Key Points
Question
Do patients with exfoliation glaucoma exhibit alterations in resting nailfold capillary blood flow?
Findings
In this cross-sectional study of 89 patients with glaucoma and 20 controls, diminished resting nailfold capillary blood flow was noted in patients with exfoliation glaucoma compared with control participants after adjusting for multiple covariables. Decreased flow was not shown in patients with high-tension glaucoma.
Meaning
Diminished resting peripheral blood flow may be a manifestation of the systemic vascular involvement in exfoliation glaucoma.
Abstract
Importance
Systemic blood flow alterations have been described using video nailfold capillaroscopy (NFC) in high-tension glaucoma (HTG) and normal-tension glaucoma (NTG) variants of primary open-angle glaucoma (POAG). To date, no previous studies have explored alterations in nailfold capillary blood flow in exfoliation glaucoma (XFG).
Objective
To investigate the measure of peripheral blood flow as a surrogate marker of systemic vascular involvement in patients with XFG, HTG, and NTG, as well as in individuals serving as controls, using NFC.
Design, Setting, and Participants
A cross-sectional clinic-based study at the New York Eye and Ear Infirmary of Mount Sinai was conducted from July 6, 2017, to May 18, 2018. A total of 111 participants (30 XFG, 30 NTG, 30 HTG, and 21 controls) received a comprehensive ophthalmic examination to confirm eligibility. Exclusion criteria were the presence of connective tissue disease, uncontrolled diabetes, history of bleeding disorders, and/or history of trauma or surgery to the nondominant hand.
Main Outcomes and Measures
Resting capillary blood flow at the nailfold of the fourth digit of the nondominant hand in patients with NTG, HTG, XFG, and controls, using NFC.
Results
Two participants were excluded owing to poor nailfold image quality, resulting in 109 participants. Sixty-two participants (57%) were women and 79 (72%) were white. Mean (SD) age of the participants was 67.9 (11.7) years. Mean (SD) resting peripheral capillary blood flow at the nailfold for controls was 70.9 (52.4) picoliters/s (pL/s); HTG, 47.5 (41.9) pL/s; NTG, 40.1 (16.6) pL/s; and XFG, 30.6 (20.0) pL/s. Multivariable analysis of the differences of flow in HTG vs control participants showed values of −18.97 (95% CI, −39.22 to 1.27; P = .07) pL/s, NTG vs controls of −25.17 (95% CI, −45.92 to −4.41; P = .02) pL/s, and XFG vs controls of −28.99 (95% CI, −51.35 to −6.63; P = .01) pL/s.
Conclusions and Relevance
Decreased resting peripheral capillary blood flow may occur in patients with XFG and NTG compared with individuals without glaucoma. These findings may contribute to understanding the possible systemic nature of glaucoma.
This cross-sectional study examines nailfold capillary blood flow in patients with normal-tension, high-tension, and exfoliative glaucoma as a possible measure of differences between these types of glaucoma.
Introduction
Glaucoma is a progressive neurodegenerative ocular disease that causes visual field loss owing to damage of the retinal ganglion cells. The most common type of glaucoma in the United States, primary open-angle glaucoma (POAG),1 has been classified into normal-tension (NTG) and high-tension glaucoma (HTG) based on the cutoff point for the statistical reference range of intraocular pressure (IOP).2 High-tension glaucoma is associated with various risk factors, such as older age, high IOP, African descent, and positive family history.3
Exfoliation syndrome (XFS), an age-related systemic condition, is the most common recognizable cause of open-angle glaucoma worldwide, comprising most cases in some countries.4 Exfoliation syndrome is characterized by the production, deposition, and progressive accumulation of a white fibrillogranular extracellular material (exfoliation material) throughout the anterior segment, most prominently on the anterior lens surface, on the zonules with their resultant weakness, and on the pupillary border. Exfoliation material is also found in posterior segment vessels, including the vortex veins, posterior ciliary arteries, and central retinal vessels. It is observed microscopically in numerous organs, including the skin, heart, lungs, and kidney, primarily in the connective tissue.5,6,7 In addition to obstructing fluid pathways, exfoliation material accumulation affects vascular endothelial cells as well as contractile pericytes, which wrap around the endothelial cells and help give blood vessel walls strength and flexibility. Such alterations at the level of endothelial cells and pericytes in XFS may explain the ocular and systemic vasculature changes, including central retinal vein occlusion,8 iris vascular ischemia,9 cardiovascular and cerebrovascular disease, aortic aneurysm, and renal artery stenosis.10,11,12 Elastic tissue is found in vasculature throughout the body.13 Exfoliation syndrome affects the extracellular matrix and is also an elastotic disorder. It has also been associated with an increased incidence of elastic tissue disorders, such as pelvic organ prolapse and inguinal hernia.14,15
Vascular dysregulation is widespread in POAG, with abnormalities found at the optic nerve head,16,17 central retinal vessels,18,19,20 macula,21,22 choroid,23 and retrobulbar vessels.24 Systemic changes in POAG are exemplified as a dysregulated response to flow-mediated vasodilation at the brachial artery in both HTG and NTG, increased incidence of nocturnal systemic hypotension, decreased cerebral blood flow, carotid artery stiffness, ischemic heart disease, and increased levels of the vasoactive peptide endothelin-1, which plays a key role in vasoconstriction.25,26,27,28,29,30,31
Nailfold capillaroscopy (NFC) is a noninvasive imaging modality that provides a highly magnified view of the capillaries at the nailfold of digits.32 This test has been used in rheumatologic studies to explore morphologic vascular changes in various connective tissue diseases.33 It has also been used in ophthalmologic research to show morphologic changes at the nailfold capillaries of patients with POAG, XFG, and XFS, helping to confirm the systemic nature of these diseases.34,35 Cousins et al36 explored resting peripheral blood flow at the nailfold in patients with POAG (HTG and NTG) and found significantly lower peripheral blood flow compared with that in controls. Lower blood cell velocity at the nailfold is also seen in patients with NTG.37 To the best of our knowledge, nailfold peripheral blood flow in patients with XFG has not previously been compared using NFC with that in individuals serving as controls. We explored the peripheral blood flow at the nailfold of patients with HTG, NTG, and XFG and compared it with that in control participants to further evaluate the possible differences between these glaucoma entities.
