Abstract
Background
Bisphosphonates are indicated in the prevention and treatment of osteoporosis. However, bone mineral density (BMD) continues to decline in up to 15% of bisphosphonate users. While randomized trials have evaluated the efficacy of concurrent bisphosphonates and vitamin D, the incremental benefit of vitamin D remains uncertain.
Methods
Using data from the Canadian Database of Osteoporosis and Osteopenia (CANDOO), we performed a 2-year observational cohort study. At baseline, all patients were prescribed a bisphosphonate and counseled on vitamin D supplementation. After one year, patients were divided into two groups based on their response to bisphosphonate treatment. Non-responders were prescribed vitamin D 1000 IU daily. Responders continued to receive counseling on vitamin D.
Results
Of 449 patients identified, 159 were non-responders to bisphosphonates. 94% of patients were women. The mean age of the entire cohort was 74.6 years (standard deviation = 5.6 years). In the cohort of non-responders, BMD at the lumbar spine increased 2.19% (p < 0.001) the year after vitamin D was prescribed compared to a decrease of 0.55% (p = 0.36) the year before. In the cohort of responders, lumbar spine BMD improved 1.45% (p = 0.014) the first year and 1.11% (p = 0.60) the second year. The difference between the two groups was statistically significant the first year (p < 0.001) but not the second (p = 0.60). Similar results were observed at the femoral neck but were not statistically significant.
Conclusion
In elderly patients with osteoporosis not responding to bisphosphonates, vitamin D 1000 IU daily may improve BMD at the lumbar spine.
Background
Osteoporosis is a skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, which consequently increase bone fragility and the susceptibility to fracture [1]. Osteoporosis strikes one in four women over the age of 50 years [2]. There are over 20 000 osteoporosis-related hip fractures in Canada annually. The mortality associated with hip fractures exceeds 25% at one year, with nearly one quarter of survivors requiring institutionalization in a long-term care facility [3].
Bisphosphonates are indicated in the prevention and treatment of Osteoporosis, as they are potent inhibitors of bone resorption. Clinical trials in primary Osteoporosis have provided conclusive evidence that in most patients therapy with this class of drug leads to improvement in bone mass and a reduction in subsequent fractures [4-19].
Despite treatment with a bisphosphonate, bone mineral density (BMD) continues to decline in up to 15% of etidronate users and 5% of alendronate users [4,13]. As a consequence, vitamin D may be recommended as adjunctive therapy in addition to increased calcium intake in those who do not to respond to bisphosphonate therapy. The vitamin D metabolite 1,25-dihydroxy vitamin D (calcitriol) is required for the active intestinal absorption of calcium and plays in concert with the parathyroid hormone (PTH), an important role in releasing calcium from bone and regulating plasma calcium [20]. Vitamin D deficiency is a risk factor for Osteoporosis [21-24]. Moreover, daily supplementation with vitamin D and calcium has been shown to reduce bone loss at the spine and femoral neck as well as the prevalence of non-vertebral fractures [25-27].
While a large number of randomized trials have evaluated the efficacy of bisphosphonate treatment with concurrent vitamin D (in doses varying between 125 to 500 IU) [4,16,28-31], the incremental benefit of vitamin D in patients taking bisphosphonates is uncertain. Therefore, from data that were collected from the Canadian Database of Osteoporosis and Osteopenia (CANDOO) patients [32], we performed a 2-year observational cohort study to determine the impact of prescribed vitamin D supplements on BMD in osteoporotic patients who had not responded to bisphosphonates and calcium therapy alone.
Methods
Study Design
Data were obtained from an analysis of computer-based patient records registered in CANDOO. The CANDOO database was designed to prospectively compile comprehensive clinical data in a cohort of patients seen in specialized tertiary care referral centres in Hamilton, Ontario, Canada. Data are aggregated using anonymous patient identifiers into a centrally maintained, fully keyed and encoded relational database. CANDOO contains data regarding patient demographics, medications and side effects, female reproductive history, diet and quality of life, BMD measurements, fracture history, and laboratory investigations. A patient record is generated at each consultation and at each follow-up visit. In addition, at each visit, patients received counseling about exercise, diet and supplementation with calcium and vitamin D.
