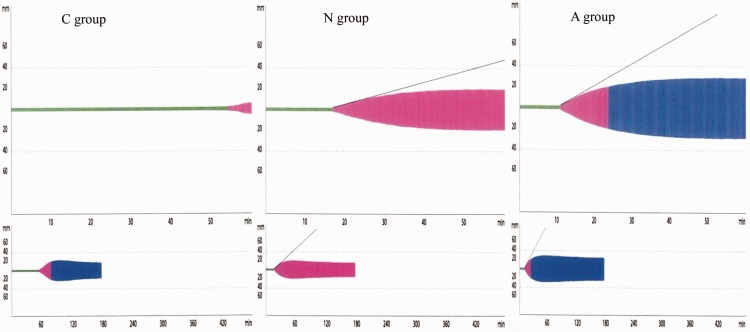
At baseline, the N group showed a significant decrease in CT and CFT compared with the C group. However, compared with the C group, the N group showed an increase (P < 0.05) in the α-angle, MCF, AUC from the start of the measurement to 6 minutes, and the CFR. The N and C groups showed no significant differences in A10 and A20 after CFT, MCF-t, ML, and LI60. However, compared with the N group, the A group showed a significant increase (P < 0.05) in the α-angle, A10 and A20 after CFT, MCF, MCF-t, AUC, and LI60, and significant a decrease (P < 0.05) in CT, CFT, MAXV-t, and ML (Table 4).
Table 4.
Results of rotational thromboelastography.
|
Baseline |
120 minutes after reperfusion |
|||||
|---|---|---|---|---|---|---|
| Group | C | N | A | C | N | A |
| CT (sec) | 905.0 (745.5, 1173.0) | 794.0 (703.5, 1017.0)a,b | 595.0 (516.0,737.0)a,b | 824.4 ± 482.7 | 735.0 ± 190.3 | 593.4 ± 151.7a |
| CFT (sec) | 650.0 (455.5, 1007.5) | 542.0 (378.0, 815.0)a,b | 407.5 (282.5, 751.0)a,b | 688.5 (488.0, 1288.5) | 706.7 ± 415.6a | 670.8 ± 325.3a |
| α-angle (°) | 26.6 ± 10.3 | 31.3 ± 9.3a,b | 36.6 ± 11.4a,b | 30.0 (19.0, 33.0) | 32.3 ± 11.6a | 31.6 ± 11.0a |
| A10 | 17.8 ± 7.6∗ | 20.7 ± 7.6b | 25.9 ± 8.5a,b | 18.0 (13.0, 21.0) | 19.0 (16.0, 25.0)a | 18.0 (17.0, 26.0)a |
| A20 | 25.3 ± 9.8 | 28.5 ± 8.8 b | 34.2 ± 10.3a,b | 26.0 (19.0, 29.0) | 27.5 (22.0, 34.0)a | 25.5 (23.5, 34.5)a |
| MCF (mm) | 31.6 ± 11.9 | 34.3 ± 10.6a,b | 38.5 ± 10.9a,b | 32.0 (23.5, 35.5) | 34.0 (26.5, 38.5)a,b | 31.5 (28.5, 40.5)a,b |
| ML (% of MCF) | 18.5 (15.0, 25.0) | 20.3 ± 7.2b | 14.8 ± 6.0a,b | 11.1 ± 5.2 | 10.7 ± 5.5b | 6.7 ± 1.3a,b |
| LI60 (% of MCF) | 96.5 (94.0, 100.0) | 96.5 (93.0, 98.0)b | 98.0 (96.0, 100.0)a,b | 100.0 (99.0, 100.0) | 100.0 (100.0, 100.0)b | 100.0 (100.0, 100.0)a,b |
| MAXV-t (sec) | 1018.0 (898.0, 1402.0) | 853.0 (780.0, 1093.0)a,b | 718.0 (623.5, 922.0)a,b | 830.5 (677.5, 964.0) | 843.0 (717.0, 999.0)a | 660.0 (550.0, 833.0)a |
| MCF-t (sec) | 2325.5 (2102.5, 2549.0) | 2105.5 (1945.5, 2360.0)b | 2384.5 (2171.5, 2575.5)b | 2597.5 (2483.0, 2897.0) | 2557.5 (2082.5, 2779.0)a,b | 2931.5 (2561.5, 3200.5)a,b |
| AUC (Ω*minutes) | 3176.6 ± 1191.8 | 3502.3 ± 974.7a,b | 3893.5 ± 1070.0a,b | 3267.5 (2473.5, 3680.0) | 3369.5 (2887.5, 3915.5)a | 3474.5 (2919.0, 4082.5)a |
| CFR (°) | 31.2 ± 9.62 | 45.7 ± 8.5a | 43.5 ± 10.3a | 38.5 (29.5, 43.0) | 44.0 (39.0, 54.0)a | 43.0 (39.0, 49.0)a |
CT, clotting time; CFT, clot formation time; A10 and A20, amplitude 10 and 20 minutes after CFT, respectively; MCF, maximum clot firmness; ML, maximum lysis; LI60, lysis index at 60 minutes; MAXV-t, time to maximum velocity of clot formation; MCF-t, MCF-time; AUC, area under curve from the start of measurement to 6 minutes; CFR, clot formation rate.