Methods
All participants were recruited at the New York Eye and Ear Infirmary of Mount Sinai, New York, and the study was conducted from July 6, 2017, to May 18, 2018. We recruited 90 patients, including 60 with POAG (30 NTG and 30 HTG), 30 with XFG, and 21 controls. One control individual and 1 patient with HTG were excluded from analysis owing to poor image quality not allowing proper capillary visualization. This cross-sectional, clinic-based, prospective study was approved by the Mount Sinai Institutional Review Board. The study conformed to the tenets of the Declaration of Helsinki,38 and all participants provided written informed consent. There was no financial compensation.
Eyes with HTG had clinical findings consistent with glaucomatous optic neuropathy (neuroretinal rim thinning and notching) with untreated IOP greater than 21 mm Hg. Patients with glaucoma had visual field defects on 3 or more consecutive examinations as measured by a standard automated perimeter (Humphrey Field Analyzer, 24-2 Swedish interactive threshold algorithm standard; Carl-Zeiss Meditec). We defined defects as a minimum of 3 abnormal points clustered in the same hemifield with a pattern deviation of less than 2% with at least 1 point of less than 1% or 2 or more adjacent points with a pattern deviation of less than 1%. Perimetry reliability was defined as false-positive and false-negative rates less than 20% and fixation losses less than 33%. Patients with NTG had signs of glaucomatous optic neuropathy with maximal untreated IOP 21 mm Hg or lower. Patients with XFG had exfoliation material on the anterior lens surface and/or pupillary margin along with evidence of glaucomatous optic neuropathy. All eyes had gonioscopically open angles.
Individuals serving as controls had IOP less than 21 mm Hg with no history of elevated IOP, cup-disc ratio of 0.6 or less, open angles on gonioscopy, and normal measures of retinal nerve fiber layer and ganglion cell complex thickness on optical coherence tomography.39,40,41
We excluded any individuals with connective tissue diseases including, but not limited to, rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis owing to their characteristic changes at the nailfold vasculature33; uncontrolled diabetes (hemoglobin A1c ≥7.0% [to convert to proportion of total hemoglobin, multiply by 0.01])42; history of bleeding disorders, current treatment with chemotherapeutic agents and/or radiotherapy; history of nail bed trauma (recent manicure or onychophagia); and/or history of a surgical procedure performed on the digit being studied.
We screened the eligible participants the day before their visits in the glaucoma clinic using an electronic medical record system and approached them prospectively on the day of their visit. Participants were given a questionnaire about general health and medication history and underwent a comprehensive ophthalmic examination at the time of enrollment, which included assessment of visual acuity, IOP measurement (Goldmann applanation tonometry), slitlamp examination of the anterior segment, and inspection of the optic nerve via ophthalmoscopy. Height, weight, pulse rate, blood pressure, and room temperature were also recorded at the time of the visit.
Video-Nailfold Capillaroscopy Procedure
A commercially available digital video nailfold capillaroscopy camera (BK-XW880; Biobase Biodustry) was used to perform capillaroscopy at ×100 magnification. The procedure was performed at the fourth digit (ring finger) of the nondominant hand, as the most medial digits have the highest skin transparency at the nailfold compared with the other digits, allowing a more detailed evaluation of the capillaries.32,43 The nondominant hand was chosen to reduce the likelihood that daily microtrauma may have affected the nailfold capillary architecture. The video capillaroscope was connected to a computer with a monitor to allow real-time visualization of the capillaries on the screen and enable storage of individual video recordings.
All recordings were made with the participants sitting with their hands at the level of their heart. Details of nailfold capillaroscopy have been previously described.44 In brief, the hand was placed on the examination table with the dorsum facing up. Two drops of neutral immersion oil (castor oil) were applied to the nailfold area of the nondominant fourth digit to facilitate the epidermal transparency of the underlying vessels. The area of the nailfold was defined as directly proximal to the cuticle. The fourth digit was then placed onto the capillaroscope scanning stage and adjustment was performed to achieve visualization of the most distal row of capillaries. The capillaroscope did not physically touch the nailfold skin.