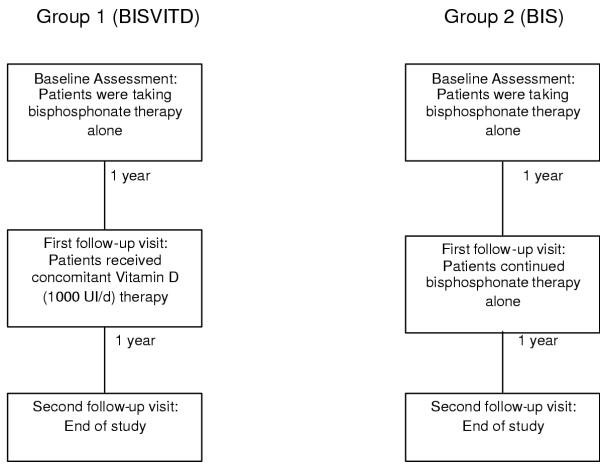
For the current analysis the database was searched for patients attending a single clinic from January 1990 to November 1997 (baseline). Bisphosphonate therapy was given as either cyclical etidronate (400 mg/d for 14 days followed by 76 days of 500 mg/d of elemental calcium) or alendronate (10 mg/day). Risedronate was not available in Canada at the time of the study. A total of 449 patients formed this cohort. These patients were then followed for two years. At the first follow-up visit (at approximately one year) patients were divided into two treatment groups depending on their response to bisphosphonate therapy in the preceding year. Group 1 (BISVITD) comprised those who did not respond to bisphosphonate therapy in the preceding year. In this cohort of patients, concomitant therapy with vitamin D (1000 lU/d) was prescribed. Treatment non-responders were defined as patients who had a negative change in lumbar spine and femoral neck BMD from baseline measurements or experienced an incident fracture while on therapy. Group 2 (BIS) comprised those who responded to bisphosphonate therapy in the preceding year and were not taking vitamin D supplements. Responders were defined as patients who had a positive change in lumbar spine and femoral neck BMD from baseline and did not sustain an incident fracture while on therapy. Both groups were then followed for an additional year (second follow-up visit) (figure 1).
Figure 1.
Time Lines
Measurements
BMD measurements were taken at baseline, the first follow-up visit and the second follow-up visit using Dual-Energy X-ray Absorptiometry (DXA) with Lunar DPX-alpha (Lunar Corporation, Madison, Wisconsin). The coefficient of variation for measurements reported for this instrument is 68% of repeat measurements falling within 1 standard deviation. All three measurements for each individual patient were performed using the same device. Baseline data extracted for analysis include age; sex; body mass index (BMI); bisphosphonate type; prior or current treatment with ovarian hormones; treatment with fluoride or calcitonin; risk factors for osteoporosis including smoking, alcohol intake, prior fractures and exposure to corticosteroids (inhaled or systemic), anti-seizure medication, thyroid hormone therapy, chemotherapy or immune suppressants; dietary and supplemental calcium intake; and serum calcium, phosphorus, and alkaline phosphatase (ALP). 25-hydroxy vitamin D (25(OH)D) levels were obtained through blood samples collected within 30 days of clinic visit and sent to a central hospital laboratory. 25(OH)D levels were determined using a radioimmunoassay kit (normal range 16–74 ng/mL; developed by Nichols Institute Diagnostics, California). Incident fractures were registered based on patient response to an item ("How many new fractures have you had since your last visit?) from the CANDOO questionnaire. Incident vertebral fractures may or may not have been confirmed by x-ray. Unblinded clinical judgment was used to determine the existence of a vertebral fracture on x-ray. Incident non-vertebral fractures included the ankle, arm, clavicle, elbow, foot, heel, hand, hip, knee, leg, nose, pelvis, rib, shoulder, sacrum, and wrist.
Statistical Analysis
Differences in baseline characteristics were analyzed using the Students' t-test. Analysis of covariance was performed to test for differences between the groups in the mean percent change from baseline to the first and second follow-up visits in BMD at the lumbar spine and femoral neck. Covariates included in the analyses were those variables that were significantly different between groups at baseline. One-way analysis of variance was performed to test for mean percent changes within groups in BMD at the lumbar spine and femoral neck. The relationship between serum 25(OH)D and response to vitamin D was analyzed with linear regression. All analyses used a two-tailed alpha level of 5% and were performed with SPSS Version 8.0 (SPSS Inc).