a: compared with C group (P < 0.05); b: compared between N and A groups (P < 0.05); C group, control group; N group, normal saline group; A group, AA group.
At 120 minutes after reperfusion, the N group showed a significant increase (P < 0.05) in CFT, α-angle, A10 and A20, MCF, MAXV-t, and AUC, and a decrease (P < 0.05) in MCF-t compared with the C group. These results are consistent with the findings observed when the baseline measurements were compared between the C and N groups, showing no decrease in fibrinolysis. Compared with the C group, all of the A group parameters showed significant changes (P < 0.05). However, the A group showed a significant decrease (P < 0.05) in MCF and ML, and a significant increase (P < 0.05) in LI60 and MCF-t compared with the N group (Table 4).
Western blot analysis
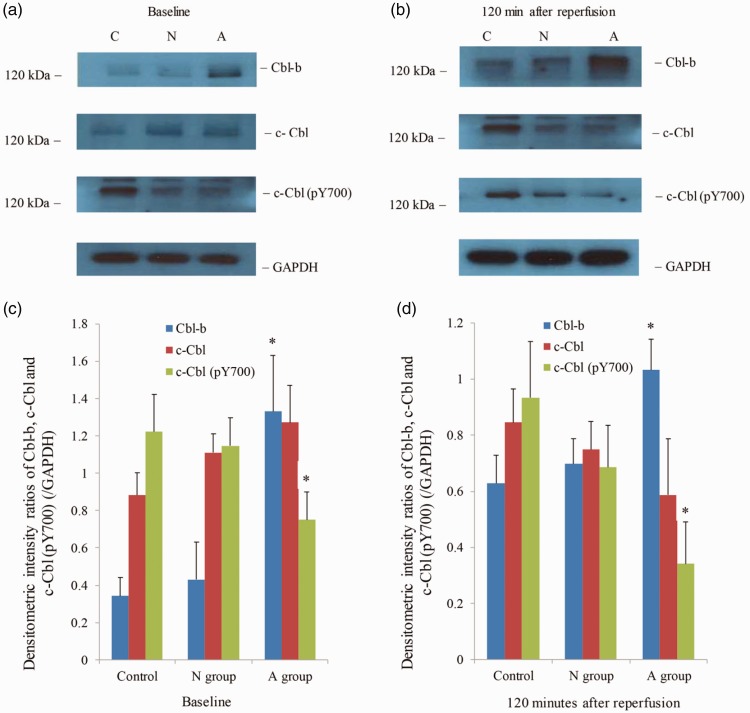
Western blotting revealed that Cbl-b expression in the A group was significantly increased at baseline and 120 minutes after reperfusion compared with the C and N groups (Figure 1). However, we could not detect significant phosphorylation of Cbl-b in any of the groups (data not shown). In our study, c-Cbl expression was not significantly changed in the N and A groups compared with the C group; however, phosphorylated c-Cbl in the A group was significantly decreased at baseline compared with that in the C group. Phosphorylated c-Cbl was most prominently decreased in the A group compared with the C and N groups at 120 minutes after reperfusion (Figure 2).
Figure 1.
A representative rotational thromboelastogram. Ascorbic acid (AA) significantly improved clot formation and decreased fibrinolysis at 120 minutes after reperfusion. C group, control group; N group, normal saline group; A group, ascorbic acid group.
Figure 2.
Changes in Cbl-b, c-Cbl, and phosphorylated forms of c-Cbl by ascorbic acid treatment. Western blot analysis of Cbl-b, c-Cbl, and phosphorylated forms of c-Cbl in C, N, and A groups at baseline (30 minutes after induction) (a) and 120 minutes after reperfusion (b), respectively, showed that ascorbic acid treatment changed the protein enzyme expression. Box plots delineate the densitometric intensity ratios of the corresponding Cbl-b, c-Cbl, and c-Cbl (pY700) western blot analysis at baseline (c) and 120 minutes after reperfusion (d). C group, control group; N group, normal saline group; A group, AA group. ∗p<0.05 compared with the control group.