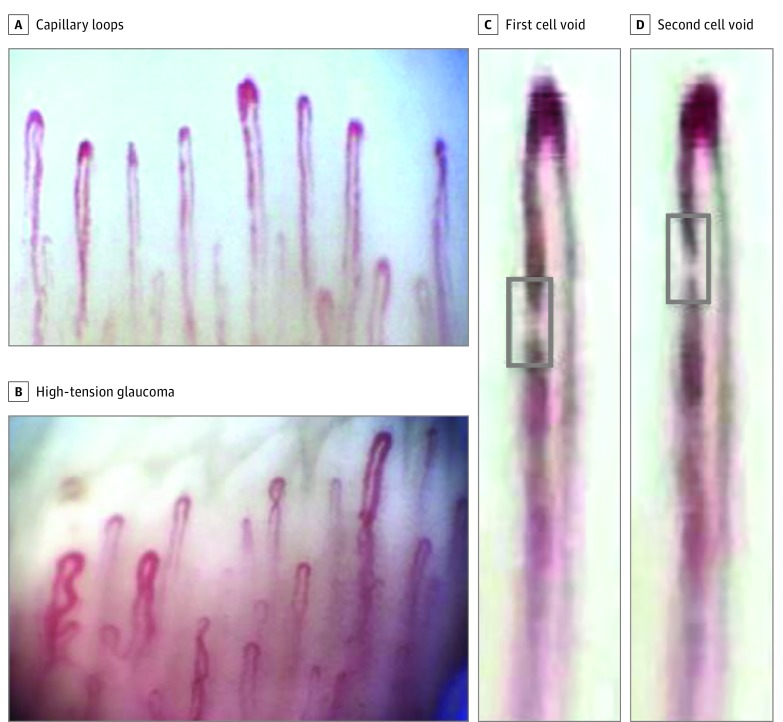
The camera was first placed above the lateral portion of the nailfold and recording was initiated. The camera was then advanced to the central/median portion and then to the medial portion of the nailfold using the same method. Videos were captured, coded, and stored (Debut Video Capture; Debut, NCH Software Inc) and then imported into video editing software (VideoPad; NCH Software Inc) and converted to an image sequence. Each image sequence was then opened in ImageJ (National Institutes of Health). The videos were first processed for stabilization using the ImageJ rigid body registration plugin. The movement in the capillary is represented as a white plasma gap (blank void) traveling in the capillary loop and was used for blood flow calculation (Figure 1). Details of blood flow analysis and how plasma gap movement in capillaries is an acceptable estimate of capillary blood movement have been previously described.36,45
Figure 1. Video Nailfold Capillaroscopy Images Showing Capillary Loops (Inverted U-Shape).
A, Control participant. B, Patient with high-tension glaucoma. C and D, Magnified capillary showing plasma gap/blood cell void (gray box and seen as the white band) movement over 2 consecutive image sequence frames in a control participant. Flow was 64.56 picoliters per second.
Nine capillaries at the fourth digit of each participant with well-visualized plasma gap movement were chosen for grading. A single experienced grader (S.P.) was masked to each patient diagnosis and graded all videos. A second masked reader (A.N.) regraded a batch of videos to assess for intergrader agreement. Videos were also assessed for intragrader reliability. Capillaroscopy videos with poor image quality not allowing capillary flow visibility and/or high levels of tortuosity not allowing proper measurement were excluded from analysis.
Statistical Analysis
Multiple linear regression analysis was used to assess the association among blood flow, diagnostic groups, ocular perfusion pressure, and mean arterial pressure (MAP). A P value <.05 indicated a statistically significant difference. Statistical analyses were performed using Stata, version 15.1 software (StataCorp, 2017).
Results
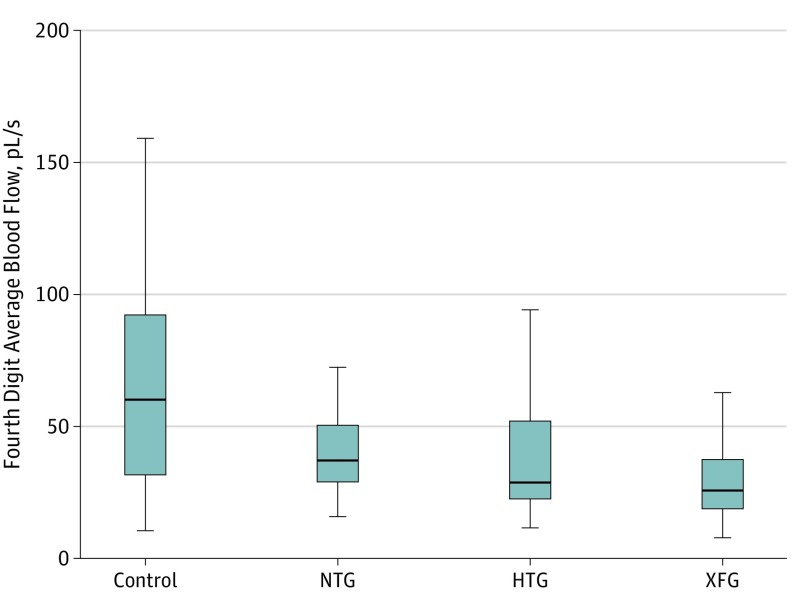
Sixty-two participants (57%) were women and 79 (72%) were white. Mean (SD) age of the participants was 67.9 (11.7) years; mean age of the participants in the XFG group was 76.3 (6.9) years. Other baseline demographic and clinical characteristics of study participants are reported in Table 1. The intraclass correlation coefficient (2-way mixed-effect model for a single rater) between 2 graders of blood flow was 86% and intragrader reliability was 92%. Patients with HTG, NTG, and XFG showed decreased peripheral blood flow at the nailfold of the fourth digit compared with control participants (HTG, 47.5 picoliters/s [pL/s]; NTG, 40.1 (16.6) pL/s; XFG, 30.6 (20.0) pL/s; and controls, 70.9 (52.4) pL/s in univariable analysis (Figure 2 and Table 1).
Table 1. Clinical and Demographic Characteristics of Study Participants.