Results
Patient characteristics are presented in Table 1. Overall, 449 patients were identified, 159 patients received a bisphosphonate and vitamin D while 290 received bisphosphonate therapy. Due to missing data, lumbar spine BMD was available for 155 (BISVITD) and 282 (BIS) patients, whereas femoral neck BMD was available for 156 and 280 patients from each treatment group, respectively. The 12 patients for whom lumbar spine data were missing had a higher BMI (29.1 vs. 25.9 kg/m2, p = 0.020) and consumed more hypnotics (33% vs 10%, p = 0.030) as compared with patients that had complete lumbar spine data. Furthermore, the 13 patients with missing femoral neck data were more likely to have received systemic steroids (46% vs 18%, p < 0.010), tamoxifen (10% vs 3%, p < 0.001) and fluoride (30% vs 8%, p = 0.005); less likely to receive anti-epileptics (10% vs 0.9%, p = 0.006); and had a higher serum ALP than the group with complete femoral neck data (120.8 vs 90.9 mmol/L, p = 0.018).
Table 1.
Baseline Characteristics
| BIS | BISVITD | P value | |
| N | 290 | 159 | - |
| Age (years) (SD*) | 75.1 (5.7) | 73.6 (5.6) | 0.011 |
| Women | 263 | 149 | 0.267 |
| Body mass index (kg/m2) (SD*) | 26.3 (4.9) | 25.3(3.7) | 0.024 |
| Bisphosphonate | |||
| - Etidronate | 89.3% | 95.0% | 0.042 |
| - Alendronate | 10.7% | 5.0% | |
| Years of bisphosphonates (SD*) | 5.9 (2.4) | 1 (0.0) | 0.001 |
| Calcium (mg/day) | |||
| - supplement | 658.2 | 757.1 | 0.002 |
| - dietary | 635.3 | 609.9 | 0.586 |
| - total | 1266.0 | 1319.0 | 0.369 |
| Previous exposure to | |||
| - estrogen | 37.6% | 30.4% | 0.127 |
| - fluoride | 9.0% | 8.8% | 0.955 |
| - calcitonin | 1.7% | 3.0% | 0.330 |
| Previous fracture | 68% | 69% | 0.786 |
| Weekly alcohol intake (ounces) | 1.63 | 1.26 | 0.537 |
| Smoking history | 32% | 35% | 0.616 |
| Corticosteroids | |||
| - systemic | 19.7% | 17.0% | 0.488 |
| - inhaled | 12.4% | 14.5% | 0.539 |
| Ever treated with | |||
| - non-steroidal anti-inflammatory | 39.7% | 35.2% | 0.356 |
| - thyroxin | 12.6% | 15.7% | 0.393 |
| β-blocker | 6.6% | 8.2% | 0.523 |
| - sedative-hypnotic | 7.2% | 6.3% | 0.704 |
| - thiazide | 7.2% | 6.3% | 0.704 |
| - calcium-channel blocker | 1.0% | 2.5% | 0.227 |
| - lipid-lowering agent | 0.3% | 2.5% | 0.036 |
| - anticonvulsants | 0.7% | 1.3% | 0.541 |
| - tamoxifen | 0.7% | 1.3% | 0.295 |
| - immunosuppressant | 0.0% | 1.9% | 0.019 |
| - chemotherapy | 0.0% | 0.0% | - |
| Laboratory values | |||
| - 25(OH)D (ng/mL) | 32.9 | 31.3 | 0.493 |
| - ALP(IU/L) | 90.7 | 94.0 | 0.474 |
| - Calcium (mmol/L) | 2.36 | 2.36 | 0.903 |
| - Phosphorus (mmol/L) | 1.13 | 1.14 | 0.539 |
| Baseline BMD (g/cm2) | |||
| - lumbar spine | 0.9287 | 0.8991 | 0.045 |
| - femoral neck | 0.7031 | 0.6850 | 0.081 |
*Standard Deviation
Of the patients evaluated in the study, 94% were women. The cohort was relatively elderly, with a mean age of 74.6 years (standard deviation = 5.6 years). There were statistically significant differences between the groups at baseline with respect to age, BMI, type of bisphosphonate, calcium supplementation and lumbar BMD. There was no difference in total calcium intake. There were significant differences in exposure to immune suppressing and lipid lowering agents, though 0.7% and 1% of all patients respectively had received such medications. Serum 25(OH)D levels were not different between the groups, but were available for 51 treatment and 115 control patients only (Table 1).
