This phase 3 randomized clinical trial evaluates the clinical benefit of taxane plus platinum regimens compared with standard doxorubicin plus cisplatin in postoperative adjuvant chemotherapy for endometrial cancer.
Key Points
Question
Will taxane plus platinum regimens show superiority over standard doxorubicin plus cisplatin in postoperative adjuvant chemotherapy treatment of patients with high-risk early stage or optimally debulked advanced-stage endometrial cancer?
Findings
This multicenter, phase 3 randomized clinical trial provides an analysis of progression-free survival in 788 patients on postoperative taxane plus platinum regimens, including docetaxel plus cisplatin or paclitaxel plus carboplatin, as compared with doxorubicin plus cisplatin. Taxane plus platinum regimens did not demonstrate a survival benefit over doxorubicin plus cisplatin, though they were well tolerated and had different profiles of toxicities from that of doxorubicin plus cisplatin.
Meaning
This study indicates that taxane plus platinum regimens can be an alternative to doxorubicin plus cisplatin in adjuvant chemotherapy for patients with endometrial cancer that has risk factors for progression.
Abstract
Importance
The efficacy of taxane plus platinum regimens has been demonstrated for advanced or recurrent endometrial cancer; however, it has not been assessed in postoperative adjuvant chemotherapy for endometrial cancer.
Objective
To evaluate the clinical benefit of taxane plus platinum compared with standard doxorubicin plus cisplatin as postoperative adjuvant chemotherapy in endometrial cancer.
Design, Setting, and Participants
In this multicenter, open-label, phase 3 randomized clinical trial, patients with endometrial cancer at high-risk stage I or II or stage III or IV that did not extend beyond the abdominal cavity and had 2 cm or greater residual tumor were included from 118 institutions in Japan from November 24, 2006, to January 7, 2011. Data was analyzed from March 15, 2017, to June 30, 2017.
Interventions
Eligible patients were randomly assigned (1:1:1) to receive 6 cycles of doxorubicin, 60 mg/m2, plus cisplatin, 50 mg/m2, on day 1; docetaxel, 70 mg/m2, plus cisplatin, 60 mg/m2, on day 1; or paclitaxel, 180 mg/m2, plus carboplatin (area under the curve, 6.0 mg/mL × min) on day 1 every 3 weeks.
Main Outcomes and Measures
The primary end point was progression-free survival. Secondary end points were overall survival, occurrence of adverse events, tolerability, and status of lymph node dissection.
Results
Among 788 eligible patients, the median (SD) age was 59 (22-74) years; 263 patients were assigned to doxorubicin plus cisplatin treatment, 263 patients to docetaxel plus cisplatin treatment, and 262 patients to paclitaxel plus carboplatin treatment. The number of patients who did not complete 6 cycles was 53 (20.1%) for the doxorubicin plus cisplatin group, 45 (17.1%) for the docetaxel plus cisplatin group, and 63 (24.0%) for the paclitaxel plus carboplatin group. Tolerability of these regimens were not statistically different. After a median follow-up period of 7 years, there was no statistical difference of progression-free survival (doxorubicin plus cisplatin, 191; docetaxel plus cisplatin, 208; paclitaxel plus carboplatin, 187; P = .12) or overall survival (doxorubicin plus cisplatin, 217; docetaxel plus cisplatin, 223; paclitaxel plus carboplatin, 215; P = .67) among the 3 groups. The 5-year progression-free survival rate was 73.3% for the doxorubicin plus cisplatin group, 79.0% for the docetaxel plus cisplatin group, and 73.9% for the paclitaxel plus carboplatin group, while the 5-year overall survival rates were 82.7%, 88.1%, and 86.1%, respectively.
Conclusions and Relevance
There was no significant difference of survival among patients receiving doxorubicin plus cisplatin, docetaxel plus cisplatin, or paclitaxel plus carboplatin as postoperative adjuvant chemotherapy for endometrial cancer. Because each regimen showed adequate tolerability but different toxic effects, taxane plus platinum regimens may be a reasonable alternative to treatment with doxorubicin plus cisplatin.