| Variable | Control (n = 20) | NTG (n = 30) | HTG (n = 29) | XFG (n = 30) |
|---|---|---|---|---|
| Age, mean (SD), y | 59.0 (12.8) | 67.3 (11.6) | 65.8 (9.7) | 76.3 (6.9) |
| Sex, No. (%) | ||||
| Women | 13 (65) | 18 (60) | 13 (45) | 18 (60) |
| Men | 7 (35) | 12 (40) | 16 (55) | 12 (40) |
| BMI, mean (SD) | 26.0 (4.8) | 23.1 (3.1) | 26.8 (4.3) | 26.2 (5.4) |
| Race/ethnicity, No. (%) | ||||
| White | 12 (60) | 21 (70) | 19 (66) | 27 (90) |
| African American | 1 (5) | 1 (3) | 4 (14) | 0 |
| South Asian | 2 (10) | 1 (3) | 3 (10) | 0 |
| East Asian | 4 (20) | 7 (23) | 2 (7) | 3 (10) |
| Hispanic | 1 (5) | 0 | 1 (3) | 0 |
| Comorbid disease, No. (%) | ||||
| Hypertension | 8 (40) | 8 (27) | 13 (45) | 14 (47) |
| Diabetes | 2 (10) | 2 (7) | 2 (7) | 0 |
| Medication, No. (%) | ||||
| Systemic antihypertensive | 5 (25) | 3 (10) | 12 (41) | 12 (40) |
| Systemic β-blocker | 2 (10) | 5 (17) | 4 (14) | 6 (20) |
| Topical β-blocker | 0 | 8 (27) | 20 (69) | 14 (47) |
| Topical prostaglandin analogue | 0 | 25 (83) | 26 (90) | 24 (80) |
| Topical α2-agonist | 0 | 11 (37) | 13 (45) | 7 (23 |
| Aspirin | 1 (5) | 9 (30) | 8 (28) | 10 (30) |
| Anticoagulant | 0 | 1 (3) | 2 (7) | 2 (7) |
| MAP, mean (SD), mm Hg | 92.1 (11.9) | 90.1 (10.3) | 94.5 (11.3) | 93.7 (13.7) |
| MOPP, right eye, mean (SD), mm Hga | 52.7 (9.3) | 51.5 (8.1) | 53.2 (6.6) | 51.4 (9.0) |
| MOPP, left eye, mean (SD), mm Hga | 52.7 (9.4) | 51.1 (7.5) | 53.3 (7.11) | 52.7 (8.6) |
| 4th-Digit blood flow, mean (SD), pL/s | 70.9 (52.4) | 40.1 (16.6) | 47.5 (41.9) | 30.6 (20.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; HTG, high-tension glaucoma; MAP, mean arterial pressure; MOPP, mean ocular perfusion pressure; NTG, normal-tension glaucoma; pL/s, picoliters per second; XFG, exfoliation glaucoma.
MOPP was determined as (2/3 [MAP – intraocular pressure]).
Figure 2. Modified Analysis of Peripheral Blood Flow at the Nailfold of the Fourth Digit.
Mean values of blood flow shown. HTG indicates high-tension glaucoma; NTG, normal-tension glaucoma; pL/s, picoliters per second; XFG, exfoliation glaucoma; and error bars, 95% CI.
Multiple covariables were analyzed and only a history of diabetes was found to be associated with the peripheral blood flow (Table 2). Multivariable analysis controlling for age, body mass index, and comorbid disease showed decrease in blood flow in patients with NTG and XFG compared with controls, with an NTG vs control difference of −25.17 (95% CI, −45.92 to −4.41; P = .02) and XFG vs control difference of −28.99 (95% CI, −51.35 to −6.63; P = .01) (Table 3). Multivariable analysis of flow between HTG and controls did not reach statistical significance, with an HTG vs control difference of −18.97 (95% CI, −39.22 to 1.27; P = .07). We also performed a subset multivariable analysis of nailfold blood flow controlling for age, body mass index, and diabetes in patients with XFG vs control participants who were aged 60 to 80 years; this analysis revealed a difference of −20.31 (95% CI, −39.86 to −0.76; P = .04).
Table 2. Association of Variables and Fourth-Digit Blood Flow.
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value | |
| Age | −1.04 (−1.59 to −0.48) | <.001 | −0.47 (−1.18 to 0.25) | .20 |
| Men vs women | −4.91 (−18.82 to 8.98) | .49 | NA | NA |
| BMI | 0.32 (−1.17 to 1.82) | .81 | NA | NA |
| Hypertension | 1.56 (−11.43 to 14.55) | .81 | NA | NA |
| Diabetes | 22.24 (2.14 to 41.48) | .025 | 32.07 (1.31 to 62.84) | .04 |
| Race/ethnicity | ||||
| White | 1 [Reference] | 1 [Reference] | ||
| African American | 17.24 (−12.21 to 46.69) | .25 | 2.21 (−26.55 to 30.98) | .88 |
| South Asian | 16.02 (−13.44 to 45.47) | .28 | −14.56 (−44.84 to 17.12) | .39 |
| East Asian | 28.08 (9.01 to 47.15) | .004 | 14.92 (−5.94 to 37.82) | .15 |
| Hispanic | −20.16 (−69.96 to 29.64) | .42 | −32.51 (−77.42 to 15.40) | .19 |
| Medication | ||||
| Systemic antihypertensive | 0.80 (−14.36 to 15.96) | .92 | NA | NA |
| Systemic β-blocker | −6.32 (−25.27 to 12.63) | .51 | NA | NA |
| Topical β-blocker | −3.96 (−18.29 to 10.35) | .58 | NA | NA |
| Topical prostaglandin analogue | −17.69 (−32420 to −2.96) | .02 | −2.04 (−22.71 to 18.63 | .85 |
| Aspirin | −13.86 (−29.40 to 1.68) | .08 | −8.56 (−23.95 to 6.23) | .25 |
| Anticoagulanta | −4.65 (−37.53 to 28.24) | .78 | NA | NA |
| Topical α2-agonist | −0.77 (−16.54 to 15.01) | .92 | NA | NA |
| MOPP, right eyeb | −0.26 (−1.09 to 0.57) | .54 | NA | NA |
| MOPP, left eyeb | −0.26 (−1.10 to 0.57) | .54 | NA | NA |
| MAP | −0.13 (−0.71 to 0.43) | .64 | NA | NA |
Abbreviations: BP, blood pressure; BMI, body mass index; IOP, intraocular pressure; MAP, mean arterial pressure; MOPP, mean ocular perfusion pressure; NA, not applicable.
Factor Xa-inhibitor or warfarin.