Lumbar Spine BMD
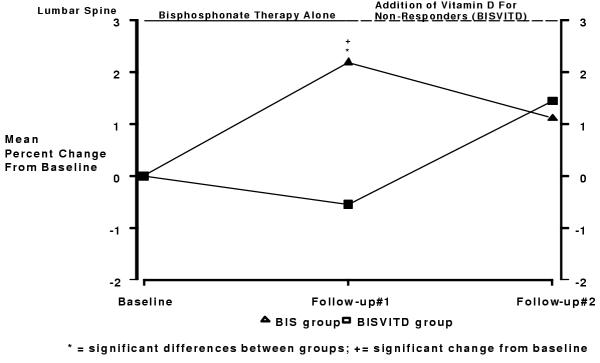
The changes in lumbar spine BMD over the 2-year trial are shown in Figure 2. Prior to the initiation of vitamin D, at the first follow-up visit, lumbar spine BMD declined from baseline measurements by 0.55% (p = 0.363) in the BISVITD group and improved 2.19% in the BIS group (p < 0.001). At the second follow-up visit, lumbar spine BMD increased from the first follow-up measurement by 1.45% (p = 0.014) in the BISVITD group and by 1.11% (p < 0.001) in the BIS group. The difference between the two treatment groups was statistically significant at the first follow-up (p < 0.001) but not at the second (p = 0.595) after adjusting for confounding variables.
Figure 2.
Changes in Lumbar Spine BMD after 2 years
Femoral Neck BMD
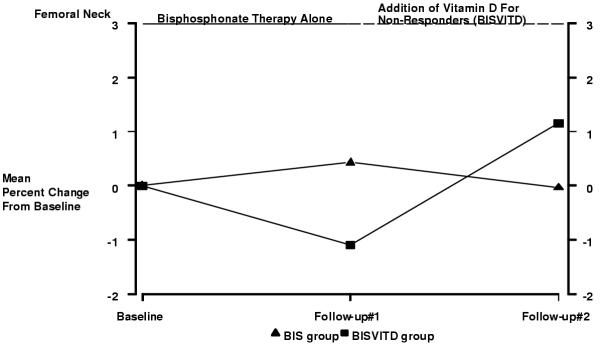
The changes in femoral neck BMD over the two-year study are depicted in Figure 3. Prior to the initiation of vitamin D, at the first follow-up visit, femoral neck BMD declined by 1.09% (p = 0.062) in the BISVITD group and increased by 0.43% (p = 0.266) in the BIS group. At the second follow-up visit, femoral neck BMD increased from the first follow-up measurement by 1.15% (p = 0.094) in the BISVITD group compared to a decline of 0.34% (p = 0.369) in the BIS group. The difference between the two treatment groups was not significant at either the first or the second follow-up (p = 0.299 and p = 0.157, respectively), after adjusting for confounding variables.
Figure 3.
Change in Femoral Neck BMD after 2 years
Effect of baseline serum 25(OH) D
In the treatment group, regression analysis did not demonstrate an association between serum 25(OH)D and change in lumbar spine or femoral BMD after vitamin D was prescribed.
Discussion
BMD continues to decline in 5 to 15% of patients with osteoporosis despite treatment with etidronate or alendronate [4,13]. These patients often receive supplementation with vitamin D, though evidence to support the value of this intervention has been lacking. In our analysis of patients not responding to stable bisphosphonate therapy, we demonstrated that the concurrent use of vitamin D (1000 lU/d) was associated with an increased lumbar spine (1.45%) and femoral neck BMD (1.15%) in those who had previously experienced a decline in BMD while on bisphosphonate therapy alone. In addition, no significant differences were found between those who initially responded to bisphosphonate therapy versus non-responders following treatment. This suggests that vitamin D supplementation may be an effective concurrent medication in those who have not responded to bisphosphonate therapy.
The power of the study to detect a 1.15% (standard deviation= 4%) change in femoral neck bone density from baseline to the second-follow-up visit in the 156 patients was approximately 95%. The absence of a statistically significant response to vitamin D at the femoral neck suggests that the lumbar spine and femoral neck may respond differently to vitamin D. Differences in bone density response between the lumbar spine and the femoral neck have been observed in other studies [33,34].