Trial Registration
UMIN-CTR identifier: UMIN000000522
Introduction
Initial treatment for endometrial cancer is surgery, including total hysterectomy. Risk factors for recurrence include deep myometrial invasion, cervical stromal invasion, lymphovascular invasion, tumor histology, extrauterine progression, and distant metastasis.1 For patients with risk factors, postoperative adjuvant therapy is indicated to reduce the risk of recurrence. In patients with early disease, postoperative radiotherapy has been shown to reduce local recurrence, but a survival benefit has not been demonstrated.2,3,4,5,6 In patients with advanced disease, postoperative systemic chemotherapy was shown to have a survival benefit comparable with radiotherapy.7
For advanced or recurrent endometrial cancer, doxorubicin plus cisplatin has been the standard treatment regimen.8,9 From the results of GOG122,7 doxorubicin plus cisplatin is also recommended as the standard treatment regimen for adjuvant chemotherapy. More recently, taxanes such as paclitaxel and docetaxel were reported to be effective,10,11 and paclitaxel has been combined with platinum to form paclitaxel with doxorubicin plus cisplatin12 and paclitaxel plus carboplatin.13 The interim analysis of the GOG209 study14 showed the noninferiority of paclitaxel plus carboplatin to paclitaxel with doxorubicin plus cisplatin. However, the role of taxanes plus platinum as adjuvant chemotherapy has not been well established. A phase 2 study15 was conducted to investigate various taxane plus platinum therapies for treatment of advanced or recurrent endometrial cancer to explore the clinical effects available as adjuvant therapy. The response rate achieved with paclitaxel plus carboplatin, docetaxel plus cisplatin, and docetaxel plus carboplatin were better than that of doxorubicin plus cisplatin as historical control. Among these regimens, paclitaxel plus carboplatin and docetaxel plus cisplatin were considered to warrant further exploration in a phase 3 study.
Establishment of evidence and validation of the optimal postoperative adjuvant chemotherapy regimen for endometrial cancer are important issues. We conducted a phase 3 randomized clinical trial to compare taxane plus platinum with doxorubicin plus cisplatin in patients with endometrial cancer that had risk factors for progression after surgery.
Methods
Study Design
This multicenter, open-label, phase 3 randomized clinical trial was performed to determine if docetaxel plus cisplatin or paclitaxel plus carboplatin was superior to doxorubicin plus cisplatin or each other as adjuvant chemotherapy in patients with endometrial cancer at a high risk of progression following surgery. The primary end point was progression-free survival (PFS), and the secondary endpoints were overall survival, occurrence of adverse events (AEs), tolerability of treatment, and status of lymph node dissection. This study was designed by the Japanese Gynecologic Oncology Group (JGOC) (protocol number: JGOG2043) and carried out by JGOG institutions.
Patients
Eligible patients had histologically confirmed endometrial cancer that was classified as having a high risk of progression. Cancer with a high risk of progression was defined as being in stages I or II16 with myometrial invasion exceeding half and histologic grade 2 or 3 (including poor prognosis histologic types such as serous, clear cell, and undifferentiated), as well as stage III and IV cancers without metastasis beyond the abdominal cavity. Other inclusion criteria were (1) patients who had undergone total abdominal hysterectomy with bilateral salpingo-oophorectomy and pelvic lymph node dissection in which the residual tumor was 2 cm or less, (2) patients scheduled to receive chemotherapy within 8 weeks postsurgery, (3) patients without prior chemotherapy or radiotherapy, (4) patients aged between 20 and 74 years at registration, (5) patients having an Eastern Cooperative Oncology Group performance status of 0 to 2, and (5) patients with adequate function of major organs (eg, bone marrow, heart, liver, kidneys). Patients were excluded if they had sarcomatous component, serious complications, concurrent infection, or a simultaneous cancer or a history of other cancer within the past 5 years.
Written informed consent was obtained from all patients prior to enrollment in the study. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and the protocol was approved by the institutional review board of each participating clinical site prior to patient enrollment.
Randomization and Treatment
Eligible patients were randomly allocated by the JGOG data center to 1 of 3 groups at a 1:1:1 ratio using surgical stage (I or II vs III or IV) and tumor histology (G1 or G2 vs G3 or histology with a poor prognosis) as stratification factors. For the doxorubicin plus cisplatin regimen, doxorubicin, 60 mg/m2, was administered intravenously followed by intravenous infusion of cisplatin, 50 mg/m2, over at least 2 hours on day 1. For the docetaxel plus cisplatin regimen, docetaxel, 70 mg/m2, was infused over 1 to 2 hours followed by intravenous infusion of cisplatin, 60 mg/m2, over at least 2 hours on day 1. For the paclitaxel plus carboplatin regimen, paclitaxel, 180 mg/m2, was infused over 3 hours followed by intravenous infusion of carboplatin (area under the curve, 6 mg/mL × min) over at least 1 hour on day 1. For calculation of doses, the upper limit of the body surface area was set as 2.0 m2. The dose of carboplatin was calculated by Calvert formula using the glomerular filtration rate as determined by Jelliffe formula, and the upper limit was set at 1000 mg. Treatment was repeated every 3 weeks for a total of 6 cycles unless disease progression or unacceptable toxicity was observed. After completion of treatment, new anticancer therapy was not permitted unless recurrence or progression occurred.