MOPP determined as (2/3 [MAP – IOP]).
Table 3. Multiple Regression Analysis Among Blood Flow, Diagnostic Groups, Ocular Perfusion Pressure, and MAP.
| Variable | Unadjusted | Adjusted for Age | Adjusted for Age, BMI, Comorbid Disease | |||
|---|---|---|---|---|---|---|
| Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value | |
| NTG vs control | −30.81 (−50.66 to −10.96) | .003 | −25.35 (−45.56 to −5.13) | .01 | −25.17 (−45.92 to −4.41) | .02 |
| HTG vs control | −23.36 (−43.34 to −3.37) | .02 | −18.93 (−39.05 to 1.20) | .07 | −18.97 (−39.22 to 1.27) | .07 |
| XFG vs control | −40.32 (−60.16 to −20.47) | <.001 | −28.97 (−51.23 to −6.72) | .01 | −28.99 (−51.35 to −6.63) | .01 |
| MOPP, right eye | −0.22 (−1.08 to 0.63) | .61 | −0.12 (−0.94 to 0.69) | .76 | −0.06 (−0.88 to 0.75) | .87 |
| MOPP, left eye | −0.28 (−1.16 to 0.60) | .53 | −0.08 (−0.92 to 0.76) | .84 | −0.005 (−0.83 to 0.83) | .99 |
| MAP | −0.09 (−0.68 to 0.49) | .76 | 0.05 (−0.52 to 0.61) | .87 | 0.09 (−0.47 to 0.65) | .74 |
Abbreviations: BMI, body mass index; HTG, high-tension glaucoma; MAP, mean arterial pressure; MOPP, mean ocular perfusion pressure; NTG, normal-tension glaucoma; XFG, exfoliation glaucoma.
Discussion
We found a lower and limited range of resting peripheral blood flow in patients with XFG compared with controls (Figure 2). This limited range may represent stiffened vasculature or impaired maximal flow in the peripheral vasculature of patients with XFG compared with controls. The limited range may also be associated with increased tortuosity in patients with XFS previously described by Cousins et al,35 albeit an NFC morphologic survey was not the focus of this work. A previous study exploring vascular changes in XFG demonstrated lower resting basal fingertip capillary blood flow measured using the cold pressor test with laser Doppler flowmetry compared with patients with POAG and controls.46 The pathophysiologic mechanism behind these flow alterations may be linked to the accumulation of exfoliation material in tissue causing degenerative fibrillopathy to normal basement cell membranes.7,47 This degeneration may cause alterations of the peripheral vessels, including elastosis, oxidative stress, and vascular endothelial dysfunction, leading to vascular stiffness.48,49
The gene encoding for LOXL1, an enzyme important for collagen cross-linking with elastin in the extracellular matrix, has 2 single-nucleotide polymorphisms in 99% of white individuals with XFS.50 Exfoliation syndrome may be associated with impaired autophagy of cellular waste, which may lead to buildup of misfolded proteins. This buildup may result in oxidative stress leading to vascular pathologic changes.51 Clusterin, a chaperone molecule involved in removing toxic protein oligomers, likely has altered expression in XFS and XFG, further affecting vascular dysregulation in exfoliation.52
Vascular stiffness is a feature of glaucoma that is possibly associated with poor regulation of the vascular smooth muscle cell by the MGP gene, leading to increased calcification.53 Restricted conduction of vasodilatory signals in the microcirculation may also be present and signify limited vessel capacity often seen in pathologic vessel states, such as hypertension, representing decreased ability to circulate blood according to bodily demands.54 Decreased blood flow to the optic nerve head and peripapillary area, a thinner choroid, and a decreased diameter of the central retinal vessels have been documented in eyes with XFS.50 Altered systemic vasoregulation is a feature of XFG and XFS reflected by the incidence of parasympathetic cardiovascular neuropathy, decreased baroreflex sensitivity, increased carotid artery stiffness, and pathologic heart rate irregularity indices, among others.48,55 These altered biomechanics may help to explain decreased peripheral blood flow in XFG.
Lower mean (SD) nailfold capillary blood flow in patients with NTG compared with controls (40.1 [16.6] vs 70.9 [52.4] pL/s; P = .02) in our study is consistent with that of previous studies.36,37 Systemic and IOP-independent vascular involvement in NTG may be noted in the context of Flammer syndrome, a primary vascular dysregulation entity with multiple manifestations that play a part in the pathogenesis of NTG. Patients with this syndrome, including those with NTG, respond differently to environmental stimuli, such as cold and emotional stress, leading to cold extremities and low blood pressure, with the latter contributing to the development of NTG.56
The HTG group did not show decreased flow compared with controls in multivariable analysis (Table 3) (−18.97; 95% CI, −39.22 to 1.27; P = .07). This result is consistent with a previous study that found a slightly decreased, but nonsignificant, difference in blood cell velocity between patients with HTG and controls.37 However, the minimal decrease is in contrast with another study that found significantly decreased blood flow in both patients with HTG and NTG compared with controls.36 This difference may be the result of a higher percentage of white participants compared with controls (72% in our study vs 91.5% in the prior study41), different methods (using blood flow only in the fourth digit in our study vs using both the fourth and fifth digits in the previous study), and subjectivity in NFC blood flow analysis between the 2 investigations. A recent study found that patients with NTG showed more signs of vascular dysfunction compared with those with HTG, further demonstrating the possible differences in the pathophysiologic mechanisms between the 2 variants.57