In this study, the improvement in BMD occurred despite no significant difference in serum 25(OH)D levels between the two treatment groups at baseline, and no relationship between baseline levels and response to prescribed vitamin D was observed. The low baseline 25(OH)D is consistent with previous observations of vitamin D deficiency in elderly persons [35], and suggests that the observed increases in BMD may partially reflect improved nutritional status.
The principal limitation of this observational study was that patients were not randomized into their respective treatment groups. The majority of patients in the intervention group were selected based on declining BMD after one year of treatment. Thus, differences between the groups were found at baseline. In addition, as the treatment group was defined by a decline in BMD after one year, regression to the mean may partially explain the improvement in BMD measured at the second follow-up [36]. The absence of blinding may have motivated patients not responding to bisphosphonates to adhere more rigorously to recommended dietary and lifestyle recommendations, confounding the effect on BMD attributed to vitamin D. Finally, sample size limitations prevent generalizability of the results to male patients and those receiving alendronate.
In summary, in elderly patients with osteoporosis who are not responding to bisphosphonates, concomitant use of vitamin D may improve BMD of the lumbar spine. Clearly, the hypothesis that vitamin D improves BMD in patients receiving bisphosphonates needs to be verified in a prospective controlled trial.
Competing Interests
None Declared.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgement
The authors wish to thank Nicole Ferko for her assistance in formatting this document for publication. This work was supported by a grant from Procter and Gamble Pharmaceuticals, Canada, Inc.. Dr. Papaioannou is a recipient of a Career Scientist award from the Ontario Ministry of Health. Dr. Heckman is a recipient of a Research Fellowship from the Heart and Stroke Foundation of Canada.
Contributor Information
George A Heckman, Email: heck0@rogers.com.
Alexandra Papaioannou, Email: papaioannou@hhsc.ca.
Rolf J Sebaldt, Email: sebaldt@mcmaster.ca.
George Ioannidis, Email: g.ioannidis@sympatico.ca.
Annie Petrie, Email: petriea@mcmaster.ca.
Charlie Goldsmith, Email: goldsmit@mcmaster.ca.
Jonathan D Adachi, Email: jd.adachi@sympatico.ca.
References
- Scientific Advisory Board, Osteoporosis Society of Canada Clinical practice guidelines for the diagnosis and management of osteoporosis. CMAJ. 1996;155:1113–1133. [PMC free article] [PubMed] [Google Scholar]
- Melton LJ, III, Chrischilles EA, Lane AW, Riggs BL. Perspective: How many women have osteoporosis? J Bone Miner Res. 1992;9:1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Wiktorowicz M, Adachi JD, Goeree MA, Papadimitropoulos E, Bédard M, et al. Mortality, independence in living, and re-fracture, one year following hip fracture in Canadians. J Soc Obs Gyne. 2000;22:591–597. [Google Scholar]
- Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med. 1990;322:1265–1271. doi: 10.1056/NEJM199005033221803. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, et al. Four-year study of intermittent-cyclic etidronate treatment of postmenopausal osteoporosis: Three years of blinded therapy followed by one year of open therapy. Am J Med. 1993;95:557–567. doi: 10.1016/0002-9343(93)90350-x. [DOI] [PubMed] [Google Scholar]
- Evans RA, Somers NM, Dunstan CR, Royle H, Kos S. The effect of low-dose cyclical etidronate and calcium on bone mass in early postmenopausal women. Osteoporos Int. 1993;3:71–75. doi: 10.1007/BF01623376. [DOI] [PubMed] [Google Scholar]
- Miller PD, Neal BJ, McIntyre DO, Yanover MJ, Anger MS, Kowalski L. Effect of cyclical therapy with phosphorus and etidronate on axial bone mineral density in postmenopausal osteoporotic women. Osteoporos Int. 1991;1:171–176. doi: 10.1007/BF01625449. [DOI] [PubMed] [Google Scholar]
- Silberstein EB, Schnur W. Cyclic oral phosphate and etidronate increase femoral and lumbar bone mineral density and reduce lumbar spine fracture rate over three years. J Nuc Med. 1992;33:1–5. [PubMed] [Google Scholar]
- Miller PD, Ericksen AL. Longterm intermittent cyclical etidronate (ICT) for postmenopausal osteoporosis (PMO). Calcif Tissue Int. 1995;56:493 (Abstract). [Google Scholar]