The protocol allowed the timing of administration and drug doses to be changed in accordance with predefined rules. Administration could be delayed up to 3 weeks to allow recovery from AEs. The dose could be reduced by about 20% and up to twice depending on the severity of AEs.
Study Assessments
Progression-free survival was defined as the time to recurrence, progression, or death from any cause, with the date of study registration for randomization being day 1. Overall survival was defined as time to death from any cause, with the date of study registration for randomization being day 1. Patients without such events or patients lost to follow-up were censored at the latest survival date. Follow-up investigation was performed every 6 months for at least 5 years.
Adverse events were documented from start of study treatment through 30 days after the last treatment and were assessed and collected based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. To assess treatment status, the following information was recorded: (1) dose reduction, delay, or discontinuation and the reasons; (2) actual total dose administrated; and (3) relative dose intensity. Relative dose intensity was calculated as the ratio of the actual total dose delivered to the predicted total dose. The number of lymph nodes dissected was also summarized.
Statistical Analysis
In accordance with the intention-to-treat principle, all randomized patients were used for efficacy analysis. The goal of this study was to perform all pairwise comparisons among 3 groups based on the primary end point, PFS. To avoid multiplicity for all of the 3 pairwise tests, the following testing procedure was planned in this study. First, the global null hypothesis (eg, the PFS functions in all 3 groups were identical) was tested using log-rank test with 2-sided P = .05 considered statistically significant. Second, only when the global null hypothesis was rejected, all pairwise comparisons among the 3 groups were performed using log-rank with 2-sided P = .05 considered statistically significant.
Using data from a previous study,17 it was estimated that the 5-year PFS would be about 75% for patients on the doxorubicin plus cisplatin regimen. Based on the results of previous studies,15,17 if the hazard ratio (HR) of the current study treatment (docetaxel plus cisplatin or paclitaxel plus carboplatin) vs doxorubicin plus cisplatin for PFS is 63%, the study treatment would be considered clinically significant. To robustly detect these clinical improvements with 80% power, which is defined by the probability that the global null hypothesis and any paired null hypotheses were rejected simultaneously, under the aforementioned procedure in an enrollment period of 4 years and a follow-up period of 5 years, 250 patients per treatment group were required in 10 000 simulation studies. The recruitment target was 260 patients with 10 dropouts per treatment group.
Progression-free survival and overall survival curves were estimated by the Kaplan-Meier method. Subgroup analysis of PFS was carried out using a Cox proportional hazards model to examine the effect of patient demographic factors on the treatment effect. All patients who received at least one dose of the study treatment were included in the safety analysis. To assess AEs, the number of patients with AEs of each grade, the number with grade 3 or higher AEs, and the incidence proportions were calculated for each treatment group. A Mantel-Haenszel test was performed for intergroup comparisons with 2-sided P = .05 considered statistically significant. To assess treatment status, intergroup comparison of each parameter was performed using Fisher exact tests. Statistical analyses were carried out using SAS, version 9.3 (SAS Institute Inc).
Results
Subjects
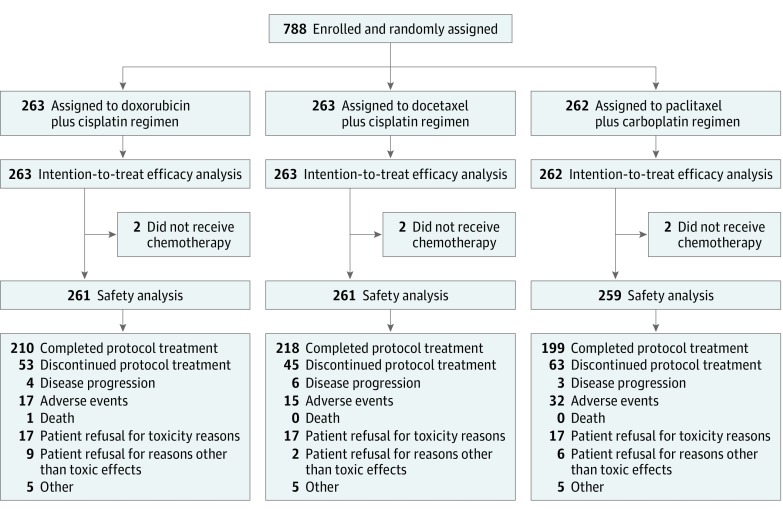
A total of 788 patients were enrolled at 118 clinical sites from November 24, 2006, through January 7, 2011. Patients were randomized as follows: 263 patients to the doxorubicin plus cisplatin treatment group, 263 patients to the docetaxel plus cisplatin treatment group, and 262 patients to the paclitaxel plus carboplatin treatment group. These patients were subjected to efficacy analysis. Seven patients did not receive chemotherapy, and safety analysis was performed in 781 patients after excluding these patients. Patient disposition is shown in Figure 1. Baseline characteristics were balanced among the 3 treatment groups, as summarized in eTable 1 in the Supplement. Most patients had endometrioid carcinoma and no residual tumor. In 454 of 788 (57.6%) patients, both pelvic and para-aortic lymph node dissection were performed.