Discussion
Coagulopathy and platelet dysfunction are common findings in patients with end-stage liver disease. The results of our study showed that AA might improve the hemostatic functions and increase the clot rigidity in recipients undergoing LDLT.
Numerous studies have demonstrated that antioxidants have an anticoagulatory function, and these inhibitory actions are explained by the broad protective effects against oxidative stress, including the cell membrane stabilization from lipid peroxidation,23 phosphatidylserine exposure on the cell surface,24 and the interaction between platelets and the endothelium25 to improve the intracellular hemostatic biological process.26–28 However, Parkinen et al.29 suggested that AA can play a role as a primary protector, preventing coagulation factor damage from photooxidative stress. Savini et al.30 reported that AA is transported to and accumulates in human platelets via Na+-dependent AA transporter 2, and intracellular AA can modulate post-aggregatory conditions, which can result in increased clot rigidity. The AA concentration is generally 40–60 µmol/L12 in plasma. We added an aliquot of 20 µmol/L of AA in the blood sample for each of the rotational thromboelastography and western blot analyses. Our results showed that the addition of AA in the A group broadly increased coagulation and decreased fibrinolysis at baseline compared with the C and N groups; specifically, AA increased CT, CFT, α-angle, A10, A20, MAXV-t, AUC, and MCF, and decreased baseline ML and LI60. However, the rotational thromboelastographic parameters measured at 120 minutes after reperfusion showed increased MCF and MCF-t, but decreased ML and LI60 in the A group compared with the N group. These findings suggest that AA may strengthen the clot firmness and reduce the fibrinolysis at baseline and 120 minutes after reperfusion.
The platelet activation signaling pathway initiated by the complex comprising glycoprotein VI and Fc receptor γ-chain is similar to the signal transduction caused by activation of the immune receptor.31,32 This signal transduction can be regulated by the E3 ubiquitin ligases c-Cbl and Cbl-b, which can serve as adapter molecules in the assembly of signaling proteins.33 Although the N-terminal regions of c-Cbl and Cbl-b share highly similar protein domain structures, c-Cbl and Cbl-b have functions that are distinct from one another. c-Cbl, which can ubiquitinate signaling molecules, is regarded as a negative regulator of the phospholipase Cγ2-dependent signal pathway. Van der Meijden et al.34 reported that platelet-deficient phospholipase Cγ2 showed a delay and suppression of phosphatidylserine exposure and thrombus formation. Daniel et al.17 reported that, in contrast to c-Cbl, which showed marked phosphorylation, Cbl-b phosphorylation was not significantly observed after stimulation by a glycoprotein VI agonist in human platelets. Although little is known about the function of Cbl-b in the platelet activation signaling pathway, it is thought that Cbl-b functions as a positive regulator, whereas c-Cbl acts as a negative regulator, of platelet activation.16,17,35 Similar to the report by Daniel et al.,17 we failed to detect phosphorylated Cbl-b. It is possible that Cbl-b phosphorylation marks it for proteosomal degradation, whereas c-Cbl is activated by phosphorylation. Our western blotting results showed that AA could improve the coagulation status by increasing the function of Cbl-b and by decreasing phosphorylation of c-Cbl. These combined effects may result in increased clot formation and clot rigidity in the baseline measurement and increased clot rigidity at 120 minutes after reperfusion. During LDLT, fibrinolysis is a key bleeding diathesis in LT recipients, which increases bleeding and the requirement for blood products.36,37 Therefore, AA use might improve hyperfibrinolysis during LDLT, when bleeding diathesis is caused by fibrinolysis.