- Cranney A, Guyatt G, Krolicki N, Welch V, Griffith L, Adachi JD, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2001;12:140–151. doi: 10.1007/s001980170147. [DOI] [PubMed] [Google Scholar]
- Adami S, Baroni MC, Broggini M, Carratelli L, Caruso I, Gnessi L, et al. Treatment of postmenopausal osteoporosis with continuous daily oral alendronate in comparison with either placebo or intranasal salmon calcitonin. Osteoporos Int. 1993;3:S21–S27. doi: 10.1007/BF01623004. [DOI] [PubMed] [Google Scholar]
- McClung M, Clemmesen B, Daifotis A, Gilchrist NL, Eisman J, Weinstein RS, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253–261. doi: 10.7326/0003-4819-128-4-199802150-00001. [DOI] [PubMed] [Google Scholar]
- Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med. 1998;338:485–492. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- Downs RW, Jr, Bone HG, McIlwain H, Baker MZ, Yates AJ, Lombardi A, et al. An open-label extension study of alendronate treatment in elderly women with osteoporosis. Caicif Tissue Int. 1999;64:463–469. doi: 10.1007/s002239900634. [DOI] [PubMed] [Google Scholar]
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt DE, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- Reginster J-Y, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and non-vertebral fractures on women with postmenopausal osteoporosis. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- McLung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D and the kidney. Kidney Int. 1987;32:912–929. doi: 10.1038/ki.1987.295. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979;64:729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Gallagher JC, DeLuca HF, Edis AJ, Lambert PW, Arnaud CD. A syndrome of osteoporosis, increased serum immunoreactive parathyroid hormone and inappropriately low serum 1,25-dihydroxy vitamin D. Mayo Clin Prod. 1978;53:701–706. [PubMed] [Google Scholar]
- Gloth FM, Tobin JD. Vitamin D deficiency in older people. J Am Geriatr Sod. 1995;43:822–828. doi: 10.1111/j.1532-5415.1995.tb07059.x. [DOI] [PubMed] [Google Scholar]
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505–1511. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- Baeksgaard L, Anderson KP, Hyldstrup L. Calcium and vitamin D supplementation increase spinal BMD in healthy postmenopausal women. Osteoporos Int. 1998;8:255–260. doi: 10.1007/s001980050062. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years or older. N EngI J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover to predict the long term response to alendronate therapy in representative elderly women: A randomized clinical trial. J Bone Miner Res. 1998;13:1431–1438. doi: 10.1359/jbmr.1998.13.9.1431. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, for the FIT Research Group Alendronate reduces the risk of vertebral fractures in women without preexisting vertebral fractures: Results of the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- Lyritis GP, Tsakalakos N, Paspati I, Skarantavos G, Galanos A, Androulakis C. The effect of a modified etidronate cyclical regimen on postmenopausal osteoporosis: a four-year study. Clin Rheumatol. 1997;16:354–360. doi: 10.1007/BF02242451. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. A four year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination in women with postmenopausal osteoporosis. Am J Med. 1998;104:219–226. doi: 10.1016/S0002-9343(98)00029-1. [DOI] [PubMed] [Google Scholar]
- Sebaldt RJ, Adachi JD. Canadian Database of Osteoporosis and Osteopenia Patients (CANDOO). COACH Conference 21 Scientific Program Proceedings, 1996. pp. 36–39.
- Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and appendicular skeleton. J Clin Invest. 1982;69:1302–1309. doi: 10.1172/JCI110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillemin A, Guillemin F, Jouanny P, Denis G, Jeandel C. Differential influence of physical activity on lumbar spine and femoral neck bone mineral density in the elderly population. J Gerontology A Biol sci med sci. 2001;56:B248–B253. doi: 10.1093/gerona/56.6.b248. [DOI] [PubMed] [Google Scholar]
- Omdahl JL, Garry PJ, Hunsaker LA, Hunt WC, Goodwin JS. Nutritional status in a healthy elderly population; vitamin D. Am J Clin Nutr. 1982;36:1225–1233. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- Lenchik L, Watts NB. Regression to the mean: What does it mean? Using bone density results to monitor treatments of osteiporosis. J Clin Densitometry. 2001;4:1–4. doi: 10.1385/JCD:4:1:01. [DOI] [PubMed] [Google Scholar]