Short abstract
Premature ovarian insufficiency is characterized by reduced ovarian function in a young woman, resulting in an earlier menopause compared with women with normal ovarian function. It is a term that reflects the variable nature of the condition and is substantially less emotive than the formerly used ‘premature ovarian failure’, which suggested a single event in time. Tripterygium glycosides can damage ovarian function and cause infertility. This case report describes a 33-year-old female patient (gravida 0, parity 0) who presented with premature ovarian insufficiency after being treated with tripterygium glycosides for nephrotic syndrome. The woman received oestrogen-progestin replacement therapy (Femoston) and ovulation induction, which resulted in a successful pregnancy. The patient delivered a healthy baby girl. This case demonstrates that it is possible for a woman with tripterygium glycoside-induced premature ovarian insufficiency to become pregnant with the correct therapy.
Keywords: Oestrogen-progestin replacement therapy, pregnancy, premature ovarian insufficiency, tripterygium glycosides
Introduction
Premature ovarian insufficiency (POI) is defined as the loss of ovarian activity in a woman before the age of 40 years with elevated gonadotrophin and decreased oestrogen.1 The diagnosis of POI is based on a serum concentration of follicle-stimulating hormone (FSH) of >25 IU/l, monitored two times with at least 1 month apart in women aged <40 years.2,3 POI is typically caused be a reduced follicle pool and follicle dysfunction in the ovary, which leads to infertility and significant health implications. It is believed that 1% of women aged <40 years and 0.1% of women aged <30 years will develop POI, which has multiple aetiologies, including genetic factors, autoimmune factors, iatrogenic factors related to chemotherapy or radiation, surgical factors and spontaneous presentation.4
Tripterygium glycosides are commonly used for therapy of refractory kidney disease, but they can cause reproductive system function impairment.5 This current case report describes a patient whose nephrotic syndrome was treated using tripterygium glycosides, but who then experienced seriously impaired ovarian function. After 1 year and 8 weeks of treatment with tripterygium glycosides, the patient experienced amenorrhea and menopausal symptoms, including tidal fever, night sweats, insomnia, dyspareunia, decreased sexual desire and vaginal dryness. She was diagnosed with ‘primary infertility, premature ovarian insufficiency, nephrotic syndrome’. Owing to her having a strong desire for a child, the patient was treated with five cycles of Femoston therapy and three cycles of letrozole ovulation induction. The patient successfully delivered a healthy baby girl. The whole therapeutic process and gestation went well and the nephrotic syndrome did not relapse.
Case report
A 33-year-old woman (gravida 0, parity 0) was diagnosed in May 2018 with ‘primary infertility, premature ovarian insufficiency, nephrotic syndrome’ in the Key Laboratory for Reproductive Medicine and Embryology, The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Lanzhou, China. In February 2007, in order to block the progression of nephrotic syndrome, the patient had received 1 year and 8 weeks of tripterygium glycosides therapy as follows: (i) 2 mg/kg per day tripterygium glycosides orally for 4 weeks; (ii) 1.5 mg/kg per day tripterygium glycosides orally for 4 weeks; (iii) 1 mg/kg per day tripterygium glycosides orally for 1 year. Before receiving tripterygium glycosides, her normal menstrual cycle was 28 or 30 days in duration and her menstrual period lasted 7 days. After she had received tripterygium glycosides therapy for 6 months, her menstrual cycle changed to 2 or 3 months in duration and her menstrual period lasted 2 days. At 6 months after the end of tripterygium glycosides treatment, amenorrhea occurred and lasted for 2 years. As the patient had a strong desire to have a child, she visited The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Lanzhou, China. The patient had a normal karyotype 46, XX and Fragile X syndrome was excluded. Hysterosalpingography confirmed bilateral tubal patency. Her husband’s semen examination was normal. Transvaginal ultrasonography revealed the following: uterus size, 5.3 × 2.7 × 4.2 cm; endometrial thickness, linear; right ovarian volume, 2.3 cm3 with two follicles; left ovarian volume, 1.9 cm3 with two follicles. Serum hormone levels were as follows: FSH, 121.5 IU/l; luteinizing hormone (LH), 39.3 IU/l; oestradiol (E2), < 5 pg/ml; and anti-Müllerian hormone (AMH), 0.06 ng/ml. After three 28-day cycles of Femoston therapy (Solvay Pharmaceuticals, Brussels, Belgium), each of which consisted of 2 mg/day oestradiol orally for 14 days followed by 2 mg/day oestradiol in combination with 10 mg/day dydrogesterone orally for 14 days, her serum hormone levels were as follows: FSH, 91.0 IU/l; LH, 40.7 IU/l; and E2, 13.2 pg/ml. After five cycles of Femoston therapy (2 mg/day oestradiol in combination with 10 mg/day dydrogesterone), her serum hormone levels were as follows: FSH, 20.2 IU/l; LH, 12.8 IU/l; and E2, 25.9 pg/ml. Transvaginal ultrasonography displayed the following: endometrial thickness, 5 mm; right ovarian follicle diameter, 7 mm; left ovarian follicle diameter, 5 mm. Then, three cycles of 2.5 mg/day letrozole (Zentiva, Guildford, UK) orally were administered for 5 days to induce ovulation. During the first cycle of ovulation induction, one follicle developed and ruptured at a diameter of 17 mm. The patient did not become pregnant after sexual intercourse. During the second cycle of ovulation induction, one follicle developed and ruptured at a diameter of 18.5 mm. The patient did not become pregnant after sexual intercourse. During the third cycle of ovulation induction, one follicle developed and ovulated at a diameter of 18 mm. The patient became pregnant during this cycle and successfully delivered a healthy baby girl. The study was approved by the Ethics Committee of the First Hospital of Lanzhou University, Lanzhou, China (no. LDYYLL2019-148) and the patient provided verbal informed consent.