Understanding the changes of the capillary endothelium, which regulates the vessel tone, is fundamental in explaining some of the blood flow alterations in glaucoma. Pasquale58 suggested a complex biochemical pathway dysfunction involving endothelial acetylcholine activation, endothelin dysfunction, and altered nitric oxide signaling in the pathogenesis of glaucoma. Investigations of blood flow in patients with POAG have found reduced ocular blood flow in comparison with individuals without POAG.17,19,59 Flammer et al59 reported that decreased ocular flow can often occur before damage and that vascular abnormalities may also occur in other systemic vasculature. Examples of the effects on systemic vasculature include incidence of nocturnal hypotension, arterial stiffness, impaired baroreflex response, and compromised flow-mediated vasodilation in both NTG and HTG.17,28,60,61 In addition, endothelin-1 level and endothelin-A receptor quantity are increased in POAG.26,27 These microvascular changes at the periphery may be part of an autoregulatory response to hypoxia in tissues.62,63,64
A history of diabetes was associated with increased blood flow in multivariable analysis and thus may have affected blood flow in our study (Table 2). However, owing to the sample size of patients with diabetes (n = 6), as displayed by a wide 95% CI, this finding may have happened by chance because of unbalanced data. Two previous studies have shown no difference in resting blood cell velocity65 and blood flow36 between patients with a history of diabetes and controls. Both of those studies also had low cohorts of patients with diabetes; thus, a larger sample size is needed to evaluate the possible vascular changes that may occur in diabetes. We adjusted for diabetes during multivariable analysis along with age and body mass index (Table 3). Our analysis also showed that, despite the use of systemic and topical β-blocker medications, this therapy was not associated with blood flow (Table 2)—a finding consistent with that of Cousins et al.36
Limitations
Several limitations exist in our study, including a low sample number (n = 109), high percentage of white participants (72%), and more patients with XFG of an older age (76.3 [6.9] years). These factors may limit the generalizability of data; however, even with a wide range of ages and body mass index across our data set, as well as accounting for multiple covariables (Table 2) through multiple linear regression analysis, our results remained significant (Table 3). Owing to the age difference, we performed a subset multivariable analysis of nailfold blood flow controlling for age, body mass index, and diabetes in patients with XFG vs control participants who were aged 60 to 80 years. We still found a difference in flow between the groups (−20.31; 95% CI, −39.86 to −0.76; P = .04). Further studies with larger sample sizes and greater variability are needed to confirm the validity of our results.
Another limitation was lack of an automated method for assessing nailfold blood flow. Our method may have been prone to some degree of error due to the manual measurement of capillary variables. We accounted for this subjectivity by use of one main grader for all videos, as well as showing a moderate intergrader reliability with an intraclass correlation coefficient of 86% for grading of a subset of videos by a second grader. In addition, intragrader reliability had an intraclass correlation coefficient greater than 90%. Graders were also masked to patient diagnosis to possibly eliminate any other subjectivity issues. Development of reliable software allowing automated processing of nailfold blood flow may be useful in future studies to overcome these factors. Our protocol was restrictive in terms of requiring proper focusing of nonobscured linear distal capillary segments. We also did not control for the time of day that the video capillaroscopy was performed and did not measure the temperature of the finger during the study. Another limitation is that we did not evaluate XFS without optic nerve damage; in addition, there is a lack of longitudinal data.
Conclusions
To the best of our knowledge, our study is the first to show diminished nonocular resting peripheral capillary blood flow as measured by NFC in patients with XFG compared with controls, even after correction for multiple covariables. Our results are in accordance with previous studies36,37 that found decreased peripheral blood flow in patients with NTG compared with controls. The vascular hemodynamic alteration at the peripheral nailfold circulation in XFG may represent the systemic nature of exfoliation glaucoma, which necessitates further studies.
References
- 1.Friedman DS, Wolfs RCW, O’Colmain BJ, et al. ; Eye Diseases Prevalence Research Group . Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532-538. doi: 10.1001/archopht.122.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colton T, Ederer F. The distribution of intraocular pressures in the general population. Surv Ophthalmol. 1980;25(3):123-129. doi: 10.1016/0039-6257(80)90086-7 [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z; EMGT Group . Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114(11):1965-1972. doi: 10.1016/j.ophtha.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 4.Ritch R. Exfoliation syndrome—the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3(2):176-177. doi: 10.1097/00061198-199400320-00018 [DOI] [PubMed] [Google Scholar]
- 5.Schlötzer-Schrehardt U, Küchle M, Naumann GO. Electron-microscopic identification of pseudoexfoliation material in extrabulbar tissue. Arch Ophthalmol. 1991;109(4):565-570. doi: 10.1001/archopht.1991.01080040133044 [DOI] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome: ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110(12):1752-1756. doi: 10.1001/archopht.1992.01080240092038 [DOI] [PubMed] [Google Scholar]
- 7.Streeten BW, Li ZY, Wallace RN, Eagle RC Jr, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110(12):1757-1762. doi: 10.1001/archopht.1992.01080240097039 [DOI] [PubMed] [Google Scholar]