Figure 1. Randomization and Follow-up of Participants.
The median number (range) of dissected lymph nodes was 24 (1-83) in the pelvic region and 13 (1-67) in the para-aortic region. Pelvic lymph node metastasis was observed in 257 (32.7%) patients, while para-aortic lymph node metastasis was found in 122 (15.5%) patients. Lymph node metastasis was detected in 93 (35.4%) patients from the doxorubicin plus cisplatin group, 95 (36.1%) patients from the docetaxel plus cisplatin group, and 93 (35.5%) patients from the paclitaxel plus carboplatin group.
Efficacy
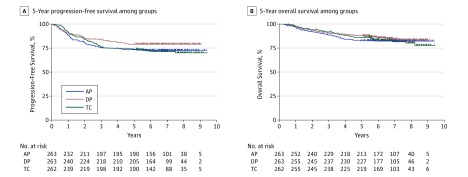
The final analysis was carried out after data cutoff on January 7, 2016. In the intention-to-treat analysis set (n = 788), the median follow-up period was 7 years. During the observation period, 202 patients showed progression or death. Death was confirmed for 133 patients. The 5-year PFS was 73.3% in the doxorubicin plus cisplatin group, 79.0% in the docetaxel plus cisplatin group, and 73.9% in the paclitaxel plus carboplatin group, with no significant differences among the 3 groups (2-sided P = .12) (Figure 2A). Because uniformity of the PFS hazard among the 3 groups was not rejected, comparison of PFS between each pair of groups was not completed.
Figure 2. Progression-Free Survival and Overall Survival of the Study Cohort.
Following a 2-sided log-rank test after a median follow-up period of 7 years, among patients in the doxorubicin plus cisplatin (AP) treatment group, docetaxel plus cisplatin (DP) treatment group, and paclitaxel plus carboplatin (TC) treatment group, there was no statistical difference of progression-free survival (A) (AP, 72 of 263 patients; DP, 55 of 263 patients; TC, 75 of 262 patients; P = .12) nor overall survival (B) (AP, 46 of 263 patients; DP, 40 of 263 patients; TC, 47 of 262 patients; P = .67).
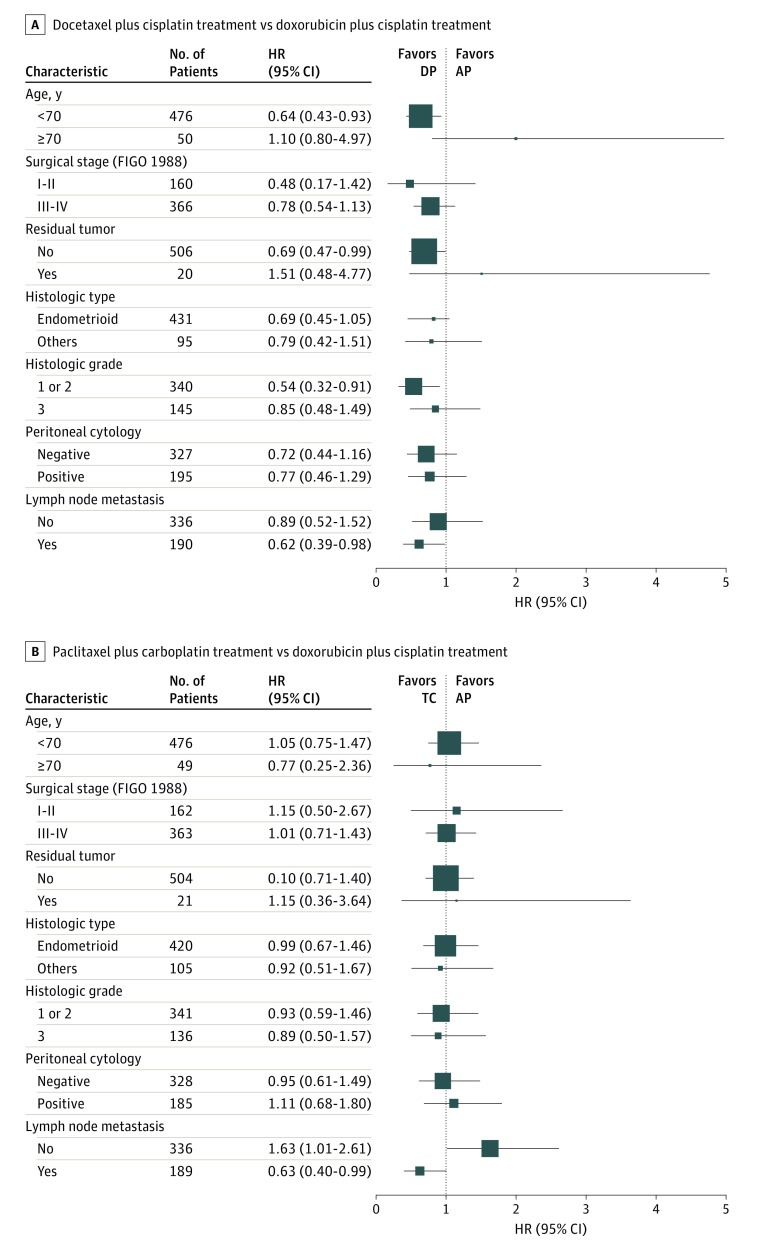
To identify subgroups responding to each study treatment, PFS was analyzed in various patient subpopulations (Figure 3). Compared with the doxorubicin plus cisplatin group, the patient subpopulations with better PFS in the docetaxel plus cisplatin group were younger than 70 years (HR, 0.64; 95% CI, 0.43-0.93), had no residual tumor (HR, 0.69; 95% CI, 0.47-0.99), had tumor histologic grade 1 or 2 (HR, 0.54; 95% CI, 0.32-0.91), and were positive for lymph node metastasis (HR, 0.62; 95% CI, 0.39-0.98). Compared with the doxorubicin plus