There are several major limitations in our study. First, we performed both the rotational thromboelastographic studies and the functional Cbl-b and c-Cbl immunoblotting assessment in vitro to avoid any consequent changes in hemostatic conditions because LDLT recipients are usually in critical condition during transplantation. The interaction between platelets and the endothelium is critically important in the hemostatic process;38,39 however, we could not assess the results from their interactions. Therefore, further study is needed to determine whether the results of our study could be applicable to clinical practice. Second, we investigated the effect of AA on the hemostatic function of platelets using a single dose of AA, and we did not measure the plasma level of AA in each LDLT recipient. Therefore, we could not determine the optimal dose for the greatest improvement in hemostatic function. Third, we did not measure ROS or RNS levels because previous studies have reported their levels.40,41 Our results showed that AA affects coagulation at the baseline measurements more extensively compared with 120 minutes after reperfusion, and AA mainly affects clot rigidity at 120 minutes after reperfusion compared with baseline. Because we did not measure the ROS or RNS levels and we used only a single dose of AA, we could not clearly postulate the effects of AA on coagulation or explain why clot rigidity was more favorably detected at 120 minutes after reperfusion during LDLT. Fourth, although hyperfibrinolysis may be most severely activated immediately after reperfusion, we could not obtain comparable rotational thromboelastographic parameters at this point in many patients to analyze the effect of AA for fibrinolysis. For this reason, we chose 120 minutes after reperfusion as our test time point.
Our results showed that AA significantly decreased fibrinolysis and improved clot rigidity. Functional changes in the intracellular E3 ubiquitin ligases Cbl-b and c-Cbl by AA might be an underlying mechanism of these findings during LDLT. Therefore, to improve hyperfibrinolysis during LDLT, AA administration might be considered when bleeding diathesis is accompanied by hyperfibrinolysis. Further study is needed to determine the optimal AA dose and to elucidate a more precise mechanism for the role of AA in coagulation. Such studies may lead to improved hemostatic function in clinical practice for better outcomes in LDLT recipients.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
Support was provided solely by institutional and/or departmental sources.
References
- 1.Czubkowski P, Socha P, Pawlowska J. Current status of oxidative stress in pediatric liver transplantation. Pediatr Transplant 2010; 14: 169–177. [DOI] [PubMed] [Google Scholar]
- 2.Elias-Miro M, Jimenez-Castro MB, Rodes J, et al. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res 2013; 47: 555–568. [DOI] [PubMed] [Google Scholar]
- 3.Muriel P. Role of free radicals in liver diseases. Hepatol Int 2009; 3: 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol 2004; 24: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 5.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2015; 309: L915–L923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iuliano L, Pedersen JZ, Pratico D, et al. Role of hydroxyl radicals in the activation of human platelets. Eur J Biochem 1994; 221: 695–704. [DOI] [PubMed] [Google Scholar]
- 7.Olas B, Wachowicz B. Role of reactive nitrogen species in blood platelet functions. Platelets 2007; 18: 555–565. [DOI] [PubMed] [Google Scholar]
- 8.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 2004; 101: 4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejaoui M, Zaouali MA, Sakly R, et al. Olprinone protects the liver from ischemia-reperfusion injury through oxidative stress prevention and protein kinase Akt activation. Can J Physiol Pharmacol 2018; 96: 227–231. [DOI] [PubMed] [Google Scholar]
- 10.Faggio C, Sureda A, Morabito S, et al. Flavonoids and platelet aggregation: a brief review. Eur J Pharmacol 2017; 807: 91–101. [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Xue F. Current antioxidant treatments in organ transplantation. Oxid Med Cell Longev 2016; 2016: 8678510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr 1982; 47: 473–482. [DOI] [PubMed] [Google Scholar]
- 13.Oldham KM, Bowen PE. Oxidative stress in critical care: is antioxidant supplementation beneficial? J Am Diet Assoc 1998; 98: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka K, Houben P, Bruns H, et al. A systematic review of pharmacological treatment options used to reduce ischemia reperfusion injury in rat liver transplantation. PLoS One 2014; 10: e0122214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keane MM, Rivero-Lezcano OM, Mitchell JA, et al. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl proto-oncogene. Oncogene 1995; 10: 2367–2377. [PubMed] [Google Scholar]
