Abstract
This cohort study of patients with advanced melanoma treated with anti–programmed cell death 1 with or without ipilimumab assessed whether cutaneous toxic effects were associated with superior clinical outcomes.
Immune checkpoint inhibitors block key mediators of immune tolerance, producing antitumor responses and autoimmunelike toxic effects.1 Toxic effects indicate immune activation against host tissues, although it remains controversial whether this off-target activity indicates concurrent antitumor immunity.2,3,4 Herein, we retrospectively studied whether cutaneous toxic effects correlated with outcomes in patients with advanced melanoma treated with immune checkpoint inhibitors.
Methods
We reviewed electronic medical records of patients treated with anti–programmed cell death 1 (anti–PD-1) with or without ipilimumab from a single center. We assessed demographics, cutaneous toxic effects, steroid administration, and outcomes by retrospective review. The Vanderbilt University Medical Center institutional review board approved the study, with a waiver of patient consent.
Results
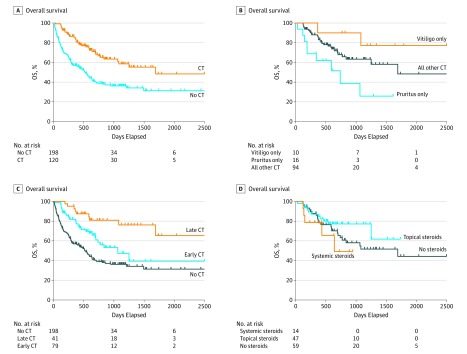
Among 318 patients (202 men [63%]; median [range] age, 63 [22-89] years) from a single center, 120 (38%) who developed cutaneous toxic effects were more likely to have received combination ipilimumab-nivolumab; had similar age, sex, stage, lactate dehydrogenase (LDH) levels; and had prior systemic therapies, including immune or targeted therapies, compared with those without cutaneous toxic effects (Table). Patients with cutaneous toxic effects had superior response rate (RR) (60.0% vs 28.6%; χ2 P < .001), progression-free survival (PFS) (median, 797 vs 112 days; log rank P < .001), and overall survival (OS) (median, 1691 vs 526 days; P < .001) (Figure, A) compared with those who did not have cutaneous toxic effects. Multivariable logistic regression, controlling for age, combination therapy, prior therapy, LDH level, and sex, confirmed an independent association of cutaneous toxic effects with superior RR (odds ratio [OR], 3.58; 95% CI, 2.17-5.90; P < .001). Notably, other clinical features were not associated with response after adjusting for cutaneous toxic effects.
Table. Comparison of Demographic and Clinical Information.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Cutaneous Toxic Effects (n = 120) | No Cutaneous Toxic Effects (n = 198) | ||
| Age at therapy start, median (range), y | 63 (25-89) | 63 (22-87) | .72 |
| Male sex | 74 (61.7) | 128 (64.6) | .59 |
| Melanoma stage | .08 | ||
| M0-M1B | 66 (55) | 89 (44.9) | |
| M1C-M1D | 54 (45) | 109 (55.1) | |
| LDH at therapy start, median (range), U/La | 200 (111-2269) | 217 (100-3587) | .20 |
| History of prior anticancer therapy | 64 (53.3) | 118 (59.6) | .27 |
| Anti–PD-1 monotherapy | 79 (65.8) | 167 (84.3) | <.001 |
| Therapy outcomes | |||
| Response rate | 72 (60.0) | 57 (28.6) | <.001 |
| PFS, d | 797 | 112 | <.001 |
| OS, d | 1691 | 526 | <.001 |
| Clinical benefitb | 88 (73.3) | 83 (41.7) | <.001 |
Abbreviations: LDH, lactate dehydrogenase; OS, overall survival; PD-1, programmed cell death 1; PFS, progression-free survival.
Twelve patients had missing LDH.
Proportion of patients with partial or complete responses plus stable disease.
Figure. Comparisons of Overall Survival Using Log-Rank Testing.