cisplatin group, the patient subpopulation with better PFS in the paclitaxel plus carboplatin group had lymph node metastasis (HR, 0.63; 95% CI, 0.40-0.99). Conversely, the subpopulation with worse PFS in the paclitaxel plus carboplatin group compared with the doxorubicin plus cisplatin group had no lymph node metastasis (HR, 1.63; 95% CI, 1.01-2.61). No significant difference in PFS was found among the 3 groups divided by cancer stages I or II (doxorubicin plus cisplatin, 70 of 80 (87.5%) patients progression free; docetaxel plus cisplatin, 75 of 80 (93.7%) patients progression free; paclitaxel plus carboplatin, 70 of 82 (85.4%) patients progression free; P = .23) and by cancer stages III or IV (doxorubicin plus cisplatin, 121 of 183 (66.1%) patients progression free; docetaxel plus cisplatin, 133 of 183 (72.7%) patients progression free; paclitaxel plus carboplatin, 117 of 180 (65.0%) patients progression free; P = .29).
Figure 3. Subgroup Analyses of Progression-Free Survival.
A, Horizontal lines represent the hazard ratio (HR) for patients assigned to docetaxel plus cisplatin (DP) treatment compared with those assigned to doxorubicin plus cisplatin (AP) treatment. B, Horizontal lines represent the HR for patients assigned to paclitaxel plus carboplatin (TC) treatment compared with those assigned to AP. FIGO 1988 indicates the International Federation of Gynecology and Obstetrics surgical staging system developed in 1988.
The 5-year overall survival was 82.7% in the doxorubicin plus cisplatin group, 88.1% in the docetaxel plus cisplatin group, and 86.1% in the paclitaxel plus carboplatin group with no significant differences among the 3 groups (2-sided P = .67) (Figure 2B).
Extent of Exposure
Delivery of treatment is shown in Figure 1. The median number of cycles was 6 (range, 0-6) in all groups. Protocol treatment was discontinued by 53 patients (20.2%; 95% CI, 15.5%-25.5%) in the doxorubicin plus cisplatin group, 45 patients (17.1%; 95% CI, 12.8%-22.2%) in the docetaxel plus cisplatin group, and 63 patients (24.0%; 95% CI, 19.0%-29.7%) in the paclitaxel plus carboplatin group (P = .14). A total of 1419 of 1578 cycles (89.9%; 95% CI, 88.3%-91.4%) were completed in the doxorubicin plus cisplatin group, 1425 of 1578 cycles (90.3%; 95% CI, 88.7%-91.7%) in the docetaxel plus cisplatin group, and 1385 of 1572 cycles (88.1%; 95% CI, 86.4%-89.7%) in the paclitaxel plus carboplatin group (P = .10).