- 16.Buitrago L, Tsygankov A, Sanjay A, et al. Cbl proteins in platelet activation. Platelets 2013; 24: 419–427. [DOI] [PubMed] [Google Scholar]
- 17.Daniel JL, Dangelmaier CA, Mada S, et al. Cbl-b is a novel physiologic regulator of glycoprotein VI-dependent platelet activation. J Biol Chem 2010; 285: 17282–17291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature 2009; 458: 438–444. [DOI] [PubMed] [Google Scholar]
- 19.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol 2004; 5: 883–890. [DOI] [PubMed] [Google Scholar]
- 20.Ng KF, Lam CC, Chan LC. In vivo effect of haemodilution with saline on coagulation: a randomized controlled trial. Br J Anaesth 2002; 88: 475–480. [DOI] [PubMed] [Google Scholar]
- 21.Singbartl K, Innerhofer P, Radvan J, et al. Hemostasis and hemodilution: a quantitative mathematical guide for clinical practice. Anesth Analg 2003; 96: 929–935, table of contents. [DOI] [PubMed] [Google Scholar]
- 22.Cazenave JP, Hemmendinger S, Beretz A, et al. [Platelet aggregation: a tool for clinical investigation and pharmacological study. Methodology]. Ann Biol Clin (Paris) 1983; 41: 167–179. [PubMed] [Google Scholar]
- 23.Cyrus T, Tang LX, Rokach J, et al. Lipid peroxidation and platelet activation in murine atherosclerosis. Circulation 2001; 104: 1940–1945. [DOI] [PubMed] [Google Scholar]
- 24.Jain SK, Palmer M, Chen Y. Effect of vitamin E and N-acetylcysteine on phosphatidylserine externalization and induction of coagulation by high-glucose-treated human erythrocytes. Metabolism 1999; 48: 957–959. [DOI] [PubMed] [Google Scholar]
- 25.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996; 94: 6–9. [DOI] [PubMed] [Google Scholar]
- 26.Chang CC, Lu WJ, Chiang CW, et al. Potent antiplatelet activity of sesamol in an in vitro and in vivo model: pivotal roles of cyclic AMP and p38 mitogen-activated protein kinase. J Nutr Biochem 2010; 21: 1214–1221. [DOI] [PubMed] [Google Scholar]
- 27.Freedman JE, Li L, Sauter R, et al. alpha-Tocopherol and protein kinase C inhibition enhance platelet-derived nitric oxide release. FASEB J 2000; 14: 2377–2379. [DOI] [PubMed] [Google Scholar]
- 28.Pignatelli P, Sanguigni V, Paola SG, et al. Vitamin C inhibits platelet expression of CD40 ligand. Free Radic Biol Med 2005; 38: 1662–1666. [DOI] [PubMed] [Google Scholar]
- 29.Parkkinen J, Vaaranen O, Vahtera E. Plasma ascorbate protects coagulation factors against photooxidation. Thromb Haemost 1996; 75: 292–297. [PubMed] [Google Scholar]
- 30.Savini I, Catani MV, Arnone R, et al. Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic Biol Med 2007; 42: 608–616. [DOI] [PubMed] [Google Scholar]
- 31.Pasquet JM, Gross B, Quek L, et al. LAT is required for tyrosine phosphorylation of phospholipase cgamma2 and platelet activation by the collagen receptor GPVI. Mol Cell Biol 1999; 19: 8326–8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quek LS, Bolen J, Watson SP. A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol 1998; 8: 1137–1140. [DOI] [PubMed] [Google Scholar]
- 33.Tsygankov AY, Teckchandani AM, Feshchenko EA, et al. Beyond the RING: CBL proteins as multivalent adapters. Oncogene 2001; 20: 6382–6402. [DOI] [PubMed] [Google Scholar]
- 34.van der Meijden PE, Munnix IC, Auger JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood 2009; 114: 881–890. [DOI] [PubMed] [Google Scholar]
- 35.Dangelmaier CA, Quinter PG, Jin J, et al. Rapid ubiquitination of Syk following GPVI activation in platelets. Blood 2005; 105: 3918–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roullet S, Freyburger G, Labrouche S, et al. Hyperfibrinolysis during liver transplantation is associated with bleeding. Thromb Haemost 2015; 113: 1145–1148. [DOI] [PubMed] [Google Scholar]
- 37.Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost 1993; 19: 191–196. [DOI] [PubMed] [Google Scholar]
- 38.Grabowski EF, Yam K, Gerace M. Evaluation of hemostasis in flowing blood. Am J Hematol 2012; 87: S51–S55. [DOI] [PubMed] [Google Scholar]
- 39.Kazmi RS, Boyce S, Lwaleed BA. Homeostasis of hemostasis: the role of endothelium. Semin Thromb Hemost 2015; 41: 549–555. [DOI] [PubMed] [Google Scholar]
- 40.Rao PN, Walsh TR, Makowka L, et al. Inhibition of free radical generation and improved survival by protection of the hepatic microvascular endothelium by targeted erythrocytes in orthotopic rat liver transplantation. Transplantation 1990; 49: 1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bzeizi KI, Dawkes R, Dodd NJ, et al. Graft dysfunction following liver transplantation: role of free radicals. J Hepatol 1997; 26: 69–74. [DOI] [PubMed] [Google Scholar]