- 8.Ritch R, Prata TS, de Moraes CGV, et al. . Association of exfoliation syndrome and central retinal vein occlusion: an ultrastructural analysis. Acta Ophthalmol. 2010;88(1):91-95. doi: 10.1111/j.1755-3768.2009.01578.x [DOI] [PubMed] [Google Scholar]
- 9.Helbig H, Schlötzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO. Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol. 1994;3(3):148-153. [PubMed] [Google Scholar]
- 10.Ritch R. Systemic associations of exfoliation syndrome. Asia Pac J Ophthalmol (Phila). 2016;5(1):45-50. doi: 10.1097/APO.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 11.Wang W, He M, Zhou M, Zhang X. Ocular pseudoexfoliation syndrome and vascular disease: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92767. doi: 10.1371/journal.pone.0092767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin LA, Albert DM. Exfoliation (psuedoexfoliation) syndrome In: Gabbedy R, Davie B, eds. Ocular Disease: Mechanisms and Management. Philadelphia, PA: Saunders/Elsevier; 2010:212-222. [Google Scholar]
- 13.Feher J. Regulation of perfusion. In: Quantitative Human Physiology. Philadelphia, PA: Elsevier; 2012:519-528. [Google Scholar]
- 14.Wirostko BM, Curtin K, Ritch R, et al. . Risk for exfoliation syndrome in women with pelvic organ prolapse: a Utah Project on Exfoliation Syndrome (UPEXS) study. JAMA Ophthalmol. 2016;134(11):1255-1262. doi: 10.1001/jamaophthalmol.2016.3411 [DOI] [PubMed] [Google Scholar]
- 15.Besch BM, Curtin K, Ritch R, Allingham RR, Wirostko BM. Association of exfoliation syndrome with risk of indirect inguinal hernia: the Utah Project on Exfoliation Syndrome. JAMA Ophthalmol. 2018;136(12):1368-1374. doi: 10.1001/jamaophthalmol.2018.4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno T, Sugiyama T, Kojima S, Nakajima M, Ikeda T. Diurnal variation in microcirculation of ocular fundus and visual field change in normal-tension glaucoma. Eye (Lond). 2004;18(7):697-702. doi: 10.1038/sj.eye.6700749 [DOI] [PubMed] [Google Scholar]
- 17.Fuchsjäger-Mayrl G, Wally B, Georgopoulos M, et al. . Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45(3):834-839. doi: 10.1167/iovs.03-0461 [DOI] [PubMed] [Google Scholar]
- 18.Kaiser HJ, Schoetzau A, Stümpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997;123(3):320-327. doi: 10.1016/S0002-9394(14)70127-8 [DOI] [PubMed] [Google Scholar]
- 19.Galambos P, Vafiadis J, Vilchez SE, et al. . Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology. 2006;113(10):1832-1836. doi: 10.1016/j.ophtha.2006.05.030 [DOI] [PubMed] [Google Scholar]
- 20.Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008;115(2):246-252. doi: 10.1016/j.ophtha.2007.04.055 [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Yu J, Kong X, Sun X, Jiang C. Macular microvasculature alterations in patients with primary open-angle glaucoma: a cross-sectional study. Medicine (Baltimore). 2016;95(33):e4341. doi: 10.1097/MD.0000000000004341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon J, Choi J, Shin JW, Lee J, Kook MS. Glaucoma diagnostic capabilities of foveal avascular zone parameters using optical coherence tomography angiography according to visual field defect location. J Glaucoma. 2017;26(12):1120-1129. doi: 10.1097/IJG.0000000000000800 [DOI] [PubMed] [Google Scholar]
- 23.Ulrich A, Ulrich C, Barth T, Ulrich WD. Detection of disturbed autoregulation of the peripapillary choroid in primary open angle glaucoma. Ophthalmic Surg Lasers. 1996;27(9):746-757. [PubMed] [Google Scholar]
- 24.Kanakamedala P, Harris A, Siesky B, et al. . Optic nerve head morphology in glaucoma patients of African descent is strongly correlated to retinal blood flow. Br J Ophthalmol. 2014;98(11):1551-1554. doi: 10.1136/bjophthalmol-2013-304393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su WW, Cheng ST, Ho WJ, Tsay PK, Wu SC, Chang SHL. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115(7):1173-1178.e1. doi: 10.1016/j.ophtha.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Cellini M, Strobbe E, Gizzi C, Balducci N, Toschi PG, Campos EC. Endothelin-1 plasma levels and vascular endothelial dysfunction in primary open angle glaucoma. Life Sci. 2012;91(13-14):699-702. doi: 10.1016/j.lfs.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 27.Mihaylova B, Petkova I, Rankova-Yotova C, et al. . Plasma endothelin-1 and endothelin-A receptor concentrations in patients with primary open-angle glaucoma. Biotechnol Equip. 2017;31(4):782-787. doi: 10.1080/13102818.2017.1334592 [DOI] [Google Scholar]
- 28.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(suppl 1):S10-S16. doi: 10.1016/S0039-6257(99)00016-8 [DOI] [PubMed] [Google Scholar]
- 29.Chen YY, Hu HY, Chu D, Chen HH, Chang CK, Chou P. Patients with primary open-angle glaucoma may develop ischemic heart disease more often than those without glaucoma: an 11-year population-based cohort study. PLoS One. 2016;11(9):e0163210. doi: 10.1371/journal.pone.0163210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visontai Z, Mersich B, Holló G. Carotid artery elasticity and baroreflex sensitivity in patients with glaucoma. J Glaucoma. 2005;14(1):30-35. doi: 10.1097/01.ijg.0000145814.46848.76 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Chen W, Qu X, et al. . Reduced cerebral blood flow in the visual cortex and its correlation with glaucomatous structural damage to the retina in patients with mild to moderate primary open angle glaucoma. J Glaucoma. 2018;27(9):816-822. doi: 10.1097/IJG.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 32.Lambova S, Hermann W, Müller-Ladner U. Capillaroscopic pattern at the toes of systemic sclerosis patients: does it “tell” more than those of fingers? J Clin Rheumatol. 2011;17(6):311-314. doi: 10.1097/RHU.0b013e31822be4e8 [DOI] [PubMed] [Google Scholar]
- 33.Cutolo M. Atlas of Capillaroscopy in Rheumatic Diseases. Milan, Italy: Elsevier; 2010. [Google Scholar]