Discussion
This current case report describes a 33-year-old woman with POI caused by tripterygium glycosides treatment for nephrotic syndrome. The AMH level of the patient was quite low and serum FSH was abnormally high prior to fertility treatment. Transvaginal ultrasonography demonstrated two follicles on each ovary. The conditions for pregnancy were not favourable, but the patient had a very strong desire to have a child, so she received the appropriate fertility treatment and successfully delivered a healthy baby girl.
Tripterygium glycosides (extracted from Chinese traditional medicine) reduce the production of immunoglobulin M by inhibiting the function of helper T-cells and B-cells, by decreasing the production of interleukin 2, by reducing the permeability of capillaries and by exerting anti-inflammatory and immunosuppressive effects.6,7 Therefore, tripterygium glycosides are widely used to treat nephrotic syndrome as they are considered to be a relatively safe Chinese medicine compared with other immunosuppressants.8 However, tripterygium glycosides can seriously damage an adult women’s fertility, which results in menstrual dysfunction or amenorrhea. A previous study found that after 16 weeks of tripterygium glycosides (12 mg/kg), the follicle number in female rats was rapidly reduced.9 Also, the oocytes shrank or disappeared, the follicular granulosa cells collapsed and the connective tissue showed hyperplasia.9
The current patient was administered tripterygium glycosides because other drugs were unable to control the progression of her nephrotic syndrome and this treatment resulted in amenorrhea after more than 1 year of therapy. Ovary protective drugs were not used during this treatment process, which finally resulted in ovarian functional insufficiency. Although the woman’s ovarian functional impairment was severe, she completed five cycles of Femoston and ovulation induction, which finally resulted in conception and a successful pregnancy. In our opinion, there were several reasons that contributed to her successful pregnancy, which will be discussed below.
The damage caused by tripterygium glycosides to the female reproductive system is closely related to age and the possibility of recovery of reproductive function after withdrawal of tripterygium glycosides is better in young women than in older women.10 Tripterygium glycosides mainly have cytotoxic effects on ovarian tissue, characterized by reduced ovarian volume, reduced number of primordium follicles, reduced number of growing follicles, thinning of the follicle granular cell layer and corpus luteum defects.11 Tripterygium glycosides also increase follicular atresia and stromal hyperplasia.12Under the electron microscope, the cells in the ovarian tissue show nuclear pyknosis, nuclear gap widening, partial disappearance of the nuclear membrane, and damaged rough endoplasmic reticulum and mitochondrial structure.11 However, the above-mentioned ovarian toxicity is time-dependent and the use of tripterygium glycosides for more than 1 year is generally believed to cause irreversible damage to the ovaries.13 Although this current patient was administered tripterygium glycosides for more than 1 year, she was young at the time of treatment and residual primordium follicles appeared to have demonstrated strong resistance to the cytotoxic effects of the drug.