The number of patients in whom treatment was delayed was 188 (72.0%; 95% CI, 66.2%-77.4%) in the doxorubicin plus cisplatin group, 108 (41.4%; 95% CI, 35.3%-47.6%) in the docetaxel plus cisplatin group, and 180 (69.5%; 95% CI, 63.5%-75.1%) in the paclitaxel plus carboplatin group (P < .001). The number of treatment cycles delayed because of AEs was 76 (29.0%; 95% CI, 26.7%-31.5%) in the doxorubicin plus cisplatin group, 23 (8.7%; 95% CI, 7.3%-10.3%) in the docetaxel plus cisplatin group, and 60 (23.0%; 95% CI, 20.8%-25.3%) in the paclitaxel plus carboplatin group (P < .001). In addition, the number of patients with dose reduction was 77 (29.5%; 95% CI, 24.0%-35.4%) in the doxorubicin plus cisplatin group, 56 (21.5%; 95% CI, 16.6%-26.9%) in the docetaxel plus cisplatin group, and 72 (27.8%; 95% CI, 22.4%-33.7%) in the paclitaxel plus carboplatin group (P = .09). A total of 99 of 1419 cycles (7.0%; 95% CI, 5.7%-8.4%) with dose reduction were completed in the doxorubicin plus cisplatin group, 65 of 1425 cycles (4.6%; 95% CI, 3.5%-5.8%) in the docetaxel plus cisplatin group, and 92 of 1385 cycles (6.6%; 95% CI, 5.4%-8.1%) in the paclitaxel plus carboplatin group (P = .01).
Mean (SD) relative dose intensity was 86.7% (11.7%) for doxorubicin and 88.5% (10.6%) for cisplatin in the doxorubicin plus cisplatin group, 92.6% (10.3%) for docetaxel and 92.9% (9.9%) for cisplatin in the docetaxel plus cisplatin group, and 86.4% (12.2%) for paclitaxel and 87.5% (11.5%) for carboplatin in the paclitaxel plus carboplatin group. For all of these indices, treatment with docetaxel plus cisplatin was superior to the other treatment groups.
Safety
One patient died of an AE (suspected myocardial infarction) in the doxorubicin plus cisplatin group. Discontinuation of treatment because of AEs was most frequent in the paclitaxel plus carboplatin group and was required in 17 (6.5%) patients in the doxorubicin plus cisplatin group, 15 (5.7%) patients in the docetaxel plus cisplatin group, and 32 (12.2%) patients in the paclitaxel plus carboplatin group. Treatment-related AEs of grade 3 or higher with a frequency of approximately 5% or greater are summarized for each treatment group in eTable 2 in the Supplement. Leukopenia, neutropenia, febrile neutropenia, and anemia were milder in the docetaxel plus cisplatin group and the paclitaxel plus carboplatin group than in the doxorubicin plus cisplatin group. However, thrombocytopenia was significantly more frequent in the paclitaxel plus carboplatin group. Gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea were more frequent with cisplatin regimen (doxorubicin plus cisplatin and docetaxel plus cisplatin) than with paclitaxel plus carboplatin treatment, while myalgia and neurotoxicity were more frequent with paclitaxel plus carboplatin treatment.
Discussion
The present study was designed to compare PFS between patients treated with taxane plus platinum regimens (docetaxel plus cisplatin and paclitaxel plus carboplatin ) and doxorubicin plus cisplatin, which is standard postoperative adjuvant chemotherapy for endometrial cancer. Although the superiority of docetaxel plus cisplatin and paclitaxel plus carboplatin over doxorubicin plus cisplatin was not demonstrated, we found that the 3 regimens were comparable in therapeutic effect. The same findings were obtained for overall survival, a secondary end point.
The factors associated with a high risk of progression in this study correspond to intermediate risk, high-intermediate risk, high risk, and part of advanced disease according to the European Society for Medical Oncology/European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology consensus conference.1 The indication for adjuvant therapy has varied in other clinical studies. In an Italian study18 and the JGOG2033 study,19 patients had stage IC cancer with myometrial invasion exceeding half through stage III. In a study of 2 randomized clinical trials,17 one trial (NSGO EC-9501/EORTC-55991) enrolled patients who had cancer in stage I, stage II, stage IIIA with positive cytology, and stage IIIC with positive pelvic lymph node metastasis, while the other trial (MaNGO ILIADE-III) enrolled patients who had cancer in stage IIB through III (excluding stage III with positive cytology). In the GOG122 study,7 patients had stage III cancer with less than 2-cm residual tumor and stage IV cancer. The present study encompassed the patient population investigated in previous phase 3 studies of adjuvant therapy. Accordingly, the results can be extrapolated to the majority of patients who require adjuvant therapy. In this study, 5-year PFS was 73.3% in the doxorubicin plus cisplatin group, and the result was within the expected range. In the Italian study,17 JGOG2033, and GOG122, 5-year PFS was 63%, 81.8%, and 42%, respectively, for the chemotherapy group.7,18,19 Integrated analysis of NSGO EC-9501/EORTC-55991 and MaNGO ILIADE-III showed 5-year PFS of 78%, and GOG184 showed 62% to 64% 3-year PFS for the radiation plus subsequent chemotherapy group.17,20 Thus, the results of the present study were favorable compared with previous investigations of adjuvant therapy.