A, Comparison of overall survival (OS) for cutaneous toxic effects (CT) vs no CT. B, Comparison of OS for vitiligo only vs pruritus only vs all other CT. C, Comparison of OS for no CT vs early CT vs late CT. D, Comparison of OS for systemic steroids vs topical steroids vs no steroids.
We assessed whether the type of cutaneous toxic effects correlated with outcomes (pruritus only vs vitiligo only vs all other cutaneous eruptions [“rash”]). Patients with vitiligo and those with rash had superior outcomes compared with those with pruritus only in terms of RR (75.0% vs 64.9% vs 25.0% for vitiligo only, rash, and pruritus only, respectively; P = .009), PFS (median 974 days vs 820 days vs 137 days; P < .001), and OS (median not reached vs 1691 days vs 728 days; P = .01) (Figure, B). Multivariate analysis confirmed superior RR for patients with vitiligo only (OR, 7.05; 95% CI, 1.69-29.42; P = .007) and rash (OR, 4.37; 95% CI, 2.51-7.60; P < .001), but not pruritus only (OR, 0.75; 95% CI, 0.23-2.45; P = .64) compared with patients who did not have cutaneous toxic effects.
To determine whether the timing of cutaneous toxic effects correlates with treatment outcomes, patients who developed toxic effects within 3 months of initiating anti–PD-1 therapy (n = 79) were compared with those developing toxic effects after 3 months (n = 41). Superior outcomes were associated with late toxic effects, followed by early toxic effects, followed by no cutaneous toxic effects in terms of RR (68.3% vs 55.7% vs 28.6%, for late, early, and none, respectively; P < .001), PFS (median not reached vs 383 days vs 112 days; P < .001) and OS (median not reached vs 1065 days vs 526 days; P < .001) (Figure, C). Multivariable analyses confirmed improved RR for late toxic effects (OR, 5.72; 95% CI, 2.72-12.03; P < .001) and early toxic effects (OR, 2.75; 95% CI, 1.55-4.89; P < .001).
We then determined whether steroid administration affected outcomes in patients with cutaneous toxic effects, specifically treatment with topical corticosteroids (n = 47), systemic corticosteroids (n = 14), or no steroids (n = 59): RR (61.7% vs 57.1% vs 59.3% for topical, systemic, and no steroids, respectively; P = .94), PFS (median 797 vs 305 vs 488 days; P = .67), and OS (median not reached vs 641 vs 1691 days; P = .30) were similar (Figure, D).
Discussion
Cutaneous toxic effects were associated with superior clinical outcomes in advanced melanoma patients treated with anti–PD-1 therapy. Specifically, vitiligo and rash were associated with improved outcomes in contrast with pruritus. This observation suggests potentially distinct mechanisms for vitiligo, pruritus, and rash, and is the first to our knowledge to dissect divergent outcomes for these distinct cutaneous manifestations.5,6 Interestingly, cutaneous toxic effects arising after 3 months on therapy was associated with the best outcomes. This is the first study to our knowledge to demonstrate this finding, highlighting a potentially unavoidable bias of toxicity-response correlations, because patients remaining on therapy have the highest risk of developing toxic effects, but are also the patients who are benefiting from therapy. Finally, in a small subset of patients (albeit largely treated with low-dose steroids), we did not observe any adverse effects of steroid administration.
References
- 1.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA. 2018;320(16):1702-1703. doi: 10.1001/jama.2018.13995 [DOI] [PubMed] [Google Scholar]
- 2.Hua C, Boussemart L, Mateus C, et al. . Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152(1):45-51. doi: 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, Hodi FS, Wolchok JD, et al. . Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol. 2017;35(7):785-792. doi: 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 4.Faje AT, Lawrence D, Flaherty K, et al. . High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706-3714. doi: 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Lacouture ME. Pruritus Associated with Targeted Anticancer Therapies and Their Management. Dermatol Clin. 2018;36(3):315-324. doi: 10.1016/j.det.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond JM, Strassner JP, Rashighi M, et al. . Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2018;(Nov):10. [DOI] [PMC free article] [PubMed] [Google Scholar]