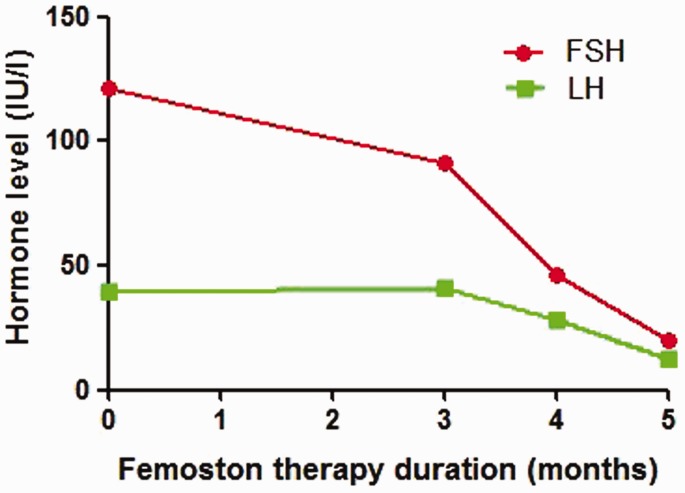
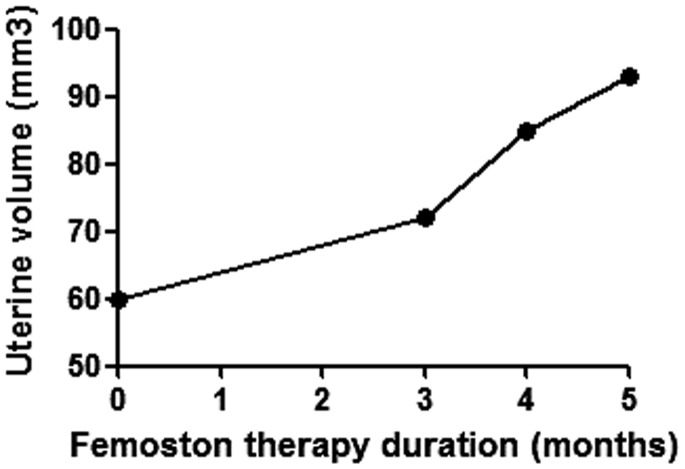
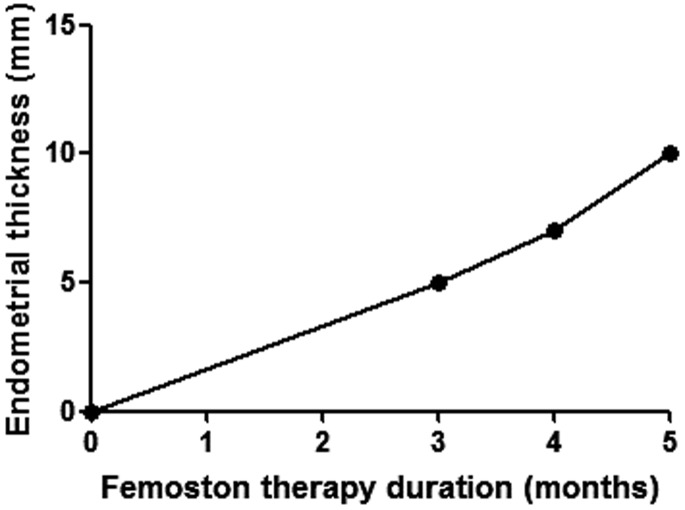
Sequential oestrogen-progestin replacement therapy effectively prevents and controls menopausal symptoms. Normal ovaries secrete oestrogen, including oestradiol, oestrone and oestriol. Oestrogen replacement therapy alleviates menopausal symptoms and improves sexual dysfunction, which is related to vaginal dryness, dyspareunia and decreased libido. Moreover, oestradiol treatment may increase the sensitivity of FSH receptors and thereby enhance the response to exogenous gonadotropins during ovulation induction.14 Progestin therapy may suppress the inappropriate luteinization of follicles caused by chronically elevated LH levels and thereby improve ovulation rates.15 Femoston contains 17-β-estradiol and 17-β-estradiol/dydroprogesterone, and it is usually used for oestrogen-progestin replacement therapy. After five cycles of oestrogen-progestin replacement therapy in this current case, the FSH level was decreased (Figure 1). Both the volume of the uterus and the endometrial thickness increased on the 11th day of Femoston therapy (Figures 2 and 3), which indicated that the uterus reacted as expected to the oestrogen-progestin replacement therapy.
Figure 1.
Effect of oestrogen-progestin replacement therapy (Femoston) on pituitary and ovarian hormone levels in a 33-year-old woman who was diagnosed with ‘primary infertility, premature ovarian insufficiency, nephrotic syndrome’ following treatment of nephrotic syndrome with tripterygium glycosides. FSH, follicle-stimulating hormone; LH, luteinizing hormone. The colour version of this figure is available at: http://imr.sagepub.com.
Figure 2.
Effect of oestrogen-progestin replacement therapy on uterine volume on day 11 of Femoston treatment in a 33-year-old woman who was diagnosed with ‘primary infertility, premature ovarian insufficiency, nephrotic syndrome’ following treatment of nephrotic syndrome with tripterygium glycosides.
Figure 3.
Effect of oestrogen-progestin replacement therapy on endometrial thickness on day 11 of Femoston treatment in a 33-year-old woman who was diagnosed with ‘primary infertility, premature ovarian insufficiency, nephrotic syndrome’ following treatment of nephrotic syndrome with tripterygium glycosides.