In previous phase 3 studies, lymph node dissection was only an option for staging,7,17,18,19,20 and lymph node dissection did not extend survival time in patients with early stage cancer.21,22 On the other hand, the number of dissected lymph nodes was correlated with detection of metastasis and a better prognosis.23,24 It was also reported that additional para-aortic lymph node dissection might contribute to an improved prognosis,25,26 and the phase 3 trial to investigate the survival benefit of para-aortic lymph node dissection is in progress.27 In this study, lymph node dissection may have contributed to improved overall prognosis, thus offsetting the expected differences between regimens. Meanwhile, subpopulation analyses showed that the PFS of patients with lymph node metastasis was better with docetaxel plus cisplatin or paclitaxel plus carboplatin treatments than doxorubicin plus cisplatin treatment. As adjuvant therapy for patients with breast cancer and lymph node metastasis, docetaxel or paclitaxel combined with anthracycline was shown to be effective, which suggests affinity of taxanes for lymph nodes.28,29,30 Extensive and careful lymph node dissection plus chemotherapy that includes a taxane may be an effective treatment option for patients with a risk of lymph node metastasis.
The incidence of AEs in each treatment group was comparable with those previously reported.7,8,9,15 Doxorubicin plus cisplatin was mainly associated with hematological and gastrointestinal toxicities. Neurotoxicity is a dose-limiting toxicity for paclitaxel plus carboplatin, and it showed a similar trend to the toxicity of paclitaxel with doxorubicin plus cisplatin.12,20,31,32 Docetaxel plus cisplatin was associated with gastrointestinal toxicity, but hematological toxicity was mild. Neutropenia is a dose-limiting toxicity of docetaxel or its combination with carboplatin,15,33 but it seems to be reduced by docetaxel combined with cisplatin.34,35 Although the completion rate in this study was similar to or better than in previous studies,7,18,19,20 docetaxel plus cisplatin appears to be more tolerated than the other regimens.
Conclusions
In conclusion, taxane plus platinum regimens did not demonstrate a survival benefit over treatment with doxorubicin plus cisplatin; therefore, doxorubicin plus cisplatin remains the standard postoperative adjuvant chemotherapy regimen for endometrial cancer at a high risk of progression. Nevertheless, considering efficacy and tolerability, taxane plus platinum regimens may be an alternative to treatment with doxorubicin plus cisplatin.
Trial Protocol.
eTable 1. Baseline patient characteristics
eTable 2. Frequency of grade 3 or higher adverse events.
Data Sharing Statement
References
- 1.Colombo N, Creutzberg C, Amant F, et al. ; ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group . ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26(1):2-30. doi: 10.1097/IGC.0000000000000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355(9213):1404-1411. doi: 10.1016/S0140-6736(00)02139-5 [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Roberts JA, Brunetto VL, et al. ; Gynecologic Oncology Group . A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744-751. doi: 10.1016/j.ygyno.2003.11.048 [DOI] [PubMed] [Google Scholar]
- 4.Blake P, Swart AM, Orton J, et al. ; ASTEC/EN.5 Study Group . Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137-146. doi: 10.1016/S0140-6736(08)61767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong A, Simera I, Collingwood M, Williams C, Kitchener H; Cochrane Gynaecological Cancer Group . Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta-analysis. Ann Oncol. 2007;18(10):1595-1604. doi: 10.1093/annonc/mdm066 [DOI] [PubMed] [Google Scholar]
- 6.Johnson N, Cornes P. Survival and recurrent disease after postoperative radiotherapy for early endometrial cancer: systematic review and meta-analysis. BJOG. 2007;114(11):1313-1320. doi: 10.1111/j.1471-0528.2007.01332.x [DOI] [PubMed] [Google Scholar]
- 7.Randall ME, Filiaci VL, Muss H, et al. ; Gynecologic Oncology Group Study . Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24(1):36-44. doi: 10.1200/JCO.2004.00.7617 [DOI] [PubMed] [Google Scholar]
- 8.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004;22(19):3902-3908. doi: 10.1200/JCO.2004.02.088 [DOI] [PubMed] [Google Scholar]
- 9.van Wijk FH, Aapro MS, Bolis G, et al. ; European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group . Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14(3):441-448. doi: 10.1093/annonc/mdg112 [DOI] [PubMed] [Google Scholar]
- 10.Ball HG, Blessing JA, Lentz SS, Mutch DG. A phase II trial of paclitaxel in patients with advanced or recurrent adenocarcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62(2):278-281. doi: 10.1006/gyno.1996.0227 [DOI] [PubMed] [Google Scholar]