- 34.Pasquale LR, Hanyuda A, Ren A, et al. . Nailfold capillary abnormalities in primary open-angle glaucoma: a multisite study. Invest Ophthalmol Vis Sci. 2015;56(12):7021-7028. doi: 10.1167/iovs.15-17860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cousins CC, Kang JH, Bovee C, et al. . Nailfold capillary morphology in exfoliation syndrome. Eye (Lond). 2017;31(5):698-707. doi: 10.1038/eye.2016.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cousins CC, Chou JC, Greenstein SH, et al. . Resting nailfold capillary blood flow in primary open-angle glaucoma. Br J Ophthalmol. 2019;103(2):203-207. doi: 10.1136/bjophthalmol-2018-311846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol. 1991;111(5):585-588. doi: 10.1016/S0002-9394(14)73703-1 [DOI] [PubMed] [Google Scholar]
- 38.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 39.Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci. 2010;51(9):4646-4651. doi: 10.1167/iovs.09-5053 [DOI] [PubMed] [Google Scholar]
- 40.Mwanza JC, Durbin MK, Budenz DL, et al. . Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119(6):1151-1158. doi: 10.1016/j.ophtha.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 41.Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(7):4422-4429. doi: 10.1167/iovs.12-11273 [DOI] [PubMed] [Google Scholar]
- 42.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2013;(suppl 1):S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutolo M, Grassi W, Matucci Cerinic M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003;48(11):3023-3030. doi: 10.1002/art.11310 [DOI] [PubMed] [Google Scholar]
- 44.Etehad Tavakol M, Fatemi A, Karbalaie A, Emrani Z, Erlandsson BE. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated? Biomed Res Int. 2015;2015:974530. doi: 10.1155/2015/974530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mugii N, Hasegawa M, Hamaguchi Y, et al. . Reduced red blood cell velocity in nail-fold capillaries as a sensitive and specific indicator of microcirculation injury in systemic sclerosis. Rheumatology (Oxford). 2009;48(6):696-703. doi: 10.1093/rheumatology/kep066 [DOI] [PubMed] [Google Scholar]
- 46.Holló G, Lakatos P, Farkas K. Cold pressor test and plasma endothelin-1 concentration in primary open-angle and capsular glaucoma. J Glaucoma. 1998;7(2):105-110. [PubMed] [Google Scholar]
- 47.Hammer T, Schlötzer-Schrehardt U, Naumann GOH. Unilateral or asymmetric pseudoexfoliation syndrome? an ultrastructural study. Arch Ophthalmol. 2001;119(7):1023-1031. doi: 10.1001/archopht.119.7.1023 [DOI] [PubMed] [Google Scholar]
- 48.Holló G. Vascular dysfunction in exfoliation syndrome: vascular dysfunction in exfoliation syndrome. J Glaucoma. 2018;27(suppl 1):S72-S74. [DOI] [PubMed] [Google Scholar]
- 49.Atalar PT, Atalar E, Kilic H, et al. . Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J. 2006;47(1):77-84. doi: 10.1536/ihj.47.77 [DOI] [PubMed] [Google Scholar]
- 50.Aboobakar IF, Johnson WM, Stamer WD, Hauser MA, Allingham RR. Major review: exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp Eye Res. 2017;154:88-103. doi: 10.1016/j.exer.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 51.Bernstein AM, Ritch R, Wolosin JM. Exfoliation syndrome: a disease of autophagy and LOXL1 proteopathy. J Glaucoma. 2018;27(suppl 1):S44-S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiggs JL, Kang JH, Fan B, Levkovitch-Verbin H, Pasquale LR. A role for clusterin in exfoliation syndrome and exfoliation glaucoma? J Glaucoma. 2018;27(suppl 1):S61-S66. doi: 10.1097/IJG.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrás T. A single gene connects stiffness in glaucoma and the vascular system. Exp Eye Res. 2017;158:13-22. doi: 10.1016/j.exer.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf). 2011;202(3):271-284. doi: 10.1111/j.1748-1716.2010.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visontai Z, Merisch B, Kollai M, Holló G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol. 2006;90(5):563-567. doi: 10.1136/bjo.2005.087908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konieczka K, Ritch R, Traverso CE, et al. . Flammer syndrome. EPMA J. 2014;5(1):11. doi: 10.1186/1878-5085-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbosa-Breda J, Van Keer K, Abegão-Pinto L, et al. . Improved discrimination between normal-tension and primary open-angle glaucoma with advanced vascular examinations—the Leuven Eye Study. Acta Ophthalmol. 2018. [DOI] [PubMed] [Google Scholar]
- 58.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27(2):94-101. doi: 10.1097/ICU.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flammer J, Orgül S, Costa VP, et al. . The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359-393. doi: 10.1016/S1350-9462(02)00008-3 [DOI] [PubMed] [Google Scholar]
- 60.Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed Res Int. 2015;2015:141905. doi: 10.1155/2015/141905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown CM, Dütsch M, Michelson G, Neundörfer B, Hilz MJ. Impaired cardiovascular responses to baroreflex stimulation in open-angle and normal-pressure glaucoma. Clin Sci (Lond). 2002;102(6):623-630. doi: 10.1042/cs1020623 [DOI] [PubMed] [Google Scholar]
- 62.Cutolo M, Sulli A, Pizzorni C, et al. . Capillaroscopy In: Matucci-CerinicM, Furst D, Fiorentino D, eds. Skin Manifestations in Rheumatic Disease. New York, NY: Springer; 2014:93-99. [Google Scholar]
- 63.Flammer J, Haefliger IO, Orgül S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8(3):212-219. doi: 10.1097/00061198-199906000-00012 [DOI] [PubMed] [Google Scholar]
- 64.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4(1):14. doi: 10.1186/1878-5085-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pazos-Moura CC, Moura EG, Bouskela E, Torres Filho IP, Breitenbach MMD. Nailfold capillaroscopy in non-insulin dependent diabetes mellitus: blood flow velocity during rest and post-occlusive reactive hyperaemia. Clin Physiol. 1990;10(5):451-461. doi: 10.1111/j.1475-097X.1990.tb00825.x [DOI] [PubMed] [Google Scholar]