In this current case, the cause of infertility was treatment with tripterygium glycosides. There was no pelvic adhesion in the patient or sperm abnormalities in her husband’s semen. Therefore, after oestrogen-progestin replacement therapy and ovulation induction, the woman conceived quickly under clinical guidance.16
Tripterygium glycosides usually cause POI in women.8 Approximately 5–10% spontaneous pregnancies and spontaneous reversibility of ovarian function have been reported in patients with POI, but the rate decreases with aging.17 In this current case, oestrogen-progestin replacement therapy improved the pelvic and uterus environment and letrozole ovulation induction reduced the time of waiting for conception. If letrozole had not been used, the patient may have ovulated naturally, but using letrozole may have helped the follicles to mature and it might have accelerated ovulation.
In conclusion, more experience should be accumulated during the diagnosis and treatment of patients with POI induced by tripterygium glycosides. This current case has demonstrated that patients in this situation can be treated successfully resulting in pregnancy. The success rate of fertility treatment depends upon the age of the patient and the time waiting for conception. Follicular monitoring and blood hormone level determination are essential for reproductive success. Also, it is important to use effective measures to protect ovarian function when using drugs that are toxic to the ovaries.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by grants from the Foundation of Gansu Natural Science (no. 18JR3RA340), the Foundation of Lanzhou Science (no. 2017-4-56), and the Foundation of the First Hospital of Lanzhou University (no. 2017-01 and no. 2017-13).
References
- 1.Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vujovic S, Brincat M, Erel T, et al. EMAS position statement: managing women with premature ovarian failure. Maturitas 2010; 67: 91–93. [DOI] [PubMed] [Google Scholar]
- 3.Panay N, Fenton A. Premature ovarian insufficiency: working towards an international database. Climacteric 2012; 15: 295–296. [DOI] [PubMed] [Google Scholar]
- 4.Podfigurna-Stopa A, Czyzyk A, Grymowicz M, et al. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest 2016; 39: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen XY, Gu C, Ma M, et al. A mouse model of premature ovarian insufficiency induced by tripterygium glycoside via subcutaneous injection. Int J Clin Exp Pathol 2014; 7: 144–151. [PMC free article] [PubMed] [Google Scholar]
- 6.Ma M, Li RX, Chen XY, et al. Premature ovarian insufficiency induced by tripterygium glycoside: does melatonin offer protection? Int J Clin Exp Med 2016; 9: 10727–10736. [Google Scholar]
- 7.Ebrahimi M, Akbari Asbagh F. Pathogenesis and causes of premature ovarian failure: an update. Int J Fertil Steril 2011; 5: 54–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Li X, Li H, et al. Comparison of tripterygium wilfordii multiglycosides and tacrolimus in the treatment of idiopathic membranous nephropathy: a prospective cohort study. BMC Nephrol 2015; 16: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Zhao Z, Wu Y, et al. Therapeutic mechanisms of Tongmai Dasheng Tablet on tripterygium glycosides induced rat model for premature ovarian failure. J Ethnopharmacol 2012; 139: 26–33. [DOI] [PubMed] [Google Scholar]
- 10.Maclaran KE, Horner E, Panay N. Premature ovarian failure: long-term sequelae. Menopause Int 2010; 16: 38–41. [DOI] [PubMed] [Google Scholar]
- 11.Ma M, Chen XY, Gu C, et al. Biochemical changes of oxidative stress in premature ovarian insufficiency induced by tripterygium glycosides . Int J Clin Exp Pathol 2014; 7: 8855.–. [PMC free article] [PubMed] [Google Scholar]
- 12.Ma M, Chen XY, Li B, et al. Melatonin protects premature ovarian insufficiency induced by tripterygium glycosides: role of SIRT1. Am J Transl Res 2017; 9: 1580–1602. [PMC free article] [PubMed] [Google Scholar]
- 13.Kurt A, Ingec M, Isaoqlu U, et al. An investigation about the inhibition of acute ischemia/reperfusion damage by dexmedetomidine in rat ovarian tissue. Gynecol Endocrinol 2013; 29: 222–225. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright B, Robinson J, Seed PT, et al. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomised controlled trial of the effects on bone mineral density. J Clin Endocrinol Metab 2016; 101: 3497–3505. [DOI] [PubMed] [Google Scholar]
- 15.Guerrieri GM, Martinez PE, Klug SP, et al. Effects of physiologic testosterone therapy on quality of life, self esteem, and mood in women with primary ovarian insufficiency. Menopause 2014; 21: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkosz P, Greggains GD, Tanbo TG, et al. Female reproductive declin is determined by remaining ovarian reserve and age. PLoS One 2014; 9: e108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins G, Patel B, Thakore S, et al. Primary Ovarian Insufficiency: Current Concepts. South Med J 2017; 110: 147.–. [DOI] [PubMed] [Google Scholar]