- 11.Katsumata N, Noda K, Nozawa S, et al. Phase II trial of docetaxel in advanced or metastatic endometrial cancer: a Japanese Cooperative Study. Br J Cancer. 2005;93(9):999-1004. doi: 10.1038/sj.bjc.6602817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22(11):2159-2166. doi: 10.1200/JCO.2004.07.184 [DOI] [PubMed] [Google Scholar]
- 13.Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol. 2001;19(20):4048-4053. doi: 10.1200/JCO.2001.19.20.4048 [DOI] [PubMed] [Google Scholar]
- 14.Miller D, Filiaci V, Fleming G, et al. Late-Breaking Abstract 1: randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125(3):771. doi: 10.1016/j.ygyno.2012.03.034 [DOI] [Google Scholar]
- 15.Nomura H, Aoki D, Takahashi F, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Ann Oncol. 2011;22(3):636-642. doi: 10.1093/annonc/mdq401 [DOI] [PubMed] [Google Scholar]
- 16.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96(8):889-892. doi: 10.1111/j.1471-0528.1989.tb03341.x [DOI] [PubMed] [Google Scholar]
- 17.Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—results from two randomised studies. Eur J Cancer. 2010;46(13):2422-2431. doi: 10.1016/j.ejca.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95(3):266-271. doi: 10.1038/sj.bjc.6603279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susumu N, Sagae S, Udagawa Y, et al. ; Japanese Gynecologic Oncology Group . Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108(1):226-233. doi: 10.1016/j.ygyno.2007.09.029 [DOI] [PubMed] [Google Scholar]
- 20.Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol. 2009;112(3):543-552. doi: 10.1016/j.ygyno.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707-1716. doi: 10.1093/jnci/djn397 [DOI] [PubMed] [Google Scholar]
- 22.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK; ASTEC study group . Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125-136. doi: 10.1016/S0140-6736(08)61766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JK, Urban R, Cheung MK, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109(12):2454-2460. doi: 10.1002/cncr.22727 [DOI] [PubMed] [Google Scholar]
- 24.Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107(8):1823-1830. doi: 10.1002/cncr.22185 [DOI] [PubMed] [Google Scholar]
- 25.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375(9721):1165-1172. doi: 10.1016/S0140-6736(09)62002-X [DOI] [PubMed] [Google Scholar]
- 26.Todo Y, Kato H, Minobe S, et al. Initial failure site according to primary treatment with or without para-aortic lymphadenectomy in endometrial cancer. Gynecol Oncol. 2011;121(2):314-318. doi: 10.1016/j.ygyno.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 27.Watari H, Katayama H, Shibata T, et al. ; Gynecologic Cancer Study Group of the Japan Clinical Oncology Group . Phase III trial to confirm the superiority of pelvic and para-aortic lymphadenectomy to pelvic lymphadenectomy alone for endometrial cancer: Japan Clinical Oncology Group Study 1412 (SEPAL-P3). Jpn J Clin Oncol. 2017;47(10):986-990. doi: 10.1093/jjco/hyx108 [DOI] [PubMed] [Google Scholar]
- 28.De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44-53. doi: 10.1200/JCO.2007.11.3787 [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Pienkowski T, Mackey J, et al. ; Breast Cancer International Research Group 001 Investigators . Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302-2313. doi: 10.1056/NEJMoa043681 [DOI] [PubMed] [Google Scholar]
- 30.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976-983. doi: 10.1200/JCO.2003.02.063 [DOI] [PubMed] [Google Scholar]
- 31.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a Gynecologic Oncology Group study. Int J Gynecol Cancer. 2007;17(2):387-393. doi: 10.1111/j.1525-1438.2007.00794.x [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Huang H, Homesley HD, et al. Patient-reported peripheral neuropathy of doxorubicin and cisplatin with and without paclitaxel in the treatment of advanced endometrial cancer: results from GOG 184. Gynecol Oncol. 2010;119(3):538-542. doi: 10.1016/j.ygyno.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 33.Vasey PA, Jayson GC, Gordon A, et al. ; Scottish Gynaecological Cancer Trials Group . Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96(22):1682-1691. doi: 10.1093/jnci/djh323 [DOI] [PubMed] [Google Scholar]
- 34.Aoki D, Watanabe Y, Jobo T, et al. Favourable prognosis with modified dosing of docetaxel and cisplatin in Japanese patients with ovarian cancer. Anticancer Res. 2009;29(2):561-566. [PubMed] [Google Scholar]
- 35.Ninomiya T, Yamagami W, Susumu N, et al. Retrospective analysis on the feasibility and efficacy of docetaxel-cisplatin therapy for recurrent endometrial cancer. Anticancer Res. 2016;36(4):1751-1758. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Baseline patient characteristics
eTable 2. Frequency of grade 3 or higher adverse events.
Data Sharing Statement